Abstract
Background
Neonatal sepsis is a leading cause of perinatal morbidity and mortality. In comparison to adults, neonates exhibit a higher susceptibility to infections. Myeloid-derived suppressor cells (MDSCs) are myeloid cells with suppressive activity on other immune cells accumulating during foetal life and controlling inflammation in neonates. Most studies investigating the mechanisms for MDSC-mediated immune suppression have been focused on T-cells. Thus far, little is known about the role of MDSC for monocyte function.
Methods
The impact of human cord blood MDSCs (CB-MDSCs) on monocytes was investigated in an in vitro model. CB-MDSCs were co-cultured with peripheral blood mononuclear cells and monocytes were analysed for expression of surface markers, T cell stimulatory and phagocytic capacity, as well as the production of intracellular cytokines by flow cytometry.
Results
CB-MDSCs increased the expression of co-inhibitory molecules and decreased the expression of major histocompatibility complex class II molecules on monocytes, leading to an impaired T-cell stimulatory capacity. Upon bacterial stimulation, expression of phagocytosis receptors, phagocytosis rates and production of tumor necrosis factor-α by monocytes was diminished by CB-MDSCs.
Conclusion
We show that CB-MDSCs profoundly modulate monocyte functions, thereby indirectly impairing T-cell activation. Further research is needed to figure out if MDSCs could be a therapeutic target for inflammatory diseases in neonates like neonatal sepsis.
Similar content being viewed by others
Introduction
Neonatal sepsis is one of the most important reasons for neonatal morbidity and mortality. About one in five very low birthweight infants undergo at least one episode of sepsis. Mortality rates still remain considerably high with 15–20% in the United States1 and in Germany.2 The incidence and mortality of neonatal sepsis is inversely related to gestational age, and comparative analysis of nosocomial infection surveillance data suggests a manifold increased risk of sepsis during the neonatal period compared to other age groups (http://www.nrz-hygiene.de/surveillance/kiss/). The high susceptibility to infections is attributed to an altered response of the neonatal immune system in comparison to adults.3 Monocytes play a pivotal role in orchestrating innate and adaptive immune responses. While also described for other immune effector cells, neonatal monocytes exhibit unique characteristics, making them key regulators of neonatal immune functions. Differences include: an impaired activation of Toll-like receptor signalling to lipopolysaccharide,4,5 diminished co-stimulatory capacity6 and altered cytokine production after innate and adaptive immune stimulation.7 However, mechanisms regulating the neonatal immune response and its adaptation towards a mature state are poorly understood.
Myeloid-derived suppressor cells (MDSCs) are myeloid cells with suppressive activity on other immune cells, accumulating during a variety of pathologic circumstances8 such as malignancies, autoimmune diseases and trauma, but also during pregnancy in the maternal and foetal organism.9,10 According to their surface phenotype, granulocytic (GR-MDSCs) and monocytic MDSCs (MO-MDSCs) can be differentiated.8 Recently, we found GR-MDSCs to be highly elevated in the cord blood (CB)10 and that GR-MDSC numbers remain elevated in the peripheral blood of preterm infants during the entire neonatal period before decreasing to adult levels.11 Furthermore, we recently showed that GR-MDSCs from CB (CB-MDSC) modulate T-helper cell responses towards an anti-inflammatory phenotype,12 and efficiently suppress natural killer cells.10 These findings point towards a role of CB-MDSCs in regulating neonatal immune responses, primarily by modulating adaptive immune responses.
Heretofore, little is known about the impact of MDSCs on innate immune functions. The present study was performed to investigate whether CB-MDSCs also modulate phenotype and function of monocytes with the hypothesis that CB-MDSCs induce a neonatal phenotype and functional state in monocytes. We focused on effects of GR-MDSCs, because in our previous studies9,10 only this MDSC subtype was elevated. In an in vitro co-culture model, we now show that CB-MDSCs profoundly modulate monocyte phenotype and function. These results underscore the role of CB-MDSCs in neonatal immune regulation and impaired host defence during the neonatal period, making them a potential target for improving immune responses in neonates.
Methods
Patients
CB was collected from healthy term neonates (≥37 + 0 gestational weeks) immediately after primary caesarean section. Infants with perinatal infections were excluded from the study. Parents gave written, informed consent and the study was approved by the local ethics committee. Peripheral blood from healthy adults was collected from trained adult volunteers.
Cell isolation and culture
Mononuclear cells (MNCs) from heparinized CB (CBMCs) and peripheral blood (PBMCs) and mature polymorphonuclear neutrophils from CB (CB-PMNs) were isolated by density gradient centrifugation (lymphocyte separation medium, Biochrom, Berlin, Germany).
CB-MDSCs were separated from the CBMC fraction by magnetic-activated cell sorting after labelling with anti-CD66b-FITC (Clone G10F5, BD Pharmingen, Heidelberg, Germany) and anti-FITC magnetic beads according to the manufacturer’s protocol (Miltenyi Biotech, Bergisch-Gladbach, Germany). The purity of CD66b+ cells was between 93 and 97%, as determined by flow cytometry. Supplementary Fig. 1a shows purity of CD66b+ cells.
