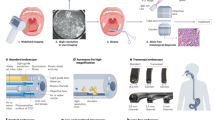
Abstract
Clinical workflows for the non-invasive detection and characterization of disease states could benefit from optical-imaging biomarkers. In this Perspective, we discuss opportunities and challenges towards the clinical implementation of optical-imaging biomarkers for the early detection of cancer by analysing two case studies: the assessment of skin lesions in primary care, and the surveillance of patients with Barrett’s oesophagus in specialist care. We stress the importance of technical and biological validations and clinical-utility assessments, and the need to address implementation bottlenecks. In addition, we define a translational roadmap for the widespread clinical implementation of optical-imaging technologies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Wax, A. et al. Angle-resolved low coherence interferometry for detection of dysplasia in Barrett’s esophagus. Gastroenterology 141, 443–447 (2011).
Imamoto, Y. & Shichida, Y. Cone visual pigments. Biochim. Biophys. Acta 1837, 664–673 (2014).
O’Connor, J. B. P. et al. Imaging biomarker roadmap for cancer studies. Nat. Rev. Clin. Oncol. 14, 169–186 (2017).
Sharma, P. et al. White Paper AGA: Advanced Imaging in Barrett’s Esophagus. Clin. Gastroenterol. Hepatol. 13, 2209–2218.
Thosani, N. et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE Preservation and Incorporation of Valuable Endoscopic Innovations thresholds for adopting real-time imaging–assisted endoscopic targeted biopsy during endoscopic surveillance. Gastrointest. Endosc. 83, 684–698.
Nikolaou, V. & Stratigos, A. J. Emerging trends in the epidemiology of melanoma. Br. J. Dermatol. 170, 11–19 (2014).
Suspected Cancer: Recognition and Referral: NICE Guideline (NG12) (NICE, 2015); https://www.nice.org.uk/guidance/ng12
Terushkin, V. & Halpern, A. C. Melanoma Early Detection. Hematol. Oncol. Clin. North Am. 23, 481–500 (2009).
Lindholm, C. et al. Invasive cutaneous malignant melanoma in Sweden, 1990–1999: A prospective, population-based study of survival and prognostic factors. Cancer 101, 2067–2078 (2004).
Breitbart, E. W. et al. Systematic skin cancer screening in Northern Germany. J. Am. Acad. Dermatol. 66, 201–211 (2012).
Katalinic, A. et al. Does skin cancer screening save lives?: an observational study comparing trends in melanoma mortality in regions with and without screening. Cancer 118, 5395–5402 (2012).
Bibbins-Domingo, K. et al. Screening for Skin Cancer. Jama 316, 429–435 (2016).
Stratigos, A. J. et al. Euromelanoma: a dermatology-led European campaign against nonmelanoma skin cancer and cutaneous melanoma. Past, present and future. Br. J. Dermatol. 167, 99–104 (2012).
Vestergaard, M. E. et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: A meta-analysis of studies performed in a clinical setting. Br. J. Dermatol. 159, 669–676 (2008).
Moncrieff, M. et al. Spectrophotometric intracutaneous analysis: a new technique for imaging pigmented skin lesions. Br. J. Dermatol. 146, 448–457 (2002).
Emery, J. D. et al. Accuracy of SIAscopy for pigmented skin lesions encountered in primary care: development and validation of a new diagnostic algorithm. BMC Dermatol. 10, 9 (2010).
Walter, F. M. et al. Protocol for the MoleMate UK Trial: a randomised controlled trial of the MoleMate system in the management of pigmented skin lesions in primary care. BMC Fam. Pract. 11, 36 (2010).
Walter, F. M. et al. Effect of adding a diagnostic aid to best practice to manage suspicious pigmented lesions in primary care: randomised controlled trial. Br. Med. J. 345, e4110 (2012).
Vasefi, F. et al. Multimode optical dermoscopy (SkinSpect) analysis for skin with melanocytic nevus. Proc. of SPIE 9711, 971110 (2016).
Vasefi., F. et al. Polarization-sensitive hyperspectral imaging in vivo: a multimode dermoscope for skin analysis. Sci. Rep. 4, 4924 (2014).
Vasefi, F. et al. Separating melanin from hemodynamics in nevi using multimode hyperspectral dermoscopy and spatial frequency domain spectroscopy. J. Biomed. Opt. 21, 114001 (2016).
MacKinnon, N. et al. In vivo skin chromophore mapping using a multimode imaging dermoscope (SkinSpect). Proc. SPIE 8587, (1–13 (2013).
Elbaum, M. et al. Automatic differentiation of melanoma from melanocytic nevi with multispectral digital dermoscopy: a feasibility study. J. Am. Acad. Dermatol. 44, 207–218 (2001).
Monheit, G. et al. The performance of MelaFind: a prospective multicenter study. Arch. Dermatol. 147, 188–194 (2011).
Hauschild, A. et al. To excise or not: impact of MelaFind on German dermatologists’ decisions to biopsy atypical lesions. J. Dtsch Dermatol. Ges. 12, 606–614 (2014).
