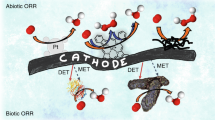
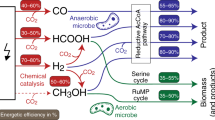
Abstract
A vast array of microorganisms from all three domains of life can produce electrical current and transfer electrons to the anodes of different types of bioelectrochemical systems. These exoelectrogens are typically iron-reducing bacteria, such as Geobacter sulfurreducens, that produce high power densities at moderate temperatures. With the right media and growth conditions, many other microorganisms ranging from common yeasts to extremophiles such as hyperthermophilic archaea can also generate high current densities. Electrotrophic microorganisms that grow by using electrons derived from the cathode are less diverse and have no common or prototypical traits, and current densities are usually well below those reported for model exoelectrogens. However, electrotrophic microorganisms can use diverse terminal electron acceptors for cell respiration, including carbon dioxide, enabling a variety of novel cathode-driven reactions. The impressive diversity of electroactive microorganisms and the conditions in which they function provide new opportunities for electrochemical devices, such as microbial fuel cells that generate electricity or microbial electrolysis cells that produce hydrogen or methane.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Potter, M. C. Electrical effects accompanying the decomposition of organic compounds. Proc. R. Soc. Lond. B Biol. Sci. 84, 260–276 (1911).
Logan, B. E. Microbial Fuel Cells (John Wiley & Sons, Inc., 2008).
Logan, B. E. & Rabaey, K. Conversion of wastes into bioelectricity and chemicals using microbial electrochemical technologies. Science 337, 686–690 (2012).
Myers, J. M. & Myers, C. R. Role for outer membrane cytochromes OmcA and OmcB of Shewanella putrefaciens MR-1 in reduction of manganese dioxide. Appl. Environ. Microbiol. 67, 260–269 (2001).
El-Naggar, M. Y. et al. Electrical transport along bacterial nanowires from Shewanella oneidensis MR-1. Proc. Nat. Acad. Sci. USA 107, 18127–18131 (2010).
Pirbadian, S. et al. Shewanella oneidensis MR-1 nanowires are outer membrane and periplasmic extensions of the extracellular electron transport components. Proc. Nat. Acad. Sci. 111, 12883–12888 (2014).
von Canstein, H., Ogawa, J., Shimizu, S. & Lloyd, J. R. Secretion of flavins by Shewanella species and their role in extracellular electron transfer. Appl. Environ. Microbiol. 74, 615–623 (2008).
Xu, S., Jangir, Y. & El-Naggar, M. Y. Disentangling the roles of free and cytochrome-bound flavins in extracellular electron transport from Shewanella oneidensis MR-1. Electrochim. Acta 198, 49–55 (2016).
Lovley, D. R. Syntrophy goes electric: direct interspecies electron transfer. Annu. Rev. Microbiol. 71, 643–664 (2017).
Lovley, D. R. Happy together: microbial communities that hook up to swap electrons. ISME J. 11, 327–336 (2017).
Light, S. H. et al. A flavin-based extracellular electron transfer mechanism in diverse Gram-positive bacteria. Nature 562, 140–144 (2018).
Reguera, G. Harnessing the power of microbial nanowires. Microb. Biotechnol. 11, 979–994 (2018).
Wang, Z., Cao, C., Zheng, Y., Chen, S. & Zhao, F. Abiotic oxygen reduction reaction catalysts used in microbial fuel cells. ChemElectroChem 1, 1813–1821 (2014).
Bond, D. R. & Lovley, D. R. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl. Environ. Microbiol. 69, 1548–1555 (2003).
Bretschger, O. et al. Current production and metal oxide reduction by Shewanella oneidensis MR-1 wild type and mutants. Appl. Environ. Microbiol. 73, 7003–7012 (2007).
Koch, C. & Harnisch, F. Is there a specific ecological niche for electroactive microorganisms? ChemElectroChem 3, 1282–1295 (2016).
Yang, W., Kim, K.-Y., Saikaly, P. E. & Logan, B. E. The impact of new cathode materials relative to baseline performance of microbial fuel cells all with the same architecture and solution chemistry. Energy Environ. Sci. 10, 1025–1033 (2017).
Qu, Y., Feng, Y., Wang, X. & Logan, B. E. Use of a coculture to enable current production by Geobacter sulfurreducens. Appl. Environ. Microbiol. 78, 3484–3487 (2012).
Oh, S. & Logan, B. E. Proton exchange membrane and electrode surface areas as factors that affect power generation in microbial fuel cells. Appl. Microbiol. Biotechnol. 70, 162–169 (2006). This paper addresses how power densities can vary depending on relative electrode sizes and the membrane cross-sectional area in two-chamber MFCs.
Yang, W. & Logan, B. E. Immobilization of a metal–nitrogen–carbon catalyst on activated carbon with enhanced cathode performance in microbial fuel cells. ChemSusChem 9, 2226–2232 (2016). This paper demonstrates high power densities using activated carbon air cathodes.
