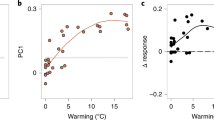
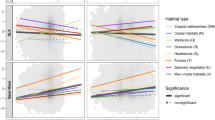
Abstract
Climate change is altering the distributions of species, which in turn causes shifts in the composition of plant communities. Specifically, rising temperatures should cause increasing relative abundances of heat-loving or heat-tolerant species (that is, ‘thermophilization’) and changes in precipitation should cause altered abundances of water-demanding species. We analysed millions of records of thousands of species and found that the plant communities in most ecoregions in North, Central and South America have experienced thermophilization over the past four decades (1970–2011). Thermophilization was fastest in ecoregions with intermediate temperatures and was positively correlated with warming rates within many biomes. Changes in the relative abundances of water-demanding species were less consistent and were not correlated with changes in precipitation, meaning that the drought sensitivity of some ecoregions may be increasing despite decreasing rainfall and increasing probabilities of drought. Climate-driven changes in plant community composition will affect the function and stability of New World ecoregions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The project was based entirely on data that are publicly available through CHELSA (http://chelsa-climate.org/), Ecoregions2017 (https://ecoregions2017.appspot.com/) and BIEN (http://bien.nceas.ucsb.edu/bien/). A list of data providers contributing plant collection and observation records to BIEN is included in the Supplementary Information.
Change history
12 February 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41558-020-00926-2
References
Zhang, T., Niinemets, Ü., Sheffield, J. & Lichstein, J. W. Shifts in tree functional composition amplify the response of forest biomass to climate. Nature 556, 99–102 (2018).
Parmesan, C. & Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 (2003).
Parmesan, C. & Hanley, M. E. Plants and climate change: complexities and surprises. Ann. Bot. 116, 849–864 (2015).
Telwala, Y., Brook, B. W., Manish, K. & Pandit, M. K. Climate-induced elevational range shifts and increase in plant species richness in a Himalayan biodiversity epicentre. PLoS ONE 8, e57103 (2013).
Jump, A. S., Huang, T. J. & Chou, C. H. Rapid altitudinal migration of mountain plants in Taiwan and its implications for high altitude biodiversity. Ecography 35, 204–210 (2012).
Angelo, C. L. & Daehler, C. C. Upward expansion of fire‐adapted grasses along a warming tropical elevation gradient. Ecography 36, 551–559 (2013).
Morueta-Holme, N. et al. Strong upslope shifts in Chimborazo’s vegetation over two centuries since Humboldt. Proc. Natl Acad. Sci. USA 112, 12741–12745 (2015).
Parolo, G. & Rossi, G. Upward migration of vascular plants following a climate warming trend in the Alps. Basic Appl. Ecol. 9, 100–107 (2008).
Chen, I.-C., Hill, J. K., Ohlemüller, R., Roy, D. B. & Thomas, C. D. Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024–1026 (2011).
Moret, P., Muriel, P., Jaramillo, R. & Dangles, O. Humboldt’s tableau physique revisited. Proc. Natl Acad. Sci. USA 116, 12889–12894 (2019).
Lenoir, J. & Svenning, J. C. Climate-related range shifts—a global multidimensional synthesis and new research directions. Ecography 38, 15–28 (2015).
Lenoir, J., Gegout, J. C., Marquet, P. A., de Ruffray, P. & Brisse, H. A significant upward shift in plant species optimum elevation during the 20th century. Science 320, 1768–1771 (2008).
Feeley, K. J. Distributional migrations, expansions, and contractions of tropical plant species as revealed in dated herbarium records. Glob. Change Biol. 18, 1335–1341 (2012).
Fei, S. et al. Divergence of species responses to climate change. Sci. Adv. 3, e1603055 (2017).
Zhu, K., Woodall, C. W. & Clark, J. S. Failure to migrate: lack of tree range expansion in response to climate change. Glob. Change Biol. 18, 1042–1052 (2012).
Crimmins, S. M., Dobrowski, S. Z., Greenberg, J. A., Abatzoglou, J. T. & Mynsberge, A. R. Changes in climatic water balance drive downhill shifts in plant species’ optimum elevations. Science 331, 324–327 (2011).
Kelly, A. E. & Goulden, M. L. Rapid shifts in plant distribution with recent climate change. Proc. Natl Acad. Sci. USA 105, 11823–11826 (2008).