Xiong, Y.-Q. et al. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: a meta-analysis. J. Cancer Res. Clin. Oncol. Springer 1627–1635 (2017).
Segura, S. et al. Development of a two-step method for the diagnosis of melanoma by reflectance confocal microscopy. J. Am. Acad. Dermatol. 61, 216–229 (2009).
Guitera, P. et al. Surveillance for treatment failure of lentigo maligna with dermoscopy and in vivo confocal microscopy: new descriptors. Br. J. Dermatol. 170, 1305–1312 (2014).
Guitera, P. et al. In vivo reflectance confocal microscopy enhances secondary evaluation of melanocytic lesions. J. Invest. Dermatol. 129, 131–138 (2009).
Stanganelli, I. et al. Integration of reflectance confocal microscopy in sequential dermoscopy follow-up improves melanoma detection accuracy. Br. J. Dermatol. 172, 365–371 (2015).
Witkowski, A. M. et al. Non-invasive diagnosis of pink basal cell carcinoma: how much can we rely on dermoscopy and reflectance confocal microscopy? Ski. Res. Technol. 22, 230–237 (2016).
Venturini, M. et al. Reflectance confocal microscopy allows in vivo real-time noninvasive assessment of the outcome of methyl aminolaevulinate photodynamic therapy of basal cell carcinoma. Br. J. Dermatol. 168, 99–105 (2013).
Langley, R. G. B. et al. The diagnostic accuracy of in vivo confocal scanning laser microscopy compared to dermoscopy of benign and malignant melanocytic lesions: a prospective study. Dermatology 215, 365–372 (2007).
Moscarella, E. et al. The role of reflectance confocal microscopy as an aid in the diagnosis of collision tumors. Dermatology 227, 109–117 (2013).
Alarcon, I. et al. Impact of in vivo reflectance confocal microscopy on the number needed to treat melanoma in doubtful lesions. Br. J. Dermatol. 170, 802–808 (2014).
VivaScope 1500 and 3000 imaging systems for detecting skin cancer lesions - guidance (DG19) (NICE, 2015).
Konig, K. & Riemann, I. High-resolution multiphoton tomography of human skin with subcellular spatial resolution and picosecond time resolution. J. Biomed. Opt. 8, 432–439 (2003).
Manfredini, M. et al. High-resolution imaging of basal cell carcinoma: a comparison between multiphoton microscopy with fluorescence lifetime imaging and reflectance confocal microscopy. Ski. Res. Technol. 19, 433–443 (2013).
Seidenari, S. et al. Multiphoton laser tomography and fluorescence lifetime imaging of melanoma: morphologic features and quantitative data for sensitive and specific non-invasive diagnostics,”. PLoS ONE 8, e70682 (2013).
Ulrich, M. et al. Dynamic optical coherence tomography in dermatology. Dermatology 232, 298–311 (2016).
Boone, M. A. L. M. et al. High-definition optical coherence tomography imaging of melanocytic lesions: a pilot study. Arch. Dermatol. Res. 306, 11–26 (2014).
Gambichler, T. et al. High-definition optical coherence tomography of melanocytic skin lesions. J. Biophotonics 8, 681–686 (2015).
Schuh, S. et al. Comparison of different optical coherence tomography devices for diagnosis of non-melanoma skin cancer. Ski. Res. Technol. 22, 395–405 (2016).
Nolan, R. C. et al. Optical coherence tomography for the neurologist. Semin. Neurol. 35, 564–577 (2015).
Tan, A. C. S. et al. An overview of the clinical applications of optical coherence tomography angiography. Eye 32, 262–268 (2017).
Liu, Q. Role of optical spectroscopy using endogenous contrasts in clinical cancer diagnosis. World J. Clin. Oncol. 2, 50–63 (2011).
Kendall, C. et al. Vibrational spectroscopy: a clinical tool for cancer diagnostics. Analyst 134, 1029–1045 (2009).
Pence, I. & Mahadevan-Jansen, A. Clinical instrumentation and applications of Raman spectroscopy. Chem. Soc. Rev. 45, 1958–1979 (2016).
Brancaleon, L. et al. In vivo fluorescence spectroscopy of nonmelanoma skin cancer. Photochem. Photobiol. 73, 178–183 (2001).
Garcia-Uribe, A. et al. Skin cancer detection by spectroscopic oblique-incidence reflectometry: classification and physiological origins. Appl. Opt. 43, 2643–2650 (2004).
Rajaram, N. et al. Pilot clinical study for quantitative spectral diagnosis of non-melanoma skin cancer. Lasers Surg. Med. 42, 716–727 (2010).
Thompson, A. J. et al. In vivo measurements of diffuse reflectance and time-resolved autofluorescence emission spectra of basal cell carcinomas. J. Biophotonics 5, 240–254 (2012).