Oliot, M. et al. Separator electrode assembly (SEA) with 3-dimensional bioanode and removable air-cathode boosts microbial fuel cell performance. J. Power Sources 356, 389–399 (2017). This paper demonstrates the highest power density for electrodes with equal projected surface area.
Liang, P., Huang, X., Fan, M.-Z., Cao, X.-X. & Wang, C. Composition and distribution of internal resistance in three types of microbial fuel cells. Appl. Microbiol. Biotechnol. 77, 551–558 (2007).
Holmes, D. E. et al. Microbial communities associated with electrodes harvesting electricity from a variety of aquatic sediments. Microb. Ecol. 48, 178–190 (2004). This is an analysis of the communities in a variety of sediments from natural system.
Kiely, P. D., Regan, J. M. & Logan, B. E. The electric picnic: synergistic requirements for exoelectrogenic microbial communities. Curr. Opin. Biotechnol. 22, 378–385 (2011).
Lovley, D. R. Bug juice: harvesting electricity with microorganisms. Nat. Rev. Microbiol. 4, 497–508 (2006).
Kiely, P. D., Rader, G., Regan, J. M. & Logan, B. E. Long-term cathode performance and the microbial communities that develop in microbial fuel cells fed different fermentation endproducts. Bioresour. Technol. 102, 361–366 (2011).
Kiely, P. D. et al. Anode microbial communities produced by changing from microbial fuel cell to microbial electrolysis cell operation using two different wastewaters. Bioresour. Technol. 102, 388–394 (2011).
Yi, H. et al. Selection of a variant of Geobacter sulfurreducens with enhanced capacity for current production in microbial fuel cells. Biosens. Bioelectron. 24, 3498–3503 (2009).
Ringeisen, B. R. et al. High power density from a miniature microbial fuel cell using Shewanella oneidensis DSP10. Environ. Sci. Technol. 40, 2629–2634 (2006).
Rosenbaum, M., Cotta, M. A. & Angenent, L. T. Aerated Shewanella oneidensis in continuously fed bioelectrochemical systems for power and hydrogen production. Biotechnol. Bioeng. 105, 880–888 (2009).
Watson, V. J. & Logan, B. E. Power production in MFCs inoculated with Shewanella oneidensis MR-1 or mixed cultures. Biotechnol. Bioeng. 105, 489–498 (2010).
Gorby, Y. A. et al. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc. Natl Acad. Sci. USA 103, 11358–11363 (2006). This is the first published report on conductive appendages of the Shewanella genus.
Subramanian, P., Pirbadian, S., El-Naggar, M. Y. & Jensen, G. J. Ultrastructure of Shewanella oneidensis MR-1 nanowires revealed by electron cryotomography. Proc. Natl Acad. Sci. USA 115, E3246–E3255 (2018). This paper provides a clear description of the appendages produced by Shewanella oneidensis MR-1.
Reguera, G. et al. Extracellular electron transfer via microbial nanowires. Nature 435, 1098–1101 (2005). This is the first description of conductive pili produced by Geobacter sp.
Myers, C. R. & Myers, J. M. Localization of cytochromes to the outer membrane of anaerobically grown Shewanella putrefaciens MR-1. J. Bacteriol. 174, 3429–3438 (1992).
Marsili, E. et al. Shewanella secretes flavins that mediate extracellular electron transfer. Proc. Natl Acad. Sci. USA 105, 3968–3973 (2008).
Call, D. F. & Logan, B. E. Lactate oxidation coupled to iron or electrode reduction by Geobacter sulfurreducens PCA. Appl. Environ. Microbiol. 77, 8791–8794 (2011). This paper provides a direct comparison of current production of G. sulfurreducens and S. oneidensis using the same substrate.
Hunt, K. A., Flynn, J. M., Naranjo, B. n., Shikhare, I. D. & Gralnick, J. A. Substrate-Level phosphorylation is the primary source of energy conservation during anaerobic respiration of Shewanella oneidensis strain MR-1. J. Bacteriol. 192, 3345–3351 (2010). This paper provides a clear explanation of the basis for current generation by a Shewanella species.
Yang, L. et al. Boosting current generation in microbial fuel cells by an order of magnitude by coating an ionic liquid polymer on carbon anodes. Biosens. Bioelectron. 91, 644–649 (2017).
Monteverde, D. R. et al. Distribution of extracellular flavins in a coastal marine basin and their relationship to redox gradients and microbial community members. Environ. Sci. Technol. 52, 12265–12274 (2018).
Cao, X., Huang, X., Zhang, X., Liang, P. & Fan, M. A mini-microbial fuel cell for voltage testing of exoelectrogenic bacteria. Front. Environ. Sci. Eng. 3, 307–312 (2009).
Li, H. et al. Power output of microbial fuel cell emphasizing interaction of anodic binder with bacteria. J. Power Sources 379, 115–122 (2018).