Wieczynski, D. J. et al. Climate shapes and shifts functional biodiversity in forests worldwide. Proc. Natl Acad. Sci. USA 116, 587–592 (2019).
Bertrand, R. et al. Changes in plant community composition lag behind climate warming in lowland forests. Nature 479, 517–520 (2011).
Blonder, B. et al. Linking environmental filtering and disequilibrium to biogeography with a community climate framework. Ecology 96, 972–985 (2015).
Gottfried, M. et al. Continent-wide response of mountain vegetation to climate change. Nat. Clim. Change 2, 111–115 (2012).
Duque, A., Stevenson, P. & Feeley, K. J. Thermophilization of adult and juvenile tree communities in the northern tropical Andes. Proc. Natl Acad. Sci. USA 112, 10744–10749 (2015).
Fadrique, B. et al. Widespread but heterogeneous responses of Andean forests to climate change. Nature 564, 207–212 (2018).
Feeley, K. J., Hurtado, J., Saatchi, S., Silman, M. R. & Clark, D. B. Compositional shifts in Costa Rican forests due to climate-driven species migrations. Glob. Change Biol. 19, 3472–3480 (2013).
Feeley, K. J. et al. Upslope migration of Andean trees. J. Biogeogr. 38, 783–791 (2011).
Esquivel‐Muelbert, A. et al. Compositional response of Amazon forests to climate change. Glob. Change Biol. 25, 39–56 (2019).
Feeley, K. J. & Silman, M. R. Biotic attrition from tropical forests correcting for truncated temperature niches. Glob. Change Biol. 16, 1830–1836 (2010).
Title, P. O. & Bemmels, J. B. ENVIREM: an expanded set of bioclimatic and topographic variables increases flexibility and improves performance of ecological niche modeling. Ecography 41, 291–307 (2018).
Santiago, L. S. et al. Coordination and trade-offs among hydraulic safety, efficiency and drought avoidance traits in Amazonian rainforest canopy tree species. New Phytol. 218, 1015–1024 (2018).
Strzepek, K., Yohe, G., Neumann, J. & Boehlert, B. Characterizing changes in drought risk for the United States from climate change. Environ. Res. Lett. 5, 044012 (2010).
Sheffield, J. & Wood, E. F. Projected changes in drought occurrence under future global warming from multi-model, multi-scenario, IPCC AR4 simulations. Clim. Dynam. 31, 79–105 (2008).
Duffy, P. B., Brando, P., Asner, G. P. & Field, C. B. Projections of future meteorological drought and wet periods in the Amazon. Proc. Natl Acad. Sci. USA 112, 13172–13177 (2015).
Conradi, T., Van Meerbeek, K., Ordonez, A. & Svenning, J. C. Biogeographic historical legacies in the net primary productivity of Northern Hemisphere forests. Ecol. Lett. 23, 800–810 (2020).
Dinerstein, E. et al. An ecoregion-based approach to protecting half the terrestrial realm. BioScience 67, 534–545 (2017).
Olson, D. M. et al. Terrestrial ecoregions of the world: a new map of life on Earth. BioScience 51, 933–938 (2001).
Anderson-Teixeira, K. J. et al. CTFS-ForestGEO: a worldwide network monitoring forests in an era of global change. Glob. Change Biol. 21, 528–549 (2015).
Dauby, G. et al. RAINBIO: a mega-database of tropical African vascular plants distributions. PhytoKeys 74, 1–18 (2016).
DeWalt, S. J., Bourdy, G., de Michel, L. R. & Quenevo, C. Ethnobotany of the Tacana: quantitative inventories of two permanent plots of Northwestern Bolivia. Econ. Bot. 53, 237–260 (1999).
Enquist, B. & Boyle, B. SALVIAS—the SALVIAS vegetation inventory database. Biodivers. Ecol. 4, 288 (2012).
Enquist, B. J., Condit, R., Peet, R. K., Schildhauer, M. & Thiers, B. M. Cyberinfrastructure for an integrated botanical information network to investigate the ecological impacts of global climate change on plant biodiversity. Preprint at https://peerj.com/preprints/2615/ (2016).
Fegraus, E. Tropical Ecology Assessment and Monitoring Network (TEAM Network). Biodivers. Ecol. 4, 287 (2012).
Maitner, B. S. et al. The BIEN R package: a tool to access the Botanical Information and Ecology Network (BIEN) database. Methods Ecol. Evol. 9, 373–379 (2018).