Garcia-Uribe, A. et al. In vivo diagnosis of melanoma and nonmelanoma skin cancer using oblique incidence diffuse reflectance spectrometry. Cancer Res. 72, 2738–2745 (2012).
Upile, T. et al. Elastic scattering spectroscopy in assessing skin lesions: an ‘in vivo’ study. Photodiagnosis Photodyn. Ther. 9, 132–141 (2012).
Lui, H. et al. Real-time raman spectroscopy for in vivo skin cancer diagnosis. Cancer Res. 72, 2491–2500 (2012).
Zhao, J. et al. Real-time Raman spectroscopy for non-invasive skin cancer detection - preliminary results. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2008, 3107–3109 (2008).
Lim, L. et al. Clinical study of noninvasive in vivo melanoma and nonmelanoma skin cancers using multimodal spectral diagnosis. J. Biomed. Opt. 19, 117003 (2014).
Schleusener, J. et al. In vivo study for the discrimination of cancerous and normal skin using fibre probe-based Raman spectroscopy. Exp. Dermatol. 24, 767–772 (2015).
Zakharov, V. P. et al. Combined Raman spectroscopy and autofluoresence imaging method for in vivo skin tumor diagnosis. Proc. SPIE 9198, (919804 (2014).
Stamnes, J. J. et al. Optical detection and monitoring of pigmented skin lesions. Biomed. Opt. Express 8, 2946–2964 (2017).
Shim, M. G. & B. C. Wilson, B. C. The effects of ex vivo handling procedures on the near-infrared Raman spectra of normal mammalian tissues. Photochem. Photobiol. 63, 662–671 (1996).
Kim, S., Byun, K. M. & Lee, S. Y. Influence of water content on Raman spectroscopy characterization of skin sample. Biomed. Opt. Express 8, 1130–1138 (2017).
Hvid-Jensen, F. et al. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N. Engl. J. Med. 365, 1375–1383 (2011).
Gatenby, P. et al. Lifetime risk of esophageal adenocarcinoma in patients with Barrett’s esophagus. World J. Gastroenterol. 20, 9611–9617 (2014).
Duits, L. C. et al. Barrett’s oesophagus patients with low-grade dysplasia can be accurately risk-stratified after histological review by an expert pathology panel. Gut 64, 700–706 (2015).
Singh, S. et al. Incidence of esophageal adenocarcinoma in Barrett’s esophagus with low-grade dysplasia: a systematic review and meta-analysis. Gastrointest. Endosc. 79, 897–909 (2014).
Fitzgerald, R. C. et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut 63, 7–42 (2014).
Spechler, S. J. et al. American Gastroenterological Association technical review on the management of Barrett’s esophagus. Gastroenterology 140, 18–52 (2011).
Wang., K. K. & Sampliner, R. E. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am. J. Gastroenterol. 103, 788–797 (2008).
Evans, J. A. et al. The role of endoscopy in Barrett’s esophagus and other premalignant conditions of the esophagus. Gastrointest. Endosc. 76, 1087–1094 (2012).
Weusten, B. et al. Endoscopic management of Barrett’s esophagus: European Society of Gastrointestinal Endoscopy (ESGE) position statement. Endoscopy 49, 191–198 (2017).
Rubenstein, J. H. et al. Effect of a prior endoscopy on outcomes of esophageal adenocarcinoma among United States veterans. Gastrointest. Endosc. 68, 849–855 (2008).
Cooper, G. S., Kou, T. D. & Chak, A. Receipt of previous diagnoses and endoscopy and outcome from esophageal adenocarcinoma: a population-based study with temporal trends. Am. J. Gastroenterol. 104, 1356–1362 (2009).
El-Serag, H. B. et al. Surveillance endoscopy is associated with improved outcomes of oesophageal adenocarcinoma detected in patients with Barrett’s oesophagus. Gut 65, 1252–1260 (2016).
Kastelein, F. et al. Impact of surveillance for Barrett’s oesophagus on tumour stage and survival of patients with neoplastic progression. Gut 65, 1–7 (2015).
Verbeek, R. E. et al. Surveillance of Barrett’s Esophagus and mortality from esophageal adenocarcinoma: a population-based cohort study. Am. J. Gastroenterol. 109, 1215–1222 (2014).
Sturm, M. B. & Wang, T. D. Emerging optical methods for surveillance of Barrett’s oesophagus. Gut 64, 1816–1823 (2015).
Chedgy, F. J. Q. et al. Acetic acid chromoendoscopy: Improving neoplasia detection in Barrett’s esophagus. World J. Gastroenterol. 22, 5753–5760 (2016).
Sharma, P. et al. The American Society for Gastrointestinal Endoscopy PIVI (preservation and incorporation of valuable endoscopic innovations) on imaging in Barrett’s Esophagus. Gastrointest. Endosc. 76, 252–254 (2012).
Lim, C. H. et al. Randomized crossover study that used methylene blue or random 4-quadrant biopsy for the diagnosis of dysplasia in Barrett’s esophagus. Gastrointest. Endosc. 64, 195–199 (2006).