Monocytes were enriched from PBMCs using the Pan Monocyte Isolation Kit (Miltenyi Biotech) according to the manufacturer’s protocol. CD4+ T cells were enriched from PBMCs using the CD4 T cell Isolation Kit (Miltenyi Biotech) according to the manufacturer’s protocol. Dendritic cells (DCs) were depleted from PBMCs using the Blood Dendritic Cell Isolation Kit II (Miltenyi Biotech) according to the manufacturer’s protocol.
PBMCs or monocyte-depleted PBMCs with the addition of isolated monocytes in a 4:1 ratio (target cells, 5 × 105 cells in 500 μl medium) were incubated alone, with CB-MDSCs (effector cells) or with CB-PMNs at different ratios (2.5 × 105 cells in 50 μl medium for a ratio of PBMC:CB-MDSC of 2:1) in RPMI-1640 with 10% foetal calf serum (FCS) in 24-well plates either with direct cell contact or separated by 0.4 μm transwells (Greiner Bio-One, Frickenhausen, Germany) at 37 °C and 5% CO2. After 4 days of culture, cells were harvested and extracellular or intracellular staining was performed. Since the aim of our study was to investigate the effects of CB-MDSCs on monocyte functions and not a general effect of MDSCs, CB-MDSCs were used as effector cells in our assays. They were mixed with adult PBMCs and not with CBMCs as target cells, and as for CB monocytes, an altered phenotype was described with diminished major histocompatibility complex class II (MHC II) expression and co-stimulatory capacity compared to adult monocytes.6,13
In vitro infection model
A clinical isolate of Escherichia coli K1, carrying the green fluorescent protein (gfp)-mut2 encoding plasmid pCD353, expressing a prokaryotic variant of GFP controlled by a lactac promoter (E. coliGFP), or the corresponding wild-type strain were grown on agar plates, either supplemented with kanamycin (50 g/ml; Sigma-Aldrich, Steinheim, Germany) and isopropyl-β-d-thiogalactopyranoside (1 mmol/l, Sigma) for GFP induction or without supplements. After 16 h, a single colony was picked and grown in Lennox L broth medium (Invitrogen, Karlsruhe, Germany) with and without supplements until early logarithmic growth.
For the analysis of phagocytosis, PBMCs (after 96 h of co-culture with CB-MDSCs, CB-PMNs or culture alone) were stimulated with E.coliGFP at a multiplicity of infection (MOI) of 1:50 for 1 h. Bacteria were removed by centrifugation through a FCS cushion. Phagocytosis of E.coliGFP by monocytes was determined by flow cytometry.
For the analysis of cytokine expression, PBMCs (after 48 h of co-culture with CB-MDSCs or culture alone) were stimulated with E. coli at an MOI of 1:50 for 4 h and brefeldin (10 μg/ml, Sigma-Aldrich, Steinheim, Germany). Bacteria was removed by centrifugation through an FCS cushion and intracellular staining for cytokines was performed.
T cell proliferation assay
PBMCs were cultured alone or with CB-MDSCs or CB-PMNs in transwells for 96 h. Afterwards, these pre-cultured PBMCs were added in a 1:1, 1:2 and 1:4 ratio (1 × 105, 5 × 104 and 2.5 × 104 cells in 100 μl medium) to freshly isolated monocyte-depleted PBMCs (after two episodes of 2 h panning) or isolated CD4+ T cells from the same donor (5 × 105 cells in 100 μl medium) stained with carboxyfluorescein-succinimidyl ester (CFSE, Invitrogen, Heidelberg, Germany) according to the manufacturer’s instructions and stimulated either with 0.5 μg/ml OKT3 (Janssen-Cilag, Neuss, Germany) or 0.5 μg/ml tetanus toxin (Merck KGaA, Darmstadt, Germany). As control, only RPMI-1640 was added to the CFSE-stained PBMCs. After 96 h of incubation, cells were harvested and stained with anti-CD4-APC and anti-CD-8-PE. CFSE fluorescence intensity was analysed by flow cytometry to determine proliferation of CD4+ and CD8+ T cells.
Flow cytometry
Antibodies used for extracellular staining of monocytes and their surface molecules were purchased from BD Pharmingen, Heidelberg, Germany (CD14 (clone M5E2), HLA-DR (clone G46-6), CD11b (clone M1-70), CD18 (clone 6.7)), Ebiosciences, San Diego, CA (CD273 (clone MIH18), CD274 (clone MIH1)), Santa Cruz Biotechnology, Dallas, TX (HLA-DP, DP11.1) and Miltenyi Biotech, Bergisch-Gladbach, Germany (HLA-DQ, clone REA303). Intracellular staining of cytokines was performed as described previously.14 Antibodies for intracellular staining were purchased from BD Biosciences. Data acquisition was performed with a FACScalibur flow cytometer (BD Bioscience) and data were analysed via CellQuest (BD Biosciences).
Statistics
Statistical analysis was done with GraphPad Prism version 5.0. Values were tested for Gaussian distribution using D’Agostino and Pearson’s omnibus normality test. As values were not normally distributed, differences in co-culture experiments were determined using the Wilcoxon’s matched-pairs signed-rank test. A p value <0.05 was considered statistically significant.