Peet, R. K. et al. Vegetation-plot database of the Carolina Vegetation Survey. Biodivers. Ecol. 4, 243–253 (2012).
Peet, R. K., Lee, M. T., Jennings, M. D. & Faber-Langendoen, D. VegBank: a permanent, open-access archive for vegetation plot data. Biodivers. Ecol. 4, 233–241 (2012).
Sosef, M. S. M. et al. Exploring the floristic diversity of tropical Africa. BMC Biol. 15, 15 (2017).
König, C. et al. Biodiversity data integration—the significance of data resolution and domain. PLoS Biol. 17, e3000183 (2019).
Karger, D. N. et al. Climatologies at high resolution for the earth’s land surface areas. Sci. Data 4, 170122 (2017).
Enquist, B. J. et al. The commonness of rarity: global and future distribution of rarity across land plants. Sci. Adv. 5, eaaz0414 (2019).
Feeley, K. J., Davies, S. J., Perez, R., Hubbell, S. P. & Foster, R. B. Directional changes in the species composition of a tropical forest. Ecology 92, 871–882 (2011).
Gosselin, F. Putting floristic thermophilization in forests into a conservation biology perspective: beyond mean trait approaches. Ann. For. Sci. 73, 215–218 (2016).
De Frenne, P. et al. Microclimate moderates plant responses to macroclimate warming. Proc. Natl Acad. Sci. USA 110, 18561–18565 (2013).
Stevens, J. T., Safford, H. D., Harrison, S. & Latimer, A. M. Forest disturbance accelerates thermophilization of understory plant communities. J. Ecol. 103, 1253–1263 (2015).
Bush, M. B., Silman, M. R. & Urrego, D. H. 48,000 years of climate and forest change in a biodiversity hot spot. Science 303, 827–829 (2004).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2014).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Soft. 67, 1–48 (2015).
Nakagawa, S. & Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed‐effects models. Methods Ecol. Evol. 4, 133–142 (2013).
Acknowledgements
This project was funded through the US National Science Foundation grant no. DEB-1350125 to K.J.F.; D.Z. was supported by the National Doctoral Scholarship COLCIENCIAS-Colombia grant no. 647, 2015-II.
Author information
Authors and Affiliations
Contributions
K.J.F. conceived and designed the project and led manuscript writing. K.J.F., C.B., B.F., T.M.P. and D.Z. analysed the data and interpreted results. K.J.F. led manuscript writing and preparation. C.B., B.F., T.M.P. and D.Z. assisted in manuscript writing and preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 The relationships between temperature and precipitation at the species, ecosystem and community levels.
The relationships between a, species’ optimal temperature (MATopt, oC) and optimal precipitation (TAPopt, mm) as based on the distribution of observation records from 1970–1985 (Pearson’s R = 0.55; d.f. = 17241; P < 0.0001), b, the average Mean Annual Temperature (MAT, oC) and Total Annual Precipitation (TAP, mm) of ecoregions from 1979–2012 (Pearson’s correlation, R = 0.58; d.f. = 189; P < 0.0001), and c) the initial (1970–1985) Community Temperature Index (CTI, oC) and Community Precipitation Index (CPI, mm) of ecoregions (Pearson’s R = 0.72; d.f. = 189; P < 0.0001). In a, each point represents a species; in b and c, each point represents an ecoregion and points are coloured according to their biome designation (see Fig. 1).
Supplementary information
Supplementary Information
Supplementary text and Figs. 1–9.
Supplementary Data
Supplementary Data 1a–f.
Rights and permissions
About this article
Cite this article
Feeley, K.J., Bravo-Avila, C., Fadrique, B. et al. Climate-driven changes in the composition of New World plant communities. Nat. Clim. Chang. 10, 965–970 (2020). https://doi.org/10.1038/s41558-020-0873-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41558-020-0873-2
This article is cited by
-
Warming underpins community turnover in temperate freshwater and terrestrial communities
Nature Communications (2024)
-
Testing the stress gradient hypothesis in soil bacterial communities associated with vegetation belts in the Andean Atacama Desert
Environmental Microbiome (2023)
-
Spatial and temporal patterns of indicators of climate change and variability in the Arab world in the past four decades
Scientific Reports (2023)
-
Regeneration in European beech forests after drought: the effects of microclimate, deadwood and browsing
European Journal of Forest Research (2023)
-
Preferential substrate use decreases priming effects in contrasting treeline soils
Biogeochemistry (2023)