Abstract
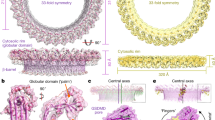
Gasdermins mediate inflammatory cell death after cleavage by caspases or other, unknown enzymes. The cleaved N-terminal fragments bind to acidic membrane lipids to form pores, but the mechanism of pore formation remains unresolved. Here we present the cryo-electron microscopy structures of the 27-fold and 28-fold single-ring pores formed by the N-terminal fragment of mouse GSDMA3 (GSDMA3-NT) at 3.8 and 4.2 Å resolutions, and of a double-ring pore at 4.6 Å resolution. In the 27-fold pore, a 108-stranded anti-parallel β-barrel is formed by two β-hairpins from each subunit capped by a globular domain. We identify a positively charged helix that interacts with the acidic lipid cardiolipin. GSDMA3-NT undergoes radical conformational changes upon membrane insertion to form long, membrane-spanning β-strands. We also observe an unexpected additional symmetric ring of GSDMA3-NT subunits that does not insert into the membrane in the double-ring pore, which may represent a pre-pore state of GSDMA3-NT. These structures provide a basis that explains the activities of several mutant gasdermins, including defective mutants that are associated with cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ding, J. et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116 (2016).
Liu, X. et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535, 153–158 (2016).
Sborgi, L. et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 35, 1766–1778 (2016).
Chen, X. et al. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 26, 1007–1020 (2016).
Russo, H. M. et al. Active caspase-1 induces plasma membrane pores that precede pyroptotic lysis and are blocked by lanthanides. J. Immunol. 197, 1353–1367 (2016).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015).
Kayagaki, N. et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015).
Aglietti, R. A. et al. GSDMD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc. Natl Acad. Sci. USA 113, 7858–7863 (2016).
Rathinam, V. A., Vanaja, S. K. & Fitzgerald, K. A. Regulation of inflammasome signaling. Nat. Immunol. 13, 333–342 (2012).
Lamkanfi, M. & Dixit, V. M. Inflammasomes and their roles in health and disease. Annu. Rev. Cell Dev. Biol. 28, 137–161 (2012).
Wang, Y. et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547, 99–103 (2017).
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Kucukelbir, A., Sigworth, F. J. & Tagare, H. D. Quantifying the local resolution of cryo-EM density maps. Nat. Methods 11, 63–65 (2014).
Jacoboni, I., Martelli, P. L., Fariselli, P., De Pinto, V. & Casadio, R. Prediction of the transmembrane regions of β-barrel membrane proteins with a neural network-based predictor. Protein Sci. 10, 779–787 (2001).
Murzin, A. G., Lesk, A. M. & Chothia, C. Principles determining the structure of β-sheet barrels in proteins. I. A theoretical analysis. J. Mol. Biol. 236, 1369–1381 (1994).
Law, R. H. et al. The structural basis for membrane binding and pore formation by lymphocyte perforin. Nature 468, 447–451 (2010).
Rossjohn, J., Feil, S. C., McKinstry, W. J., Tweten, R. K. & Parker, M. W. Structure of a cholesterol-binding, thiol-activated cytolysin and a model of its membrane form. Cell 89, 685–692 (1997).
Köster, S. et al. Crystal structure of listeriolysin O reveals molecular details of oligomerization and pore formation. Nat. Commun. 5, 3690 (2014).
van Pee, K. et al. CryoEM structures of membrane pore and prepore complex reveal cytolytic mechanism of pneumolysin. eLife 6, e23644 (2017).
Marshall, J. E. et al. The crystal structure of pneumolysin at 2.0 Å resolution reveals the molecular packing of the pre-pore complex. Sci. Rep. 5, 13293 (2015).
Holm, L. & Sander, C. Dali: a network tool for protein structure comparison. Trends Biochem. Sci. 20, 478–480 (1995).
Rosado, C. J. et al. A common fold mediates vertebrate defense and bacterial attack. Science 317, 1548–1551 (2007).
Degiacomi, M. T. et al. Molecular assembly of the aerolysin pore reveals a swirling membrane-insertion mechanism. Nat. Chem. Biol. 9, 623–629 (2013).
Iacovache, I. et al. Cryo-EM structure of aerolysin variants reveals a novel protein fold and the pore-formation process. Nat. Commun. 7, 12062 (2016).
Idone, V. et al. Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J. Cell Biol. 180, 905–914 (2008).
Keefe, D. et al. Perforin triggers a plasma membrane-repair response that facilitates CTL induction of apoptosis. Immunity 23, 249–262 (2005).
Evavold, C. L. et al. The pore-forming protein gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity 48, 35–44 (2018).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Moriya, T. et al. High-resolution single particle analysis from electron cryo-microscopy images using SPHIRE. J. Vis. Exp. 123, https://doi.org/10.3791/55448 (2017).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Acknowledgements
This work was supported by US NIH grant DP1HD087988 (H.W.), R01Al124491 (H.W.), R01AI123265 (J.L.) and Charles A. King Trust Postdoctoral Fellowship Program (J.R., X.L.). We thank the University of Massachusetts Cryo-EM Core Facility and NCI National Cryo-EM Facility at the Frederick National Laboratory for Cancer Research for data collection, and D. Ni and M. Liao for discussions.