Results
CB-MDSC induce co-inhibitory molecules and decrease expression of MHC class II molecules on monocytes
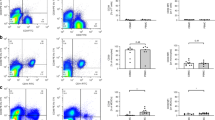
First, we asked whether CB-MDSCs’ influence the expression of co-inhibitory molecules and/or the expression of MHC class II molecules on monocytes, thereby modulating their capacity to stimulate T-cell proliferation. CB-MDSCs were enriched and assessed for their suppressive activity as described previously.15 Supplementary Fig. 1b, c shows percentages of GR-MDSCs in adult blood and CB, as well as the gating strategy for and phenotype of CB-MDSCs. CB-MDSCs were then co-cultured with freshly isolated PBMCs of healthy adult donors for 96 h and then analysed the expression of PD-L1 (CD274), PD-L2 (CD273), HLA-DR, HLA-DQ and HLA-DP. We found an almost 6-fold increase in the expression of PD-L1 (mean fluorescence intensity (MFI) median 1730 vs. 290, n = 7, p = 0.008, Fig. 1a, c) and a 1.5-fold increase in the expression of PD-L2 on monocytes (MFI median 450 vs. 270, n = 7, p = 0.02, Fig. 1d) after co-culture with CB-MDSCs, while expression of HLA-DR, HLA-DQ and HLA-DP strongly decreased (MFI median 140 vs. 730, n = 7, p = 0.008 for HLA-DR, MFI median 165 vs. 1430, n = 6, p = 0.02 for HLA-DQ and MFI median 140 vs. 535, n = 6, p = 0.02 for HLA-DP, Fig. 1b, g–i). Effects of CB-MDSCs on the expression of co-inhibitory molecules and MHC class II molecules were concentration-dependent (Supplementary Fig. 2a, b) and co-culture with mature granulocytes (PMNs) did not have such effects (Supplementary Fig. 2c–g). In order to deduce if alteration of expression of co-inhibitory molecules and MHC class II molecules on monocytes by CB-MDSCs was cell-contact-dependent, we next performed transwell experiments. Separation of CB-MDSCs and PBMCs by a membrane did not abrogate upregulation of co-inhibitory molecules and downregulation of MHC class II molecules, suggesting that soluble factors mediate these effects (Fig. 1e–f, j–l). A migration of CB-MDSCs from the upper to the lower chamber was excluded by CFSE staining of CB-MDSCs (Supplementary Fig. 2h). Further, it was tested whether alteration of monocyte phenotype by CB-MDSCs was dependent on cell types other than monocytes by conducting co-culture experiments with CD14-enriched monocytes instead of full PBMCs. In contrast to the effects observed in CB-MDSC/PBMC co-cultures, the addition of CB-MDSCs to CD14-enriched monocytes led to neither a change in the expression of PD-L1 nor of HLA-DR on monocytes (Fig. 1m, n), pointing towards an indirect impact of CB-MDSCs on monocytes mediated through other cell types in the culture. Interestingly, when using DC-depleted PBMCs as target cells, no upregulation, not even a downregulation of PD-L1 and PD-L2 expression on monocytes, could be observed, suggesting that an interaction between DCs and monocytes is needed for the stimulatory effect of CB-MDSCs on co-inhibitory molecule expression of monocytes (Supplementary Fig. 2i, j).
Expression of co-inhibitory molecules and major histocompatibility complex (MHC) class II molecules on monocytes after co-culture with cord blood myeloid-derived suppressor cells (CB-MDSCs). CB-MDSCs were enriched from cord blood mononuclear cells (CBMCs) and added to peripheral blood mononuclear cells (PBMCs) isolated from a healthy adult control either in direct cell–cell contact or in transwells. After 4 days culture surface staining was performed. a, b Representative density plots showing the expression of PD-L1 (a) and HLA-DR (b) on monocytes with or without co-culture with CB-MDSCs. c–l Scatter plots with bars show the mean fluorescent intensity (MFI) for expression of PD-L1 (c + e), PD-L2 (d + f), HLA-DR (g + j), HLA-DQ (h + k) and HLA-DP (i + l) on monocytes without the addition of CB-MDSCs (white bars) and with the addition of CB-MDSCs (grey bars) in direct cell–cell contact and the mean fluorescent intensity (MFI) for the expression of PD-L1 (c), PD-L2 (d), HLA-DR (g), HLA-DQ (h) and HLA-DP (i) on monocytes without the addition of CB-MDSCs (white bars), with the addition of CB-MDSCs in direct cell–cell contact (grey bars) and with the addition of CB-MDSCs in transwells (checkered bars). Bars represent pooled data from five to seven independent experiments and each point represents an individual sample. *p < 0.05, **p < 0.01, n.s.: not significant; Wilcoxon’s matched-pairs signed-rank test. m, n CB-MDSCs were enriched from CBMCs and added to CD14-enriched monocytes isolated from a healthy adult control. After 4 days of culture surface staining was performed. Scatter plots with bars show the MFI for expression of PD-L1 (m) and HLA-DR (n) on monocytes without the addition of CB-MDSCs (white bars) and with the addition of CB-MDSCs (grey bars). Bars represent pooled data from eight independent experiments and each point represents an individual sample. n.s.: Not significant; Wilcoxon’s matched-pairs signed-rank test