Doyle, L. E. & Marsili, E. Weak electricigens: a new avenue for bioelectrochemical research. Bioresour. Technol. 258, 354–364 (2018).
Rabaey, K., Lissens, G., Siciliano, S. D. & Verstraete, W. A microbial fuel cell capable of converting glucose to electricity at high rate and efficiency. Biotechnol. Lett. 25, 1531–1535 (2003).
Rabaey, K., Boon, N., Siciliano, S. D., Verhaege, M. & Verstraete, W. Biofuel cells select for microbial consortia that self-mediate electron transfer. Appl. Environ. Microbiol. 70, 5373–5382 (2004).
Rabaey, K., Boon, N., Hofte, M. & Verstraete, W. Microbial phenazine production enhances electron transfer in biofuel cells. Environ. Sci. Technol. 39, 3401–3408 (2005). This paper demonstrates the role of phenazines for mediating electron transfer.
Pham, T. H. et al. Metabolites produced by Pseudomonas sp. enable a Gram positive bacterium to achieve extracellular electron transfer. Appl. Microbiol. Biotechnol. 77, 1119–1129 (2008).
Kiely, P. D., Call, D. F., Yates, M. D., Regan, J. R. & Logan, B. E. Anodic biofilms in microbial fuel cells harbor low numbers of higher-power producing bacteria than abundant genera. Appl. Microbiol. Biotechnol. 88, 371–380 (2010).
Zhang, T. et al. A novel mediatorless microbial fuel cell based on biocatalysis of Escherichia coli. Chem. Commun. (Camb.) 21, 2257–2259 (2006).
Sayed, E. T., Saito, Y., Tsujiguchi, T. & Nakagawa, N. Catalytic activity of yeast extract in biofuel cell. J. Biosci. Bioeng. 114, 521–525 (2012).
Holmes, D. E., Nicoll, J. S., Bond, D. R. & Lovley, D. R. Potential role of a novel psychrotolerant member of the family Geobacteraceae, Geopsychrobacter electrodiphilus gen. nov., sp. nov., in electricity production by a marine sediment fuel cell. Appl. Environ. Microbiol. 70, 6023–6030 (2004); erratum 75, 885 (2009).
Logan, B. E. Exoelectrogenic bacteria that power microbial fuel cells. Nat. Rev. Microbiol 7, 375–381 (2009).
Samrot, A. V. et al. Electricity generation by Enterobacter cloacae SU-1 in mediator less microbial fuel cell. Int. J. Hydrogen Energy 35, 7723–7729 (2010).
Angenent, L. et al. Comments on “Electricity generation by Enterobacter cloacae SU-1 in mediator less microbial fuel cell” by Samrot et al., Int. J. Hydrogen Energy, 35 (15) 2010, 7723–7729. Int. J. Hydrogen Energy 36, 9396–9397 (2011).
Samrot, A. V. et al. Retraction notice to: Electricity generation by Enterobacter cloacae SU-1 in mediator less microbial fuel cell [Int J Hydrogen Energy (2010) 33:7723–7729]. Int. J. Hydrogen Energy 37, 728 (2012).
Kargi, F. & Eker, S. High power generation with simultaneous COD removal using a circulating column microbial fuel cell. J. Chem. Technol. Biotechnol. 84, 961–965 (2009).
Zhu, X. & Logan, B. E. Copper anode corrosion affects power generation in microbial fuel cells. J. Chem. Technol. Biotechnol. 89, 471–474 (2014).
Baudler, A., Schmidt, I., Langner, M., Greiner, A. & Schroder, U. Does it have to be carbon? Metal anodes in microbial fuel cells and related bioelectrochemical systems. Energy Environ. Sci. 8, 2048–2055 (2015).
Sekar, N., Wu, C.-H., Adams, M. W. W. & Ramasamy, R. P. Electricity generation by Pyrococcus furiosus in microbial fuel cells operated at 90 °C. Biotechnol. Bioeng. 114, 1419–1427 (2017).
Yilmazel, Y. D., Zhu, X., Kim, K.-Y., Holmes, D. E. & Logan, B. E. Electrical current generation in microbial electrolysis cells by hyperthermophilic archaea Ferroglobus placidus and Geoglobus ahangari. Bioelectrochemistry 119, 142–149 (2016). This paper demonstrates electricity production at very high temperatures by iron-reducing hyperthermophiles.
Chen, S. & Smith, A. L. Methane-driven microbial fuel cells recover energy and mitigate dissolved methane emissions from anaerobic effluents. Environ. Sci. (Camb.) 4, 67–79 (2018).
Myung, J., Saikaly, P. E. & Logan, B. E. A two-staged system to generate electricity in microbial fuel cells using methane. Chem. Eng. J. 352, 262–267 (2018). This paper uses methane to produce electricity in a two-stage process.