Horwhat, J. D. et al. A randomized comparison of methylene blue-directed biopsy versus conventional four-quadrant biopsy for the detection of intestinal metaplasia and dysplasia in patients with long-segment Barrett’s esophagus. Am. J. Gastroenterol. 103, 546–554 (2008).
Coletta, M. et al. Acetic acid chromoendoscopy for the diagnosis of early neoplasia and specialized intestinal metaplasia in Barrett’s esophagus: a meta-analysis. Gastrointest. Endosc. 83, 57–67 (2016).
Tholoor, S. et al. Acetic acid chromoendoscopy in Barrett’s esophagus surveillance is superior to the standardized random biopsy protocol: results from a large cohort study. Gastrointest. Endosc. 80, 417–424 (2014).
Olliver, J. R. et al. Chromoendoscopy with methylene blue and associated DNA damage in Barrett’s oesophagus. Lancet 362, 373–374 (2003).
Sturmey, R. G., Wild, C. P. & Hardie, L. J. Removal of red light minimizes methylene blue-stimulated DNA damage in oesophageal cells: implications for chromoendoscopy. Mutagenesis 24, 253–258 (2009).
Kovacic, P. & Somanathan, R. Toxicity of imine-iminium dyes and pigments: electron transfer, radicals, oxidative stress and other physiological effects. J. Appl. Toxicol. 34, 825–834 (2014).
James, M. L. & Gambhir, S. S. A molecular imaging primer: modalities, imaging agents, and applications. Physiol. Rev. 92, 897–965 (2012).
Lee, J. H. & Wang, T. D. Molecular endoscopy for targeted imaging in the digestive tract. Lancet Gastroenterol. Hepatol. 1, 147–155 (2016).
Sturm, M. B. et al. Targeted imaging of esophageal neoplasia with a fluorescently labeled peptide: first-in-human results. Sci. Transl. Med. 5, 184ra61 (2013).
Joshi, B. P. et al. Multimodal endoscope can quantify wide-field fluorescence detection of Barrett’s neoplasia. Endoscopy 48, 1–13 (2015).
Bird-Lieberman, E. L. et al. Molecular imaging using fluorescent lectins permits rapid endoscopic identification of dysplasia in Barrett’s esophagus. Nat. Med. 18, 315–321 (2012).
Nagengast, W. B. et al. Near-infrared fluorescence molecular endoscopy detects dysplastic oesophageal lesions using topical and systemic tracer of vascular endothelial growth factor A. Gut 68, 7–10 (2017).
Kaneko, K. et al. Effect of novel bright image enhanced endoscopy using blue laser imaging (BLI). Endosc. Int. Open 2, E212–E219 (2014).
Yoshida, N. et al. Ability of a novel blue laser imaging system for the diagnosis of colorectal polyps. Dig. Endosc. 26, 250–258 (2014).
Miyake, Y. et al. Development of new electronic endoscopes using the spectral images of an internal organ. In Proc. IS&T/SID’s Thirteen Color Imaging Conf. 261–269 (Society for Imaging Science and Technology, 2005).
Kodashima, S. & Fujishiro, M. Novel image-enhanced endoscopy with i-scan technology. World J. Gastroenterol. 16, 1043–1049 (2010).
Li, C. Q. et al. Magnified and enhanced computed virtual chromoendoscopy in gastric neoplasia: a feasibility study. World J. Gastroenterol. 19, 4221–4227 (2013).
Osawa, H. et al. Diagnosis of depressed-type early gastric cancer using small-caliber endoscopy with flexible spectral imaging color enhancement. Dig. Endosc. 24, 231–236 (2012).
Osawa, H. et al. Diagnosis of endoscopic Barrett’s esophagus by transnasal flexible spectral imaging color enhancement. J. Gastroenterol. 44, 1125–1132 (2009).
Aminalai, A. et al. Live image processing does not increase adenoma detection rate during colonoscopy: a randomized comparison between FICE and conventional imaging (Berlin Colonoscopy Project 5, BECOP-5). Am. J. Gastroenterol. 105, 2383–2388 (2010).
Chung, S. J. et al. Efficacy of computed virtual chromoendoscopy on colorectal cancer screening: a prospective, randomized, back-to-back trial of Fuji Intelligent Color Enhancement versus conventional colonoscopy to compare adenoma miss rates. Gastrointest. Endosc. 72, 136–142 (2010).
Basford, P. J. et al. High-definition endoscopy with i-Scan for evaluation of small colon polyps: the HiSCOPE study. Gastrointest. Endosc. 79, 111–118 (2014).
Hong, S. N. et al. Prospective, randomized, back-to-back trial evaluating the usefulness of i-SCAN in screening colonoscopy. Gastrointest. Endosc. 75, 1011–1021 (2012).