CB-MDSCs impair the ability of monocytes to stimulate antigen-independent and antigen-dependent T-cell proliferation
Next, it was analysed if the altered phenotype of monocytes after co-culture with CB-MDSCs has an impact on their ability to stimulate T-cell proliferation. Therefore, we tested the ability of CB-MDSC-incubated monocytes to stimulate antigen-independent as well as antigen-dependent T-cell proliferation. Since it was shown that expression of co-inhibitory molecules and MHC class II molecules was altered only when full PBMCs (and not CD14-enriched monocytes) were co-cultured with CB-MDSCs, full PBMCs had to be used as effector cells in these proliferation experiments. Supplementary Fig. 3a shows that percentages of monocytes did not differ in PBMC cultures with or without preincubation with CB-MDSCs. Afterwards, freshly isolated CFSE-stained, OKT3-stimulated and monocyte-depleted PBMCs (target cells) were added to PBMCs with and without prior incubation with CB-MDSCs (transwell) from the same donor (effector cells), to analyse the capacity of monocytes to stimulate antigen-independent T-cell proliferation. Stimulation of CD4+ as well as CD8+ T-cell proliferation was lower in cultures where CB-MDSC-pre-incubated monocytes served as effector cells than in cultures where untreated monocytes served as effector cells (proliferation 51.2 ± 19.3% vs. 61.8 ± 21.3% for CD4+ and 60.0 ± 17.7% vs. 70.6 ± 16.3% for CD8+, n = 5, both p < 0.05, Fig. 2a–c). Supplementary Fig. 3b, c shows concentration dependency of antigen-independent T-cell proliferation in the presence of CB-MDSC-pre-incubated PBMCs. Preincubation of PBMCs with CB-PMN led to an even higher T-cell stimulatory capacity, arguing against an unspecific effect (Supplementary Fig. 3f). In order to confirm the experiments with minimized confounding factors from other cells in the culture, monocytes were first depleted from PBMCs and afterwards added back in defined ratios to the monocyte-depleted PBMCs. These cells were incubated with CB-MDSCs and after 4 days the non-adherent PBMCs were removed and the adherent cells were used as effector cells in our proliferation assay. As target cells enriched CD4+ T cells were used. Also under these conditions, CB-MDSCs led to a decreased T cell stimulatory capacity of monocytes (Supplementary Fig. 3g, h).
Stimulation of antigen-independent and antigen-dependent T-cell proliferation by monocytes after co-culture with cord blood myeloid-derived suppressor cells (CB-MDSCs). CB-MDSCs were enriched from cord blood mononuclear cells (CBMCs) and added to peripheral blood mononuclear cells (PBMCs) isolated from a healthy adult control in transwells. After 4 days of culture PBMCs (effector cells) were added to carboxyfluorescein-succinimidyl ester (CFSE)-stained and OKT3-stimulated (a–c) or tetanus toxin-stimulated (d–f) freshly isolated and monocyte-depleted PBMCs from the same donor (target cells). a, d Representative histogram plots show proliferation of CD4+ T-cells (left histograms) and CD8+ T-cells (right histograms) after stimulation with OKT3 (a) or tetanus toxin (d) and without the addition of effector cells (w/o ECs) or with the addition of control effector cells (w ctrl ECs) or CB-MDSC-incubated monocytes (w MDSC-incubated ECs). b, c, e, f Scatter plots with connection lines show proliferation of CD4+ T-cells (b, e) and CD8+ T-cells (c, f) with the addition of control effector cells and with the addition of CB-MDSC-incubated monocytes under stimulation with OKT3 (b, c) or tetanus toxin (e, f). Diagrams represent data from five independent experiments; each point represents an individual sample. *p < 0.05; Wilcoxon’s matched-pairs signed-rank test
In order to test the capacity of monocytes to stimulate antigen-dependent T-cell proliferation, freshly isolated CFSE-stained and monocyte-depleted PBMCs (target cells) were added to PBMCs previously incubated with CB-MDSCs from the same donor (effector cells) and cultures were stimulated with tetanus toxin. Again, stimulation of CD4+ as well as CD8+ T-cell proliferation was lower in cultures where CB-MDSC-pre-incubated monocytes served as effector cells than in cultures where untreated monocytes served as effector cells (proliferation 17.6 ± 7.0% vs. 33.4 ± 14.9% for CD4+ and 21.2 ± 8.4% vs. 31.2 ± 5.4% for CD8+, n = 5, both p < 0.05, Fig. 2d–f). Supplementary Fig. 3d, e shows concentration dependency of antigen-dependent T cell proliferation in the presence of pre-incubated PBMCs.