McAnulty, M. J. et al. Electricity from methane by reversing methanogenesis. Nat. Commun. 8, 15419 (2017).
Hubenova, Y. & Mitov, M. Extracellular electron transfer in yeast-based biofuel cells: a review. Bioelectrochemistry 106 (Pt A), 177–185 (2015).
Raghavulu, S. V., Goud, R. K., Sarma, P. N. & Mohan, S. V. Saccharomyces cerevisiae as anodic biocatalyst for power generation in biofuel cell: Influence of redox condition and substrate load. Bioresour. Technol. 102, 2751–2757 (2011).
Hubenova, Y. & Mitov, M. Mitochondrial origin of extracelullar transferred electrons in yeast-based biofuel cells. Bioelectrochemistry 106 (Pt A), 232–239 (2015).
Hubenova, Y. V. et al. Improvement of yeast−biofuel cell output by electrode modifications. Ind. Engin. Chem. Res. 50, 557–564 (2011).
Sayed, E. T., Tsujiguchi, T. & Nakagawa, N. Catalytic activity of baker’s yeast in a mediatorless microbial fuel cell. Bioelectrochemistry 86, 97–101 (2012).
Wu, S. et al. Extracellular electron transfer mediated by flavins in gram-positive Bacillus sp. WS-XY1 and yeast Pichia stipitis. Electrochim. Acta 146, 564–567 (2014).
Dexter, S. C. & Gao, G. Y. Effect of seawater biofilms on corrosion potential and oxygen reduction of stainless steel. Corros. Sci. 44, 717–723 (1988).
Hasvold, Ø. et al. Sea-water battery for subsea control systems. J. Power Sources 65, 253–261 (1997). This paper provides evidence that bacteria on cathodes can improve oxygen reduction by the electrode.
Gregory, K. B., Bond, D. R. & Lovley, D. R. Graphite electrodes as electron donors for anaerobic repiration. Environ. Microbiol. 6, 596–604 (2004).
Clauwaert, P. et al. Biological denitrification in microbial fuel cells. Environ. Sci. Technol. 41, 3354–3360 (2007). This paper demonstrates the complete denitrification in an MFC.
Puig, S. et al. Autotrophic denitrification in microbial fuel cells treating low ionic strength waters. Environ. Sci. Technol. 46, 2309–2315 (2012).
Jiang, X. et al. Electrochemical study of enhanced nitrate removal in wastewater treatment using biofilm electrode. Bioresour. Technol. 252, 134–142 (2018).
Beese-Vasbender, P. F., Nayak, S., Erbe, A., Stratmann, M. & Mayrhofer, K. J. J. Electrochemical characterization of direct electron uptake in electrical microbially influenced corrosion of iron by the lithoautotrophic SRB Desulfopila corrodens strain IS4. Electrochim. Acta 167, 321–329 (2015).
Cordas, C. M., Guerra, L. T., Xavier, C. & Moura, J. J. G. Electroactive biofilms of sulphate reducing bacteria. Electrochim. Acta 54, 29–34 (2008).
Aulenta, F., Catapano, L., Snip, L., Villano, M. & Majone, M. Linking bacterial metabolism to graphite cathodes: electrochemical insights into the H2-producing capability of Desulfovibrio sp. ChemSusChem 5, 1080–1085 (2012).
Rhoads, A., Beyenal, H. & Lewandowski, Z. Microbial fuel cell using anaerobic respiration as an anodic reaction and biomineralied manganese as a cathodic reactant. Environ. Sci. Technol. 39, 4666–4671 (2005).
Bergel, A., Feron, D. & Mollica, A. Catalysis of oxygen reduction in PEM fuel cell by seawater biofilm. Electrochem. Commun. 7, 900–904 (2005).
Erable, B. et al. Marine aerobic biofilm as biocathode catalyst. Bioelectrochemistry 78, 51–56 (2010).
Debuy, S., Pecastaings, S., Bergel, A. & Erable, B. Oxygen-reducing biocathodes designed with pure cultures of microbial strains isolated from seawater biofilms. Int. Biodeterior. Biodegradation 103, 16–22 (2015).
Malanoski, A. P. et al. Relative abundance of ‘Candidatus Tenderia electrophaga’ is linked to cathodic current in an aerobic biocathode community. Microb. Biotechnol. 11, 98–111 (2018).
Lu, Z. et al. Behavior of metal ions in bioelectrochemical systems: a review. J. Power Sources 275, 243–260 (2015).
Yates, M. D., Cusick, R. D. & Logan, B. E. Extracellular palladium nanoparticle production using Geobacter sulfurreducens. ACS Sustain. Chem. Eng. 1, 1165–1171 (2013).
Ishii, T., Kawaichi, S., Nakagawa, H., Hashimoto, K. & Nakamura, R. From chemolithoautotrophs to electrolithoautotrophs: CO2 fixation by Fe(II)-oxidizing bacteria coupled with direct uptake of electrons from solid electron sources. Front. Microbiol. 6, 994 (2015).
