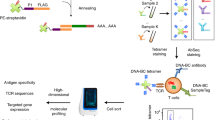
Abstract
The activation of T cells by the T cell antigen receptor (TCR) results in the formation of signaling protein complexes (signalosomes), the composition of which has not been analyzed at a systems level. Here, we isolated primary CD4+ T cells from 15 gene-targeted mice, each expressing one tagged form of a canonical protein of the TCR-signaling pathway. Using affinity purification coupled with mass spectrometry, we analyzed the composition and dynamics of the signalosomes assembling around each of the tagged proteins over 600 s of TCR engagement. We showed that the TCR signal-transduction network comprises at least 277 unique proteins involved in 366 high-confidence interactions, and that TCR signals diversify extensively at the level of the plasma membrane. Integrating the cellular abundance of the interacting proteins and their interaction stoichiometry provided a quantitative and contextual view of each documented interaction, permitting anticipation of whether ablation of a single interacting protein can impinge on the whole TCR signal-transduction network.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon request. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository (http://www.ebi.ac.uk/pride) with the dataset identifiers PXD012826, PXD007660 and PXD003972.
References
Chakraborty, A. K. & Weiss, A. Insights into the initiation of TCR signaling. Nat. Immunol. 15, 798–807 (2014).
Brownlie, R. J. & Zamoyska, R. T cell receptor signalling networks: branched, diversified and bounded. Nat. Rev. Immunol. 13, 257–269 (2013).
Shah, N. H. et al. An electrostatic selection mechanism controls sequential kinase signaling downstream of the T cell receptor. eLife 5, e20105 (2016).
Roncagalli, R. et al. Quantitative proteomics analysis of signalosome dynamics in primary T cells identifies the surface receptor CD6 as a Lat adaptor-independent TCR signaling hub. Nat. Immunol. 15, 384–392 (2014).
Astoul, E., Edmunds, C., Cantrell, D. A. & Ward, S. G. PI 3-K and T-cell activation: limitations of T-leukemic cell lines as signaling models. Trends Immunol. 22, 490–496 (2001).
Hein, M. Y. et al. A human interactome in three quantitative dimensions organized by stoichiometries and abundances. Cell 163, 712–723 (2015).
Caron, E. et al. Precise temporal profiling of signaling complexes in primary cells using SWATH mass spectrometry. Cell Rep. 18, 3219–3226 (2017).
Gaud, G. et al. The costimulatory molecule CD226 signals through VAV1 to amplify TCR signals and promote IL-17 production by CD4+ T cells. Sci. Signal. 11, eaar3083 (2018).
Voisinne, G. et al. Co-recruitment analysis of the CBL and CBLB signalosomes in primary T cells identifies CD5 as a key regulator of TCR-induced ubiquitylation. Mol. Syst. Biol. 12, 876 (2016).
Schwanhausser, B. et al. Global quantification of mammalian gene expression control. Nature 473, 337–342 (2011).
Wisniewski, J. R., Hein, M. Y., Cox, J. & Mann, M. A “proteomic ruler” for protein copy number and concentration estimation without spike-in standards. Mol. Cell. Proteomics 13, 3497–3506 (2014).
Ruepp, A. et al. CORUM: the comprehensive resource of mammalian protein complexes—2009. Nucleic Acids Res. 38, D497–D501 (2010).
Huang, Y. et al. Deficiency of ADAP/Fyb/SLAP-130 destabilizes SKAP55 in Jurkat T cells. J. Biol. Chem. 280, 23576–23583 (2005).
Seet, B. T. et al. Efficient T-cell receptor signaling requires a high-affinity interaction between the Gads C-SH3 domain and the SLP-76 RxxK motif. EMBO J. 26, 678–689 (2007).
Giuriato, S. et al. SH2-containing inositol 5-phosphatases 1 and 2 in blood platelets: their interactions and roles in the control of phosphatidylinositol 3,4,5-trisphosphate levels. Biochem. J. 376, 199–207 (2003).
Liu, S. L. et al. Quantitative lipid imaging reveals a new signaling function of phosphatidylinositol-3,4-bisphophate: isoform- and site-specific activation of Akt. Mol. Cell 71, 1092–1104.e5 (2018).
Goulden, B. D. et al. A high-avidity biosensor reveals plasma membrane PI(3,4)P2 is predominantly a class I PI3K signaling product. J. Cell. Biol. 218, 1066–1079 (2018).
Yang, M. et al. K33-linked polyubiquitination of Zap70 by Nrdp1 controls CD8 T cell activation. Nat. Immunol. 16, 1253–1262 (2015).
Paster, W. et al. GRB2-mediated recruitment of THEMIS to LAT is essential for thymocyte development. J. Immunol. 190, 3749–3756 (2013).
Balagopalan, L., Kortum, R. L., Coussens, N. P., Barr, V. A. & Samelson, L. E. The linker for activation of T cells (LAT) signaling hub: from signaling complexes to microclusters. J. Biol. Chem. 290, 26422–26429 (2015).
Kumari, S. et al. Actin foci facilitate activation of the phospholipase C-gamma in primary T lymphocytes via the WASP pathway. eLife 4, e04953 (2015).
Ditliev, J. A. et al. A composition-dependent molecular clutch between T cell signaling condensates and actin. eLife 8, e42695 (2019).
Choi, S. et al. THEMIS enhances TCR signaling and enables positive selection by selective inhibition of the phosphatase SHP-1. Nat. Immunol. 18, 433–441 (2017).