CB-MDSCs inhibit phagocytic activity of monocytes
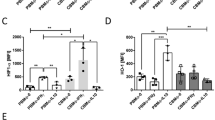
Concerning the effect of CB-MDSCs on phagocytosis of pathogens by monocytes, we first quantified the expression of phagocytosis receptors CD11b and CD18 on monocytes after co-culture with CB-MDSCs. Expression of both CD11b and CD18 was diminished on monocytes after co-culture with CB-MDSCs (MFI median 281 vs. 407 for CD11b and 569 vs. 630 for CD18, both n = 5, p = 0.03, Fig. 3a, b). Supplementary Fig. 4a shows concentration dependency for CD11b expression on monocytes after co-culture with CB-MDSCs. Afterwards, monocytes after culture with or without CB-MDSCs were infected for 1 h with E. coliGFP and quantified phagocytic activity by flow cytometry. Phagocytic capacity was decreased after co-culture with CB-MDSCs (GFP-MFI median 184 vs. 249, n = 5, p = 0.03, Fig. 3c, d), while percentages of phagocyting monocytes were unchanged (62% vs. 70%, n = 5, not significant, Fig. 3e). These effects were specific for CB-MDSCs, as co-culture with mature granulocytes did not change the expression of phagocytosis receptors or phagocytic activity of monocytes (Supplementary Fig. 4b–e). The effect was dependent on other cell types in the culture, as co-culture of CB-MDSCs with CD14-enriched monocytes instead of full PBMCs did not show downregulation of CD11b on monocytes (Supplementary Fig. 4f).
Expression of phagocytosis receptors and phagocytic activity of monocytes under the influence of cord blood myeloid-derived suppressor cells (CB-MDSCs). CB-MDSCs were enriched from cord blood mononuclear cells (CBMCs) and added to peripheral blood mononuclear cells (PBMCs) isolated from a healthy adult control. After 4 days of culture surface staining was performed (a + b) or GFP-expressing E. coli (E.coliGFP) were added to the culture for 1 h. a, b Scatter plots with bars show the mean fluorescent intensity (MFI) for expression of CD11b (a) and CD18 (b) on monocytes without the addition of CB-MDSCs (white bars) and with the addition of CB-MDSCs (grey bars). Bars represent pooled data from five independent experiments and each point represents an individual sample. *p < 0.05; Wilcoxon’s matched-pairs signed-rank test. c Representative histogram plot shows the expression of GFP on monocytes without the addition of CB-MDSCs (white histogram) and with the addition of CB-MDSCs (grey histogram). d, e Scatter plots with bars show the mean fluorescent intensity (MFI) for expression of GFP on monocytes (d) and the percentages of GFP-expressing monocytes (e) without the addition of CB-MDSC (white bars) and with the addition of CB-MDSC (grey bars). Bars represent pooled data from five independent experiments and each point represents an individual sample. n.s. Not significant; *p < 0.05; Wilcoxon’s matched-pairs signed-rank test
CB-MDSCs suppress TNF-α production of monocytes after bacterial stimulation
Lastly, the influence of CB-MDSCs on the production of pro-inflammatory cytokines by monocytes after bacterial stimulation was analysed. One hour after infection with E. coli, production of tumor necrosis factor-α (TNF-α) by monocytes previously co-cultured with CB-MDSCs was reduced in comparison to monocytes cultured without CB-MDSCs (MFI median 44 vs. 71, n = 7, p = 0.008, Fig. 4a, d), while production of interleukin-8 (IL-8) was increased (MFI median 383 vs. 176, n = 7, p = 0.008, Fig. 4b, e) and production of IL-1β remained unchanged (Fig. 4c, f). Interestingly, the effect on TNF-α production was cell-contact-independent, while effect on IL-8 production could not be reproduced in transwell experiments (Supplementary Fig. 5a, b).
Cytokine expression of monocytes under the influence of cord blood myeloid-derived suppressor cells (CB-MDSCs). CB-MDSCs were enriched from CBMCs and added to peripheral blood mononuclear cells (PBMCs) isolated from a healthy adult control. After 2 days of culture, cells were stimulated for 4 h with E. coli and brefeldin and intracellular cytokine staining was performed. a, b Representative histogram plots show expression of tumor necrosis factor-α (TNF-α) (a), interleukin-8 (IL-8) (b) and IL-1β (c) on monocytes without the addition of CB-MDSCs (white histogram) and with the addition of CB-MDSCs (grey histogram). d–f Scatter plots with bars show the mean fluorescent intensity (MFI) for expression of TNF-α (d), IL-8 (e) and IL-1β (f) on monocytes without the addition of CB-MDSCs (white bars) and with the addition of CB-MDSCs (grey bars). Bars represent pooled data from six to seven independent experiments and each point represents an individual sample. *p < 0.05, **p < 0.01; Wilcoxon’s matched-pairs signed-rank test
Effects of cord blood myeloid-derived suppressor cells (CB-MDSCs) on monocytes. In our in vitro experiments, we could show that co-culture of CB-MDSCs with peripheral blood mononuclear cells (PBMCs) led to a downregulation of major histocompatibility complex (MHC) class II molecules and phagocytosis receptors on monocytes, while expression of co-inhibitory molecules on monocytes was increased in CB-MDSCs. This led to a diminished capacity of monocytes to stimulate T cell proliferation and phagocytosis of pathogens. Furthermore, CB-MDSCs inhibited the production of the pro-inflammatory cytokine tumor necrosis factor-α (TNF-α) by monocytes
Discussion
Recent evidence suggests that MDSCs not only modulate immune responses during pathological processes like cancer, infections or autoimmune diseases16 but also under physiological conditions such as pregnancy and foetal/neonatal life.17,18,19 While they seem to facilitate maternal–foetal tolerance during pregnancy,14,19 their role during immune adaptation of the neonate is not yet clearly defined. On the one hand, MDSCs may control inflammation during early microbial colonization,18 on the other hand, they may contribute to the high neonatal susceptibility to infections.11 Thus far, suppression and modulation of T cell responses have been described as the main effector mechanisms by which MDSCs exert their immune-modulatory properties in neonates,10,15,18 while little is known about interactions between MDSCs and monocytes in neonates. Consequently, effects of CB-MDSCs on basic monocyte functions like co-stimulation, antigen presentation, phagocytosis and cytokine production were analysed in an in vitro co-culture model. We found that co-culture with CB-MDSCs (1) led to increased expression of co-inhibitory molecules and decreased expression of MHC class II molecules on monocytes, (2) reduced the ability of monocytes to stimulate antigen-independent and antigen-dependent T cell proliferation, (3) caused diminished expression of phagocytosis receptors on monocytes as well as reduced phagocytic activity and (4) altered cytokine expression of monocytes after bacterial stimulation (Fig. 5).