Rozendal, R. A., Jeremiasse, A. W., Hamelers, H. V. M. & Buisman, C. J. N. Hydrogen production with a microbial biocathode. Environ. Sci. Technol. 42, 629–634 (2008).
Geelhoed, J. S. & Stams, A. J. M. Electricity-assisted biological hydrogen production from acetate by Geobacter sulfurreducens. Environ. Sci. Technol. 45, 815–820 (2011).
Bajracharya, S. et al. Biotransformation of carbon dioxide in bioelectrochemical systems: state of the art and future prospects. J. Power Sources 356, 256–273 (2017).
Jiang, Y. et al. Carbon dioxide and organic waste valorization by microbial electrosynthesis and electro-fermentation. Water Res. 149, 42–55 (2019).
Nevin, K. P., Woodard, T. L., Franks, A. E., Summers, A. M. & Lovley, D. R. Microbial electrosynthesis: feeding microbes electricity to convert carbon dioxide and water to multicarbon extracellular organic compounds. mBio 1, e00103–10 (2010). This paper is one of the first studies showing chemical production from a biocathode.
Nevin, K. P. et al. Electrosynthesis of organic compounds from carbon dioxide is catalyzed by a diversity of acetogenic microorganisms. Appl. Environ. Microbiol. 77, 2882–2886 (2011).
Bajracharya, S. et al. Carbon dioxide reduction by mixed and pure cultures in microbial electrosynthesis using an assembly of graphite felt and stainless steel as a cathode. Bioresour. Technol. 195, 14–24 (2015).
Aryal, N., Tremblay, P.-L., Lizak, D. M. & Zhang, T. Performance of different Sporomusa species for the microbial electrosynthesis of acetate from carbon dioxide. Bioresour. Technol. 233, 184–190 (2017).
Zhang, T. et al. Improved cathode materials for microbial electrosynthesis. Energy Environ. Sci. 6, 217–224 (2013).
Gildemyn, S., Rozendal, R. A. & Rabaey, K. A. Gibbs free energy-based assessment of microbial electrocatalysis. Trends Biotechnol. 35, 393–406 (2017).
Clauwaert, P. & Verstraete, W. Methanogenesis in membraneless microbial electrolysis cells. Appl. Microbiol. Biotechnol. 82, 829–836 (2008).
Sato, K., Kawaguchi, H. & Kobayashi, H. Bio-electrochemical conversion of carbon dioxide to methane in geological storage reservoirs. Energy Convers. Manag. 66, 343–350 (2013).
Jiang, Y. et al. Bioelectrochemical systems for simultaneously production of methane and acetate from carbon dioxide at relatively high rate. Int. J. Hydrogen Energy 38, 3497–3502 (2013).
Cheng, S., Xing, D., Call, D. F. & Logan, B. E. Direct biological conversion of electrons into methane by electromethanogenesis. Environ. Sci. Technol. 43, 3953–3958 (2009). This paper provides the first reported evidence that methanogens could be using electrons directly from the cathode to produce methane.
Deutzmann, J. S., Sahin, M. & Spormann, A. M. Extracellular enzymes facilitate electron uptake in biocorrosion and bioelectrosynthesis. mBio 6, e00496–15 (2015). This paper provides clear evidence that M. maripaludis releases enzymes that facilitate electron transfer from the cathode.
Van Eerten-Jansen, M. C. A. A. et al. Microbial community analysis of a methane-producing biocathode in a bioelectrochemical system. Archaea 2013, 12 (2013).
Siegert, M., Yates, M. D., Spormann, A. M. & Logan, B. E. Methanobacterium dominates biocathodic Archaeal communities in methanogenic microbial electrolysis cells. ACS Sustain. Chem. Eng. 3, 1668–1676 (2015). This paper shows that Methanobacterium spp. predominate on cathodes that poorly catalyse hydrogen production, but they do not predominate on platinum-catalysed cathodes.
Summers, Z. M. et al. Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria. Science 330, 1413–1415 (2010).
Rotaru, A.-E. et al. Direct interspecies electron transfer between Geobacter metallireducens and Methanosarcina barkeri. Appl. Environ. Microbiol. 80, 4599–4605 (2014).
Rotaru, A.-E. et al. A new model for electron flow during anaerobic digestion: direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy Environ. Sci. 7, 408–415 (2014).
Kato, S., Hashimoto, K. & Watanabe, K. Methanogenesis facilitated by electric syntrophy via (semi)conductive iron-oxide minerals. Environ. Microbiol. 14, 1646–1654 (2012).
Liu, F. et al. Magnetite compensates for the lack of a pilin-associated c-type cytochrome in extracellular electron exchange. Environ. Microbiol. 17, 648–655 (2015).