Lee, C. K., Lee, S. H. & Hwangbo, Y. Narrow-band imaging versus I-Scan for the real-time histological prediction of diminutive colonic polyps: A prospective comparative study by using the simple unified endoscopic classification. Gastrointest. Endosc. 74, 603–609 (2011).
Yoshida, Y. et al. A randomized crossover open trial of the adenoma miss rate for narrow band imaging (NBI) versus flexible spectral imaging color enhancement (FICE). Int. J. Colorectal Dis. 28, 1511–1516 (2013).
Chung, S. J. et al. Comparison of detection and miss rates of narrow band imaging, flexible spectral imaging chromoendoscopy and white light at screening colonoscopy: a randomised controlled back-to-back study. Gut 63, 785–791 (2014).
Masci, E. et al. Interobserver agreement among endoscopists on evaluation of polypoid colorectal lesions visualized with the Pentax i-Scan technique. Dig. Liver Dis. 45, 207–210 (2013).
Pigò, F. et al. I-Scan high-definition white light endoscopy and colorectal polyps: prediction of histology, interobserver and intraobserver agreement. Int. J. Colorectal Dis. 28, 399–406 (2013).
Sharma, P. et al. Development and validation of a classification system to identify high-grade dysplasia and esophageal adenocarcinoma in Barrett’s Esophagus using narrow band imaging. Gastroenterology 150, 591–598 (2016).
Haringsma, J. & Tytgat, G. N. J. Fluorescence and autofluorescence. Clin. Gastroenterol. 13, 1–10 (1999).
Kara, M. A. et al. Endoscopic video autofluorescence imaging may improve the detection of early neoplasia in patients with Barrett’s esophagus. Gastrointest. Endosc. 61, 679–685 (2005).
Curvers, W. L. et al. Endoscopic trimodal imaging versus standard video endoscopy for detection of early Barrett’s neoplasia: a multicenter, randomized, crossover study in general practice. Gastrointest. Endosc. 73, 195–203 (2011).
Boerwinkel, D. F. et al. Endoscopic TriModal imaging and biomarkers for neoplasia conjoined: a feasibility study in Barrett’s esophagus. Dis. Esophagus 27, 435–443 (2014).
Curvers, W. L. et al. Endoscopic tri-modal imaging is more effective than standard endoscopy in identifying early-stage neoplasia in Barrett’s esophagus. Gastroenterology 139, 1106–1114 (2010).
Wallace, M. et al. Miami classification for probe-based confocal laser endomicroscopy. Endoscopy 43, 882–891 (2011).
Kiesslich, R. et al. In vivo histology of Barrett’s esophagus and associated neoplasia by confocal laser endomicroscopy. Clin. Gastroenterol. Hepatol. 4, 979–987 (2006).
Vakoc, B. J. et al. Comprehensive esophageal microscopy by using optical frequency-domain imaging. Gastrointest. Endosc. 65, 898–905 (2007).
Gora, M. J. et al. Tethered capsule endomicroscopy enables less invasive imaging of gastrointestinal tract microstructure. Nat. Med. 19, 238–240 (2013).
Yun, S. H. et al. Comprehensive volumetric optical microscopy in vivo. Nat. Med. 12, 1429–1433 (2006).
Wolfsen, H. C. et al. Safety and feasibility of volumetric laser endomicroscopy in patients with Barrett’s esophagus. Gastrointest. Endosc. 82, 631–640 (2015).
Swager, A. F. et al. Detection of buried Barrett’s glands after radiofrequency ablation with volumetric laser endomicroscopy. Gastrointest. Endosc. 83, 80–88 (2016).
Swager, A. et al. Volumetric laser endomicroscopy in Barrett’s esophagus: a feasibility study on histological correlation. Dis. Esophagus 29, 505–512 (2015).
Trindade, A. J. et al. Volumetric laser endomicroscopy can target neoplasia not detected by conventional endoscopic measures in long segment Barrett’s esophagus. Endosc. Int. 4, 318–322 (2016).
Leggett, C. L. et al. Comparative diagnostic performance of volumetric laser endomicroscopy and confocal laser endomicroscopy in the detection of dysplasia associated with Barrett’s esophagus. Gastrointest. Endosc. 83, 880–888 (2015).
Poneros, J. M. et al. Diagnosis of specialized intestinal metaplasia by optical coherence tomography. Gastroenterology 120, 7–12 (2001).
Evans, J. A. et al. Optical coherence tomography to identify intramucosal carcinoma and high-grade dysplasia in Barrett’s esophagus. Clin. Gastroenterol. Hepatol. 4, 38–43 (2006).
Evans, J. A. et al. Identifying intestinal metaplasia at the squamocolumnar junction by using optical coherence tomography. Gastrointest. Endosc. 65, 50–56 (2007).
Sauk, J. et al. Interobserver agreement for the detection of barrett’s esophagus with optical frequency domain imaging. Dig. Dis. Sci. 58, 2261–2265 (2013).
Suter, M. J. et al. Esophageal-guided biopsy with volumetric laser endomicroscopy and laser cautery marking: a pilot clinical study. Gastrointest. Endosc. 79, 886–896 (2014).