To date, the expression of PD-L1 and PD-L2 on foetal compared to adult monocytes is unknown, while data on the expression of the co-stimulatory molecules B7-1 and B7-2 on CB monocytes are conflicting.20,21 It was shown that upon stimulation with interferon-γ (IFNγ), upregulation of B7-1 and B7-2 was diminished in CB compared to adult monocytes6 and that B7-2 expression was diminished on neonatal monocytes in a humanized mouse model.13 Together with the present results, it can be hypothesized that neonatal monocytes exhibit a more inhibitory rather than stimulatory phenotype. Intriguingly, induction of T-regulatory cells (Tregs) in neonates was found to be mediated through the PD-1/PD-L1 axis.22 Thus, increased expression of PD-L1 on monocytes, induced by high levels of MDSCs, could be a mechanism for elevated Treg numbers in neonates.23 Intriguingly, in our experimental setting, the presence of DCs seemed to play a role in PD-L1 and PD-L2 induction by CB-MDSCs, since depletion of DCs led to PD-L1 and PD-L2 downregulation. While potential mechanisms remain elusive, several cytokines and metabolites, which might be regulated by DCs, such as TNF-α24 and reactive oxygen species (ROS),25 might be interesting candidates for future research.
The finding that CB-MDSCs inhibit expression of the MHC class II molecules HLA-DR, HLA-DQ and HLA-DP on monocytes is in line with previous studies showing that MHC class II expression and antigen presentation may be reduced in neonatal compared to adult monocytes13 and that MHC class II expression was positively correlated with gestational age.26 Some studies described diminished HLA-DR expression on monocytes in neonatal patients with sepsis or respiratory distress syndrome.26,27 Recently, an increased GR-MDSC accumulation was shown in preterm infants with sepsis,11 potentially explaining the low HLA-DR expression. However, it is not yet clear if GR-MDSC accumulation during neonatal sepsis is protective or harmful to the infant. This question will be addressed in ongoing studies. As MO-MDSCs in humans are phenotypically defined as CD14+/HLA-DRlow/− 8 cells, one may hypothesize that CB-MDSCs lead to an induction of MO-MDSCs from monocytes. The proliferation experiments of the present study, however, argue against this hypothesis, as they showed that CB-MDSC-pre-incubated monocytes still exhibited stimulatory, not inhibitory, properties on T-cells, albeit to a lower extent than monocytes not pre-incubated with CB-MDSCs.
A former study of our group showed that T-cell proliferation in CBMCs was lower than T-cell proliferation in PBMCs. Co-culture of monocytes isolated from CB with monocyte-depleted MNCs from adult blood led to a lower T-cell proliferation compared to co-culture between adult monocytes and adult monocyte-depleted MNCs. Inversely co-culture of monocytes isolated from adult blood with monocyte-depleted MNCs from CB led to a decreased T-cell proliferation compared to co-culture between CB monocytes and CB monocyte-depleted MNCs.6 The results of the present study showing that CB-MDSCs reduce the T-cell stimulatory capacity of monocytes now provide a possible explanation for these effects.
There exists a disparity between the reduced expression of phagocytosis receptors and reduced phagocytic activity of monocytes after co-culture with CB-MDSCs and previous results investigating phagocytic activity in neonates, suggesting a similar phagocytic activity of neonatal compared to adult monocytes;28,29 phagocytic activity even seemed to increase with lower gestational age (reviewed in ref. 30). Yet, there are also studies showing a lower phagocytic activity in neonatal than in adult monocytes.31 We have shown that GR-MDSCs were capable of taking up bacteria by phagocytosis.32 In the present study, mature PMNs had no suppressive effect on phagocytic activity of monocytes, excluding a competition for bacteria between GR-MDSCs and monocytes as the cause of the lower phagocytic activity observed. The inhibition of monocyte phagocytosis by CB-MDSCs could be a cause for the higher bacterial load observed during neonatal compared to adult sepsis.33
Regarding the cytokine response of neonatal monocytes, now study has yet used bacterial stimuli, making it difficult to compare them to our results. Most studies analysed immune cells from preterm infants (reviewed in ref. 30) Studies comparing neonatal with adult monocytes showed lower or similar expression of TNF-α, IL-8 and IL-1β.34,35,36,37 As TNF-α is one of the most important cytokine-mediating pro-inflammatory immune responses with several functions such as immune cell recruitment, angiogenesis, increasing phagocytic activities, triggering production of other cytokines and others,38 it fits the context of anti-inflammatorily acting CB-MDSCs downregulate TNF-α expression. The main function of IL-8 is chemotaxis of neutrophilic cells to inflamed tissue;39 MDSCs are also attracted by IL-8.40 Upregulated IL-8 receptors CXCR1 and CXCR2 were described on activated placental GR-MDSCs, so that it could be hypothesized that increasing the monocytic IL-8 production by CB-MDSCs acts as a positive feedback mechanism for further recruitment and activation of MDSCs.