Martins, G., Salvador, A. F., Pereira, L. & Alves, M. M. Methane production and conductive materials: a critical review. Environ. Sci. Technol. 52, 10241–10253 (2018). This is a good critical review of the complicating factors involved in understanding how conductive materials can influence methane production in bioreactors.
Bourdakos, N., Marsili, E. & Mahadevan, R. A defined co-culture of Geobacter sulfurreducens and Escherichia coli in a membrane-less microbial fuel cell. Biotechnol. Bioeng. 111, 709–718 (2014).
Karthikeyan, R. Sathish kumar, K., Murugesan, M., Berchmans, S. & Yegnaraman, V. Bioelectrocatalysis of Acetobacter aceti and Gluconobacter roseus for current generation. Environ. Sci. Technol. 43, 8684–8689 (2009).
Ren, Z., Ward, T. E. & Regan, J. M. Electricity production from cellulose in a microbial fuel cell using a defined binary culture and an undefined mixed culture. Environ. Sci. Technol. 41, 4781–4786 (2007).
Venkataraman, A., Rosenbaum, M. A., Perkins, S. D., Werner, J. J. & Angenent, L. T. Metabolite-based mutualism between Pseudomonas aeruginosa PA14 and Enterobacter aerogenes enhances current generation in bioelectrochemical systems. Energy Environ. Sci. 4, 4550–4559 (2011).
Malvankar, N. S. et al. Electrical conductivity in a mixed-species biofilm. Appl. Environ. Microbiol. 78, 5967–5971 (2012).
Lovley, D. R. Electromicrobiology. Annu. Rev. Microbiol. 66, 391–409 (2012).
Walker, D. J. F. et al. Electrically conductive pili from pilin genes of phylogenetically diverse microorganisms. ISME J. 12, 48 (2017).
Li, F. et al. Engineering Shewanella oneidensis enables xylose-fed microbial fuel cell. Biotechnol. Biofuels 10, 196 (2017).
Choi, D. et al. Metabolically engineered glucose-utilizing Shewanella strains under anaerobic conditions. Bioresour. Technol. 154, 59–66 (2014).
Flynn, J. M., Ross, D. E., Hunt, K. A., Bond, D. R. & Gralnick, J. A. Enabling unbalanced fermentations by using engineered electrode-interfaced bacteria. mBio 1, e00190–10 (2010).
Johnson, E. T. et al. Enhancement of survival and electricity production in an engineered bacterium by light-driven proton pumping. Appl. Environ. Microbiol. 76, 4123–4129 (2010).
Leang, C., Malvankar, N. S., Franks, A. E., Nevin, K. P. & Lovley, D. R. Engineering Geobacter sulfurreducens to produce a highly cohesive conductive matrix with enhanced capacity for current production. Energy Environ. Sci. 6, 1901–1908 (2013).
Kouzuma, A., Oba, H., Tajima, N., Hashimoto, K. & Watanabe, K. Electrochemical selection and characterization of a high current-generating Shewanella oneidensis mutant with altered cell-surface morphology and biofilm-related gene expression. BMC Microbiol. 14, 190–190 (2014).
Liu, T. et al. Enhanced Shewanella biofilm promotes bioelectricity generation. Biotechnol. Bioeng. 112, 2051–2059 (2015).
Ueki, T. et al. Construction of a Geobacter strain with exceptional growth on cathodes. Front. Microbiol. 9, 1512 (2018).
Yang, Y. et al. Enhancing bidirectional electron transfer of Shewanella oneidensis by a synthetic flavin pathway. ACS Synth. Biol. 4, 815–823 (2015).
Cao, Y., Li, X., Li, F. & Song, H. CRISPRi–sRNA: transcriptional–translational regulation of extracellular electron transfer in Shewanella oneidensis. ACS Synth. Biol. 6, 1679–1690 (2017).
TerAvest, M. A., Zajdel, T. J. & Ajo-Franklin, C. M. The mtr pathway of Shewanella oneidensis MR-1 couples substrate utilization to current production in Escherichia coli. ChemElectroChem 1, 1874–1879 (2014).
Feng, J. et al. Enhancing the performance of Escherichia coli-inoculated microbial fuel cells by introduction of the phenazine-1-carboxylic acid pathway. J. Biotechnol. 275, 1–6 (2018).
Liu, T., Yu, Y.-Y., Chen, T. & Chen, W. N. A synthetic microbial consortium of Shewanella and Bacillus for enhanced generation of bioelectricity. Biotechnol. Bioeng. 114, 526–532 (2017).
Liu, Y. et al. A three-species microbial consortium for power generation. Energy Environ. Sci. 10, 1600–1609 (2017). This paper reports on a consortium of microorganisms developed to produce current from a specific substrate.
Kim, T., Logan, B. E. & Gorski, C. A. A. pH-gradient flow cell for converting waste CO2 into electricity. Environ. Sci. Technol. Lett. 4, 49–53 (2017). This paper demonstrates that only concentration differences are needed to produce current.