Reviewer information
Nature thanks H. Saibil, J. Whisstock and B. Zuber for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
J.R., H.W. and J.L. conceived the study. J.R., S.X. and H.W. designed the experiments and analysed the data. J.R. reconstituted the pores, performed cryo-EM experiments and refined the structure. J.R. and S.X. analysed structure-based mutants. J.R., H.W. and S.X. analysed the structure. H.W., J.R., S.X. and J.L. wrote the manuscript. X.L. provided advice and comments on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Reconstitution of GSDMA3 pores in vitro.
a, A general procedure for GSDMA3 pore reconstitution. b, Size-exclusion chromatography of GSDMA3 pores extracted from liposomes (top) and Coomassie blue-stained SDS–PAGE of the collected fractions. c, Representative negative-stain electron microscopy images of GSDMA3. d, Representative negative-stain electron microscopy images of human GSDMD pores with double-rimmed side views. e, f, Representative cryo-EM images of GSDMA3 pores with double-rimmed side views (e) and HgCl2-treated GSDMA3 pores (f). Scale bar, 50 nm (c–f). All results were confirmed at least three times as technical replicates.
Extended Data Fig. 2 Cryo-EM analysis of double- and single-ring GSDMA3 pores.
a–c, Gold-standard FSC plots from two half-reconstructions refined separately in RELION for the 27-fold double-ring pore map (a), the 27-fold single-ring pore map (b) and the 28-fold single-ring pore map (c). Model-to-map correlations are shown for the 27-fold single-ring pore (b). d, e, Local resolution estimation generated by ResMap on the two half maps separately refined in a gold-standard procedure in RELION. Local resolutions are colour-coded on the densities. Highest resolution is observed at the β-barrel domain for both the single-ring pore (d) and the membrane-inserted ring of the double-ring pore (e). The globular domains and the additional ring exhibit relatively low resolution.
Extended Data Fig. 3 Cryo-EM density validation.
Close-up views of GDSMA3 subunit model fitted into the cryo-EM density map at four locations denoted by residue numbers.
Extended Data Fig. 4 Aligned sequences in the N-terminal region among GSDMA3, human GSDMA, mouse GSDMD and human GSDMD.
Secondary structures are shown for both the auto-inhibited conformation1 and the pore conformation of GSDMA3. Dotted lines represent disordered regions. Residue numbers are shown as dots every ten residues above the alignment for GSDMA3 and below for human GSDMD. Blue highlights, potential positively charged residues for acidic lipid binding; blue, tested residues that did not affect pore formation; magenta and cyan highlights, residues involved in the oligomerization or membrane insertion of the two adjacent subunits; red and green highlights, previously reported and cancer-associated mutations localized on the oligomerization interface of the globular domains (green) and β-barrel domain (red).
Extended Data Fig. 5 Lipid binding by GSDM.
a, Effect of the GSDMA3 R132A/R145A mutation on the liposome-leakage activity, monitored by measuring DPA-chelating-induced fluorescence of released Tb3+ ion (n = 3 biological replicates). Error bars denote mean ± s.d. b, Equal processing of GSDMA3 by the 3C protease for the wild type and the α1 mutant. A representative gel of two independent experiments is shown.
Extended Data Fig. 6 Two-residue shift in the hydrogen-bonding pattern in each GSDMA3 subunit.
The shift results in a shear number of 27 × 2 = 54 for a 27-fold symmetric pore.
Extended Data Fig. 7 Structure-based sequence alignment.
The N-terminal region of GSDMA3 crystal structure (PDB ID: 5B5R) aligned with structures of perfringolysin O (5DIM), pneumolysin (4QQQ), haemolysin (3HVN), perforin-1 (3NSJ), complement C8 alpha (2QQH) and Plu-MAPCF (2QP2) and using the distance alignment matrix method (DALI). Helices are coloured in green, strands in cyan and loops in yellow. Vertical yellow bars indicate disordered regions in the GSDMA3-NT structure. Vertical red bars denote gaps in alignment of Plu-MACPF. Identical residues conserved among at least three proteins are highlighted in yellow. Glycines conserved among MACPF/CDCs are not conserved in GSDMA3, as highlighted in deep red.
Supplementary information
Supplementary Video 1
The GSDMA3-NT pore structure Rotational view of the 3.8 Å cryo-EM density (grey) superimposed with the atomic model (cyan) of the Hg-treated, 27-fold symmetric GSDMA3-NT pore.
Supplementary Video 2
Conformational change of GSDMA3-NT during pore formation The α3’, β3, β4, β5 region including disordered loops (ED1) in the autoinhibited structure1 extends into the first transmembrane β-hairpin (HP1) in the pore conformation. The β7, α4, β8 region including disordered loops (ED2) in the autoinhibited structure stretches to form the second transmembrane β-hairpin in the pore conformation (HP2).
Supplementary Video 3
Conformational change of pneumolysin during pore formation Long β-hairpins in pore-form pneumolysin (PDB: 5YL6)22 are formed by the straightening of a cluster of helices connected to the central β-sheet (PDB: 5CR6)23.
Rights and permissions
About this article
Cite this article
Ruan, J., Xia, S., Liu, X. et al. Cryo-EM structure of the gasdermin A3 membrane pore. Nature 557, 62–67 (2018). https://doi.org/10.1038/s41586-018-0058-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0058-6
This article is cited by
-
Multiomics characterization of pyroptosis in the tumor microenvironment and therapeutic relevance in metastatic melanoma
BMC Medicine (2024)
-
Structure and assembly of a bacterial gasdermin pore
Nature (2024)
-
Mechanistic insights from inflammasome structures
Nature Reviews Immunology (2024)
-
The gasdermin family: emerging therapeutic targets in diseases
Signal Transduction and Targeted Therapy (2024)
-
Transcription factor Sp1 transcriptionally enhances GSDME expression for pyroptosis
Cell Death & Disease (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.