In conclusion, CB-MDSCs have impact on basic functions of monocytes, co-stimulation and antigen presentation of T-cells, phagocytosis and cytokine production. Thereby, a novel mechanism by which CB-MDSCs may influence the neonatal immune response is illustrated. Moreover, we present here an explanation for mechanisms leading to the distinct neonatal monocyte phenotype. During pregnancy, foetal MDSCs may be essential for immune tolerance towards maternal antigens as it has been described on the maternal side.41,42,43 After birth, neonatal MDSCs seem to prevent autoimmune inflammation during immune adaptation,18 but also may contribute to the increased susceptibility to infections in neonates. More studies are needed to better understand the complex role of MDSCs in neonatal immune regulation, and to develop therapeutic strategies targeting MDSC functions.
References
Stoll, B. J. et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 126, 443–456 (2010).
Stichtenoth, G. et al. Major contributors to hospital mortality in very-low-birth-weight infants: data of the birth year 2010 cohort of the German Neonatal Network. Klin. Padiatr. 224, 276–281 (2012).
Dowling, D. J. & Levy, O. Ontogeny of early life immunity. Trends Immunol. 35, 299–310 (2014).
Sadeghi, K. et al. Immaturity of infection control in preterm and term newborns is associated with impaired toll-like receptor signaling. J. Infect. Dis. 195, 296–302 (2007).
Yan, S. R. et al. Role of MyD88 in diminished tumor necrosis factor alpha production by newborn mononuclear cells in response to lipopolysaccharide. Infect. Immun. 72, 1223–1229 (2004).
Orlikowsky, T. W. et al. Expression and regulation of B7 family molecules on macrophages (MPhi) in preterm and term neonatal cord blood and peripheral blood of adults. Cytom. B 53, 40–47 (2003).
Adkins, B. Peripheral CD4+ lymphocytes derived from fetal versus adult thymic precursors differ phenotypically and functionally. J. Immunol. 171, 5157–5164 (2003).
Gabrilovich, D. I. & Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174 (2009).
Kostlin, N. et al. Granulocytic myeloid derived suppressor cells expand in human pregnancy and modulate T-cell responses. Eur. J. Immunol. 44, 2582–2591 (2014).
Rieber, N. et al. Neutrophilic myeloid-derived suppressor cells in cord blood modulate innate and adaptive immune responses. Clin. Exp. Immunol. 174, 45–52 (2013).
Schwarz, J. et al. Granulocytic myeloid-derived suppressor cells (GR-MDSC) accumulate in cord blood of preterm infants and remain elevated during the neonatal period. Clin. Exp. Immunol. 191, 328–337 (2017).
Kostlin, N. et al. Granulocytic myeloid derived suppressor cells from human cord blood modulate T-helper-cell response towards an anti-inflammatory phenotype. Immunology 152, 89–101 (2017).
Gille, C. et al. Monocytes derived from humanized neonatal NOD/SCID/IL2Rgamma(null) mice are phenotypically immature and exhibit functional impairments. Hum. Immunol. 73, 346–354 (2012).
Kostlin, N. et al. Granulocytic myeloid-derived suppressor cells accumulate in human placenta and polarize toward a Th2 phenotype. J. Immunol. 196, 1132–1145 (2016).
Kostlin, N. et al. Granulocytic myeloid-derived suppressor cells from human cord blood modulate T-helper cell response towards an anti-inflammatory phenotype. Immunology 152, 89–101 (2017).
Greten, T. F., Manns, M. P. & Korangy, F. Myeloid derived suppressor cells in human diseases. Int. J. Immunopharmacol. 11, 802–807 (2011).
Kostlin, N. et al. Granulocytic myeloid-derived suppressor cells (GR-MDSC) in breast milk (BM); GR-MDSC accumulate in human BM and modulate T-cell and monocyte function. Front. Immunol. 9, 1098 (2018).
He, Y. M. et al. Transitory presence of myeloid-derived suppressor cells in neonates is critical for control of inflammation. Nat. Med. 24, 224–231 (2018).
Ostrand-Rosenberg, S. et al. Frontline science: myeloid-derived suppressor cells (MDSCs) facilitate maternal-fetal tolerance in mice. J. Leukoc. Biol. 101, 1091–1101 (2017).
Grozdics, E. et al. B7 costimulation and intracellular indoleamine 2,3-dioxygenase expression in umbilical cord blood and adult peripheral blood. Biol. Blood Marrow Transplant. 20, 1659–1665 (2014).
Jones, C. A., Holloway, J. A. & Warner, J. O. Phenotype of fetal monocytes and B lymphocytes during the third trimester of pregnancy. J. Reprod. Immunol. 56, 45–60 (2002).