Park, D. H. & Zeikus, J. G. Electricity generation in microbial fuel cells using neutral red as an electronophore. Appl. Environ. Microbiol. 66, 1292–1297 (2000).
Katz, E., Shipway, A. N. & Willner, I. in Handbook of Fuel Cells – Fundamentals, Technology and Applications Vol. 1 (eds Vielstich, W., Gasteiger, H. A. & Lamm, A.) 1–27 (John Wiley & Sons, Ltd, 2003).
Kim, B.-H. et al. Electrochemical activity of an Fe(III)-reducing bacterium, Shewanella putrefaciens IR-1, in the presence of alternative electron acceptors. Biotechnol. Tech. 13, 475–478 (1999).
Kim, H.-J., Hyun, M.-S., Chang, I. S. & Kim, B.-H. A microbial fuel cell type lactate biosensor using a metal-reducing bacterium, Shewanella putrefaciens. J. Microbiol. Biotechnol. 9, 365–367 (1999).
Kim, B. H., Park, D. H., Shin, P. K., Chang, I. S. & Kim, H. J. Mediator-less biofuel cell. US Patent 5976719 (1999). This is the beginning of MFCs without mediators.
Kim, B. H., Kim, H.-J., Hyun, M.-S. & Park, D.-H. Direct electrode reaction of Fe(III)-reducing bacterium, Shewanella putrefaciens. J. Microbiol. Biotechnol. 9, 127–131 (1999).
Reimers, C. E., Tender, L. M., Fertig, S. & Wang, W. Harvesting energy from the marine sediment-water interface. Environ. Sci. Technol. 35, 192–195 (2001). This is the first demonstration of how bacteria in sediments can be used to produce electrical power.
Tender, L. M. et al. Harnessing microbially generated power on the seafloor. Nat. Biotechnol. 20, 821–825 (2002).
Bond, D. R., Holmes, D. E., Tender, L. M. & Lovley, D. R. Electrode-reducing microorganisms that harvest energy from marine sediments. Science 295, 483–485 (2002).
Liu, H., Ramnarayanan, R. & Logan, B. E. Production of electricity during wastewater treatment using a single chamber microbial fuel cell. Environ. Sci. Technol. 38, 2281–2285 (2004). This paper introduces the concept of using MFCs for waste water treatment.
He, Z., Minteer, S. D. & Angenent, L. T. Electricity generation from artificial wastewater using an upflow microbial fuel cell. Environ. Sci. Technol. 39, 5262–5267 (2005).
Fu, Q. et al. A thermophilic Gram-negative nitrate-reducing bacterium, Calditerrivibrio nitroreducens, exhibiting electricity generation capability. Environ. Sci. Technol. 47, 12583–12590 (2013).
Parameswaran, P. et al. Kinetic, electrochemical, and microscopic characterization of the thermophilic, anode-respiring bacterium Thermincola ferriacetica. Environ. Sci. Technol. 47, 4934–4940 (2013).
Malki, M., Lacey, A. L. D., Rodríguez, N., Amils, R. & Fernandez, V. M. Preferential use of an anode as an electron acceptor by an acidophilic bacterium in the presence of oxygen. Appl. Environ. Microbiol. 74, 4472–4476 (2008).
Badalamenti, J. P., Krajmalnik-Brown, R. & Torres, C. I. Generation of high current densities by pure cultures of anode-respiring Geoalkalibacter spp. under alkaline and saline conditions in microbial electrochemical cells. mBio 4, e00144–13 (2013).
Kashefi, K. et al. Geoglobus ahangari gen. nov., sp. nov., a novel hyperthermophilic archaeon capable of oxidizing organic acids and growing autotrophically on hydrogen with Fe(III) serving as the sole electron acceptor. Int. J. Syst. Evol. Microbiol. 52, 719–728 (2002).
Nielsen, L. P. & Risgaard-Petersen, N. Rethinking sediment biogeochemistry after the discovery of electric currents. Ann. Rev. Mar. Sci. 7, 425–442 (2015). This is a thoughtful review of how cable bacteria could be greatly impacting the biogeochemical processes in marine sediments.
Trojan, D. et al. A taxonomic framework for cable bacteria and proposal of the candidate genera Electrothrix and Electronema. Syst. Appl. Microbiol. 39, 297–306 (2016).
Reimers, C. E., Li, C., Graw, M. F., Schrader, P. S. & Wolf, M. The identification of cable bacteria attached to the anode of a benthic microbial fuel cell: evidence of long distance extracellular electron transport to electrodes. Front. Microbiol. 8, 2055 (2017).
Vilajeliu-Pons, A. et al. Microbial electricity driven anoxic ammonium removal. Water Res. 130, 168–175 (2018).
Di Domenico, E. G. et al. Development of electroactive and anaerobic ammonium-oxidizing (anammox) biofilms from digestate in microbial fuel cells. Biomed. Res. Int. 2015, 351014 (2015).