Gora, M. J. et al. Imaging the upper gastrointestinal tract in unsedated patients using tethered capsule endomicroscopy. Gastroenterology 145, 723–725 (2013).
Ughi, G. J. et al. Automated segmentation and characterization of esophageal wall in vivo by tethered capsule optical coherence tomography endomicroscopy. Biomed. Opt. Express 7, 660–665 (2016).
Gora, M. et al. Tethered capsule endomicroscopy : from bench to bedside at a primary care practice tethered capsule endomicroscopy : from bench to bedside at a primary care practice. J. Biomed. Opt. 21, 104001 (2016).
Ntziachristos, V. ESOTRAC https://www.esotrac2020.eu/ (2018).
Bergholt, M. S. et al. Fiberoptic confocal raman spectroscopy for real-time in vivo diagnosis of dysplasia in Barrett’s esophagus. Gastroenterology 146, 27–32 (2014).
Almond, L. M. et al. Endoscopic Raman spectroscopy enables objective diagnosis of dysplasia in Barrett’s esophagus. Gastrointest. Endosc. 79, 37–45 (2014).
Van Norman, G. A. Drugs and devices: comparison of European and U. S. approval processes. JACC Basic Transl. Sci. 1, 399–412 (2016).
Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. Official J. European Union L117, 1–175, https://eur-lex.europa.eu/legal-content/ENG/TXT/PDF/?uri=CELEX:32017R0745 (2017).
Beswick, D. M., Kaushik, A. & Beinart, D. Biomedical device innovation methodology: applications in biophotonics. J. Biomed. Opt. 23, 1–7 (2017).
Laudicella, M. et al. Cost of care for cancer patients in England: evidence from population-based patient-level data. Br. J. Cancer 114, 1286–1292 (2016).
Trivedi, P. J. & Braden, B. Indications, stains and techniques in chromoendoscopy. QJM 106, 117–131 (2013).
Sevick-Muraca, E. M. et al. Advancing the translation of optical imaging agents for clinical imaging. Biomed. Opt. Express 4, 160–170 (2013).
Tummers, W. S. et al. Regulatory aspects of optical methods and exogenous targets for cancer detection. Cancer Res. 77, 2197–2206 (2017).
US FDA. Overview of Device Regulation. US Department of Health and Human Services https://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/Overview/default.htm (2017).
European Commission. Medical devices. European Union https://ec.europa.eu/growth/sectors/medical-devices_en (2017).
Marcu, L. et al. Biophotonics: the big picture. J. Biomed. Opt. 23, 1–7 (2017).
Boerwinkel, D. F. et al. Third-generation autofluorescence endoscopy for the detection of early neoplasia in Barrett’s esophagus: a pilot study. Dis. Esophagus 27, 276–284 (2014).
Alvarez Herrero, L. et al. Zooming in on Barrett oesophagus using narrow-band imaging: an international observer agreement study. Eur. J. Gastroenterol. Hepatol. 21, 1068–1075 (2009).
Curvers, W. L. et al. Mucosal morphology in Barrett’s esophagus: interobserver agreement and role of narrow band imaging. Endoscopy 40, 799–805 (2008).
Silva, F. B. et al. Endoscopic assessment and grading of Barrett’s esophagus using magnification endoscopy and narrow-band imaging: accuracy and interobserver agreement of different classification systems. Gastrointest. Endosc. 73, 7–14 (2011).
Kara, M. A. et al. Detection and classification of the mucosal and vascular patterns (mucosal morphology) in Barrett’s esophagus by using narrow band imaging. Gastrointest. Endosc. 64, 155–166 (2006).
Singh, R. et al. Narrow-band imaging with magnification in Barrett’s esophagus: validation of a simplified grading system of mucosal morphology patterns against histology. Endoscopy 40, 457–463 (2008).
Sharma, P. et al. The utility of a novel narrow band imaging endoscopy system in patients with Barrett’s esophagus. Gastrointest. Endosc. 64, 167–175 (2006).
de Bruijne, M. Machine learning approaches in medical image analysis: From detection to diagnosis. Med. Image Anal. 33, 94–97 (2016).
Suzuki, K. Overview of deep learning in medical imaging. Radiol. Phys. Technol. 10, 1–17 (2017).
Garcia-Allende, P. B. et al. Towards clinically translatable NIR fluorescence molecular guidance for colonoscopy. Biomed. Opt. Express 5, 78–92 (2013).
Pelargos, P. E. et al. Utilizing virtual and augmented reality for educational and clinical enhancements in neurosurgery. J. Clin. Neurosci. 35, 1–4 (2016).
Nicolau, S. et al. Augmented reality in laparoscopic surgical oncology. Surg. Oncol. 20, 189–201 (2011).
Kandiah, K. et al. OC-054 Development and validation of a classification system to identify Barrett’s neoplasia using acetic acid chromoendoscopy: the predict classification. Gut 65(31), 1–31 (2016).