Rabe, H. et al. Staphylococcus aureus convert neonatal conventional CD4(+) T cells into FOXP3(+) CD25(+) CD127(low) T cells via the PD-1/PD-L1 axis. Immunology 141, 467–481 (2014).
Pagel, J. et al. Regulatory T cell frequencies are increased in preterm infants with clinical early-onset sepsis. Clin. Exp. Immunol. 185, 219–227 (2016).
Hartley, G. et al. Regulation of PD-L1 expression on murine tumor-associated monocytes and macrophages by locally produced TNF-alpha. Cancer Immunol. Immunother. 66, 523–535 (2017).
Roux, C. et al. Reactive oxygen species modulate macrophage immunosuppressive phenotype through the up-regulation of PD-L1. Proc. Natl Acad. Sci. USA. 116, 4326–4335 (2019).
Palojarvi, A. et al. Low monocyte HLA-DR expression as an indicator of immunodepression in very low birth weight infants. Pediatr. Res. 73, 469–475 (2013).
Juskewitch, J. E. et al. Monocyte HLA-DR expression and neutrophil CD64 expression as biomarkers of infection in critically ill neonates and infants. Pediatr. Res. 78, 683–690 (2015).
Gille, C. et al. Phagocytosis and postphagocytic reaction of cord blood and adult blood monocyte after infection with green fluorescent protein-labeled Escherichia coli and group B Streptococci. Cytom. B 76B, 271–284 (2009).
Gille, C. et al. Diminished phagocytosis-induced cell death (PICD) in neonatal monocytes upon infection with Escherichia coli. Pediatr. Res. 63, 33–38 (2008).
de Jong, E. et al. The phenotype and function of preterm infant monocytes: implications for susceptibility to infection. J. Leukoc. Biol. 102, 645–656 (2017).
Silveira-Lessa, A. L. et al. TLR expression, phagocytosis and oxidative burst in healthy and septic newborns in response to Gram-negative and Gram-positive rods. Hum. Immunol. 77, 972–980 (2016).
Leiber, A. et al. Neonatal myeloid derived suppressor cells show reduced apoptosis and immunosuppressive activity upon infection with Escherichia coli. Eur. J. Immunol. 47, 1009–1021 (2017).
Elahi, S. et al. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature 504, 158–162 (2013).
Hartel, C. et al. Cytokine responses correlate differentially with age in infancy and early childhood. Clin. Exp. Immunol. 142, 446–453 (2005).
Granland, C. et al. NOD1 and NOD2 expression and function in very preterm infant mononuclear cells. Acta Paediatr. 103, e212–e218 (2014).
Strunk, T. et al. Responsiveness of human monocytes to the commensal bacterium Staphylococcus epidermidis develops late in gestation. Pediatr. Res. 72, 10–18 (2012).
Wisgrill, L. et al. Reduced TNF-alpha response in preterm neonates is associated with impaired nonclassic monocyte function. J. Leukoc. Biol. 100, 607–612 (2016).
Semenzato, G. Tumour necrosis factor: a cytokine with multiple biological activities. Br. J. Cancer 61, 354–361 (1990).
David, J. M. et al. The IL-8/IL-8R axis: a double agent in tumor immune resistance. Vaccines 4, 22 (2016).
Alfaro, C. et al. Tumor-produced interleukin-8 attracts human myeloid-derived suppressor cells and elicits extrusion of neutrophil extracellular traps (NETs). Clin. Cancer Res. 22, 3924–3936 (2016).
Kostlin, N. et al. HLA-G promotes myeloid-derived suppressor cell accumulation and suppressive activity during human pregnancy through engagement of the receptor ILT4. Eur. J. Immunol. 47, 374–384 (2017).
Kostlin-Gille, N. et al. HIF-1alpha-deficiency in myeloid cells leads to a disturbed accumulation of myeloid derived suppressor cells (MDSC) during pregnancy and to an increased abortion rate in mice. Front. Immunol. 10, 161 (2019).
Pan, T. et al. Myeloid-derived suppressor cells are essential for maintaining feto-maternal immunotolerance via STAT3 signaling in mice. J. Leukoc. Biol. 100, 499–511 (2016).
Acknowledgements
This work has been supported by the research funds from the Medical Faculty of Tuebingen University (grant no. E.05.00433), the Federal Ministry of Education and Research (BMBF), the Ministry for Science, Research and Art of Baden-Württemberg (MWKBW), the European Social Fund (ESF) and the German Centre for Infection Research (DZIF).
Author information
Authors and Affiliations
Contributions
N.K.-G. and C.G. conceptualized and designed the study. N.K.-G., S.D., J.S., M.V., K.M. and B.S. performed experiments. N.K.-G., S.D., M.V. and C.G. analysed data. N.K.-G. drafted the initial manuscript. N.K.-G., T.W.O., C.F.P., F.W., U.H. and C.G. reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Dietz, S., Schwarz, J., Vogelmann, M. et al. Cord blood granulocytic myeloid-derived suppressor cells impair monocyte T cell stimulatory capacity and response to bacterial stimulation. Pediatr Res 86, 608–615 (2019). https://doi.org/10.1038/s41390-019-0504-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0504-7
This article is cited by
-
Impaired functional capacity of polarised neonatal macrophages
Scientific Reports (2020)