Yin, X., Qiao, S., Zhou, J. & Quan, X. Using three-bio-electrode reactor to enhance the activity of anammox biomass. Bioresour. Technol. 196, 376–382 (2015).
Shaw, D. R., Ali, M., Katuri, K. P. & Saikaly, P. E. in ISMET 6 - General Meeting of the International Society for Microbial Electrochemistry and Technology (ISMET, Lisbon 2017).
Ruiz-Urigüen, M., Shuai, W. & Jaffé, P. R. Feammox Acidimicrobiaceae sp. A6, a lithoautotrophic electrode-colonizing bacterium. Appl. Environ. Microbiol. https://doi.org/10.1128/AEM.02029-18 (2018).
Qu, B., Fan, B., Zhu, S. & Zheng, Y. Anaerobic ammonium oxidation with an anode as the electron acceptor. Env. Microbiol. Rep. 6, 100–105 (2014).
Ishii, S. i. et al. Functionally stable and phylogenetically diverse microbial enrichments from microbial fuel cells during wastewater treatment. PLOS ONE 7, e30495 (2012).
Finster, K. & Bak, F. & Pfennig, N. Desulfuromonas acetexigens sp. nov., a dissimilatory sulfur-reducing eubacterium from anoxic freshwater sediments. Arch. Microbiol. 161, 328–332 (1994).
Kumar, A. et al. The ins and outs of microorganism–electrode electron transfer reactions. Nat. Rev. Chem. 1, 0024 (2017).
Katuri, K. P., Albertsen, M. & Saikaly, P. E. Draft genome sequence of Desulfuromonas acetexigens strain 2873, a novel anode-respiring bacterium. Genome Announc. 5, e01522–01516 (2017).
Lovley, D. R. Live wires: direct extracellular electron exchange for bioenergy and the bioremediation of energy-related contamination. Energy Environ. Sci. 4, 4896–4906 (2011).
Li, Z., Jinlian, M., Zhen, Y., Yueqiang, W. & Jia, T. Magnetite accelerates syntrophic acetate oxidation in methanogenic systems with high ammonia concentrations. Microb. Biotechnol. 11, 710–720 (2018).
Liu, F. et al. Promoting direct interspecies electron transfer with activated carbon. Energy Environ. Sci. 5, 8982–8989 (2012).
Acknowledgements
The authors acknowledge funding by the US Department of Energy (DOE) Energy Efficiency and Renewable Energy (EERE) Fuel Cell Technologies Office through a contract from the National Renewable Energy Laboratory (NREL), Project #21263, and by the Environmental Security Technology Certification Program via cooperative research agreement W9132T-16-2-0014 through the US Army Engineer Research and Development Center.
Author information
Authors and Affiliations
Contributions
All authors researched data, wrote the article and reviewed and edited the manuscript before submission. B.E.L. and R.R. prepared drafts for figures 1 and 3–5, and P.E.S. and A.R. prepared figure 2.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Bioelectrochemical systems
-
Devices that contain microorganisms that donate to or accept electrons from an electrode.
- Microbial electrochemical technologies
-
(METs). Bioelectrochemical systems that are used for a specific purpose, for example, microbial fuel cells used to produce electricity.
- Catholyte
-
An electrolyte that surrounds the cathode when a bioelectrochemical system is divided into two chambers; if there is only one chamber, the cathode is exposed to the anolyte.
- Exoelectrogenic
-
The ability of microorganisms to transfer electrons outside the cell.
- Electrotrophic
-
The ability of microorganisms to accept electrons into the cell from external sources.
- Single-chamber
-
A single-chamber microbial fuel cell (MFC) is an MFC with an air cathode that is exposed to air on one side and water on the other side. By contrast, two-chamber systems are reactors with a membrane that separates the anode and cathode chambers.
- Anolyte
-
The electrolyte that surrounds the anode in a bioelectrochemical system. In one-chamber systems, the cathode is exposed to the same electrolyte.
- Biocathodes
-
Cathodes that transfer electrons to microorganisms on the electrode surface.
Rights and permissions
About this article
Cite this article
Logan, B.E., Rossi, R., Ragab, A. et al. Electroactive microorganisms in bioelectrochemical systems. Nat Rev Microbiol 17, 307–319 (2019). https://doi.org/10.1038/s41579-019-0173-x
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41579-019-0173-x
This article is cited by
-
A hybrid transistor with transcriptionally controlled computation and plasticity
Nature Communications (2024)
-
Programmed microalgae-gel promotes chronic wound healing in diabetes
Nature Communications (2024)
-
Electrochemically coupled CH4 and CO2 consumption driven by microbial processes
Nature Communications (2024)
-
Critical evaluation of electroactive wetlands: traditional and modern advances
Environmental Science and Pollution Research (2024)
-
Performance evaluation of a dual-chamber plant microbial fuel cell developed for electricity generation and wastewater treatment
International Journal of Environmental Science and Technology (2024)