Longcroft-Wheaton, G. et al. Duration of acetowhitening as a novel objective tool for diagnosing high risk neoplasia in Barrett’s esophagus: a prospective cohort trial. Endoscopy 45, 426–432 (2013).
Wilson, B. C., Jermyn, M. & Leblond, F. Challenges and opportunities in clinical translation of biomedical optical spectroscopy and imaging. J. Biomed. Opt. 23, 1–13 (2018).
ICNIRP. ICNIRP guidelines on limits of exposure to incoherent visible and infrared radiation. Health Phys. 71, 804–819 (2013).
Brookner, C. K. et al. Safety analysis: relative risks of ultraviolet exposure from fluorescence spectroscopy and colposcopy are comparable. Photochem. Photobiol. 65, 1020–1025 (1997).
EU. Directive 2006/25/EC of the European Parliament and of the Council of 5th of April 2006. Official J. European Union L114, 38–60 (2006); https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:114:0038:0059:en:PDF
Performance Measurements of Positron Emission Tomographs (PETs) (NEMA, 2013); https://www.nema.org/Standards/Pages/Performance-Measurements-of-Positron-Emission-Tomographs.aspx
Boellaard, R. et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur. J. Nucl. Med. Mol. Imaging 42, 328–354 (2014).
Zhu, B. et al. Determining the performance of fluorescence molecular imaging devices using traceable working standards with SI units of radiance. IEEE Trans. Med. Imaging 0062, 1 (2015).
Downs-Kelly, E. et al. Poor interobserver agreement in the distinction of high-grade dysplasia and adenocarcinoma in pretreatment Barrett’s esophagus biopsies. Am. J. Gastroenterol. 103, 2333–2340 (2008).
O’Connor, J. P. B. et al. Imaging intratumor heterogeneity: role in therapy response, resistance, and clinical outcome. Clin. Cancer Res. 21, 249–257 (2015).
Usher-Smith, J. A., Sharp, S. J. & Griffin, S. J. The spectrum effect in tests for risk prediction, screening, and diagnosis. BMJ 353, i3139 (2016).
Calin, M. A. et al. Hyperspectral imaging in the medical field: present and future. Appl. Spectrosc. Rev. 49, 435–447 (2014).
Grosenick, D. et al. Review of optical breast imaging and spectroscopy. J. Biomed. Opt. 21, 091311 (2016).
Fuks, D. et al. Intraoperative confocal laser endomicroscopy for real-time in vivo tissue characterization during surgical procedures. Surg. Endosc. https://doi.org/10.1007/s00464-018-6442-3 (2018).
Kassianos, A. P. et al. Smartphone applications for melanoma detection by community, patient and generalist clinician users: a review. Br. J. Dermatol. 172, 1507–1518 (2015).
Farahani, N. et al. International telepathology: promises and pitfalls. Pathobiology 83, 121–126 (2016).
Tensen, E. et al. Two decades of teledermatology: current status and integration in national healthcare systems. Curr. Dermatol. Rep. 5, 96–104 (2016).
Cohen, J. D. et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 359, 926–930 (2018).
Chi, C. et al. Intraoperative imaging-guided cancer surgery: from current fluorescence molecular imaging methods to future multi-modality imaging technology. Theranostics 4, 1072–1084 (2014).
De Boer, E. et al. Optical innovations in surgery. Br. J. Surg. 102, 56–72 (2015).
Heinzmann, K. et al. Multiplexed imaging for diagnosis and therapy. Nat. Biomed. Eng. 1, 697–713 (2017).
Bhat, Y. M. et al. High-definition and high-magnification endoscopes. Gastrointest. Endosc. 80, 919–927 (2014).
Medical Systems (Olympus Technologies, 2017); https://www.olympus-europa.com/medical/en/medical_systems/technologies/narrow_band_imaging__nbi_1/technologies_nbi.jsp
FICE Dual Mode (Fujifilm Europe, 2017); https://www.fujifilm.eu/eu/products/medical-systems/endoscopy/technology/fice-dual-mode.
i-Scan Imaging (Pentax Medical, 2017); https://www.pentaxmedical.com/pentax/en/95/1/i-scan-imaging
About SIMSYS-MoleMate (MedX Health, 2017); http://medxhealth.com/Our-Products/SIAscopytrade;/overview.aspx.
MelaFind http://www.melafind.com (accessed 26 April 2017).
Manfredi, M. A. et al. Electronic chromoendoscopy. Gastrointest. Endosc. 81, 249–261 (2015).
Rameshshanker, R. & Wilson, A. Electronic imaging in colonoscopy: clinical applications and future prospects. Curr. Treat. Options Gastroenterol. 14, 140–151 (2016).
Lu, G. & Fei, B. Medical hyperspectral imaging: a review. J. Biomed. Opt. 19, 10901 (2014).
Jang, J. The past, present, and future of image-enhanced endoscopy. Clin. Endosc. 48, 466–475 (2015).
DERMA Medical Systems https://www.dermamedicalsystems.com/index.php?menu_id=117 (accessed 2 May 2017).
Alali, S. & Vitkin, A. Polarized light imaging in biomedicine: emerging Mueller matrix methodologies for bulk tissue assessment. J. Biomed. Opt. 20, 61104 (2015).
Cellvizio: Our Flagship Product (Mauna Kea Technologies, 2017); http://www.maunakeatech.com/en/hospital-administrators/cellvizio-solution
OptiScan ViewnVivo. http://viewnvivo.com/ (accessed 26 April 2017).
Clinical Applications: Noninvasive cellular imaging of the skin (Caliber ID, 2017); http://www.caliberid.com/clinical.html
Wong Kee Song, L. M. et al. Autofluorescence imaging. Gastrointest. Endosc. 73, 647–650 (2011).
DermaInspect (Jenlab, 2017); http://www.jenlab.de/DermaInspect.29.0.html
Hanson, K. M. & Bardeen, C. J. Application of nonlinear optical microscopy for imaging skin. Photochem. Photobiol. 85, 33–44 (2009).
Fu, L. & Gu, M. Fibre-optic nonlinear optical microscopy and endoscopy. J. Microsc. 226, 195–206 (2007).
Thomas, G. et al. Advances and challenges in label-free nonlinear optical imaging using two-photon excitation fluorescence and second harmonic generation for cancer research. J. Photochem. Photobiol. B Biol. 141, 128–138 (2014).
NvisionVLE Imaging System (NinePoint Medical Inc, 2017); http://www.ninepointmedical.com/nvisionvle-imaging-system/
Vivosight (Michelson Diagnostics, 2017); https://vivosight.com/about-us/product/
A. J. Trindade, A. J., Smith, M. S. & Pleskow, D. K. The new kid on the block for advanced imaging in Barrett’s esophagus: a review of volumetric laser endomicroscopy. Therap. Adv. Gastroenterol. 9, 408–416 (2016).
Kallaway, C. et al. Advances in the clinical application of Raman spectroscopy for cancer diagnostics. Photodiagnosis Photodyn. Ther. 10, 207–219 (2013).
Wang, W. et al. Real-time in vivo cancer diagnosis using raman spectroscopy. J. Biophotonics 8, 527–545 (2015).
Tu, Q. & Chang, C. Diagnostic applications of Raman spectroscopy. Nanomed. Nanotechnol. Biol. Med. 8, 545–558 (2012).
SpectraScience and PENTAX Medical Announce Expanded Distribution of WavSTAT4 Optical Biopsy System in Europe. PENTAX Medical (24 April 2013).
Benes, Z. & Antos, Z. Optical biopsy system distinguishing between hyperplastic and adenomatous polyps in the colon during colonoscopy. Anticancer Res. 29, 4737–4739 (2009).
Boerwinkel, D. F. et al. Fluorescence spectroscopy incorporated in an Optical Biopsy System for the detection of early neoplasia in Barrett’s esophagus. Dis. Esophagus 28, 345–351 (2014).
Yu, G. Near-infrared diffuse correlation spectroscopy in cancer diagnosis and therapy monitoring. J. Biomed. Opt. 17, 010901 (2012).
Acknowledgements
We would like to thank F. Walter of the University of Cambridge for helpful comments on our manuscript. D.J.W., C.R.M.F. and S.E.B. are financially supported by CRUK (C14303/A17197, C47594/A16267, C47594/A21102, C55962/A24669) and EPSRC (C197/A16465, EP/N014588/1).
Author information
Authors and Affiliations
Contributions
D.J.W., J.P.B.O. and S.E.B. conceived the manuscript. D.J.W. researched and wrote the manuscript together with C.R.M.F. B.W.P. reviewed and edited the manuscript. All authors discussed and agreed with the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
S.E.B. receives research support from iThera Medical GmbH and PreXion Inc., and chairs the International Photoacoustic Standardisation Consortium (IPASC).
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Waterhouse, D.J., Fitzpatrick, C.R.M., Pogue, B.W. et al. A roadmap for the clinical implementation of optical-imaging biomarkers. Nat Biomed Eng 3, 339–353 (2019). https://doi.org/10.1038/s41551-019-0392-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-019-0392-5
This article is cited by
-
Molecular radio afterglow probes for cancer radiodynamic theranostics
Nature Materials (2023)
-
Surgical polarimetric endoscopy for the detection of laryngeal cancer
Nature Biomedical Engineering (2023)
-
Criteria for the design of tissue-mimicking phantoms for the standardization of biophotonic instrumentation
Nature Biomedical Engineering (2022)
-
Discrimination of normal and cancerous human skin tissues based on laser-induced spectral shift fluorescence microscopy
Scientific Reports (2022)
-
Non-invasive monitoring of blood oxygenation in human placentas via concurrent diffuse optical spectroscopy and ultrasound imaging
Nature Biomedical Engineering (2022)