Mehta, M. et al. Themis-associated phosphatase activity controls signaling in T cell development. Proc. Natl Acad. Sci. USA 115, E11331–E11340 (2018).
Zvezdova, E. et al. Themis1 enhances T cell receptor signaling during thymocyte development by promoting Vav1 activity and Grb2 stability. Sci. Signal. 9, ra51 (2016).
Murphy, T. L. & Murphy, K. M. Slow down and survive: enigmatic immunoregulation by BTLA and HVEM. Annu. Rev. Immunol. 28, 389–411 (2010).
Celis-Gutierrez, J. et al. Quantitative interactomics in primary T cells provides a rationale for concomitant PD-1 and BTLA coinhibitor blockade in cancer immunotherapy. Cell Rep. 27, 3315–3330.e7 (2019).
Seki, A. & Rutz, S. Optimized RNP transfection for highly efficient CRISPR/Cas9-mediated gene knockout in primary T cells. J. Exp. Med. 215, 985–997 (2018).
de Kreuk, B. J. et al. The human minor histocompatibility antigen 1 is a RhoGAP. PLoS ONE 8, e73962 (2013).
Huse, M. et al. Spatial and temporal dynamics of T cell receptor signaling with a photoactivatable agonist. Immunity 27, 76–88 (2007).
Lasserre, R. et al. Release of serine/threonine-phosphorylated adaptors from signaling microclusters down-regulates T cell activation. J. Cell. Biol. 195, 839–853 (2011).
Hashimoto-Tane, A. & Saito, T. Dynamic regulation of TCR-microclusters and the microsynapse for T cell activation. Front. Immunol. 7, 255 (2016).
Yi, J., Balagopalan, L., Nguyen, T., McIntire, K. M. & Samelson, L. E. TCR microclusters form spatially segregated domains and sequentially assemble in calcium-dependent kinetic steps. Nat. Commun. 10, 277 (2019).
Andreotti, A. H., Joseph, R. E., Conley, J. M., Iwasa, J. & Berg, L. J. Multidomain control over TEC kinase activation state tunes the T cell response. Annu. Rev. Immunol. 36, 549–578 (2018).
Bouchet, J. et al. Rab11-FIP3 regulation of lck endosomal traffic controls TCR signal transduction. J. Immunol. 198, 2967–2978 (2017).
Geng, L., Raab, M. & Rudd, C. E. Cutting edge: SLP-76 cooperativity with FYB/FYN-T in the up-regulation of TCR-driven IL-2 transcription requires SLP-76 binding to FYB at Tyr595 and Tyr651. J. Immunol. 163, 5753–5757 (1999).
Veale, M. et al. Novel isoform of lymphoid adaptor FYN-T-binding protein (FYB-130) interacts with SLP-76 and up-regulates interleukin 2 production. J. Biol. Chem. 274, 28427–28435 (1999).
Marcos, T. et al. Proline-serine-threonine phosphatase interacting protein 1 inhibition of T-cell receptor signaling depends on its SH3 domain. FEBS J. 281, 3844–3854 (2014).
Stanford, S. M., Mustelin, T. M. & Bottini, N. Lymphoid tyrosine phosphatase and autoimmunity: human genetics rediscovers tyrosine phosphatases. Semin. Immunopathol. 32, 127–136 (2010).
Xu, Q. et al. Phosphatase PP2A is essential for TH17 differentiation. Proc. Natl Acad. Sci. USA 116, 982–987 (2019).
Clauset, A., Newman, M. E. & Moore, C. Finding community structure in very large networks. Phys. Rev. E 70, 066111 (2004).
Acknowledgements
We thank D. Mori, C. Wülfing (University of Bristol) and A. Zarubica for discussions and E. Bergot, S. Li, T. Chao, S. Durand and the late F. Danjan for technical help. This work was supported by CNRS, INSERM, the European Research Council (ERC) under FP7 program (grant agreement no. 322465 (INTEGRATE), to B.M.) and the European Union’s Horizon 2020 research and innovation program (grant agreement no. 787300 (BASILIC), to B.M.), Agence Nationale de la Recherche (BASILIC project, to M.M.), the MSDAVENIR Fund (to B.M.), the Investissement d’Avenir program of the French Ministry of Research ProFI (Proteomics French Infrastructure, ANR-10-INBS-08, to O.B.-S.), and PHENOMIN (French National Infrastructure for mouse Phenogenomics; ANR-10-INBS-07, to B.M.), the National Natural Science Foundation of China (grant nos 81471595 and 31400759, to Y.L.) and the Education Department of Henan Province, China (16HASTIT030, to Y.L.) and by fellowships from the INTEGRATE (to M.G.M, G.V., K.K. and K.C.), MSDAVENIR (to Y.O.) and PHENOMIN (to L.G.) projects.
Author information
Authors and Affiliations
Contributions
B.M., R.R. and G.V. conceived the project. B.M. and F.F supervised the construction of OST-tagged mice. R.R. and M.M. performed the experiments shown in Supplementary Figs. 1–3 with the help of L.G., Y.O. and M.G.M. K.K. characterized the SHIP1OST, PLC-γ1OST and PTPN6OST mice with the help of J.C. A.G.de P., K.C. and O.B.-S. performed the MS experiments. G.V. designed the computational and bioinformatics analysis. Y.L., M.M., H.L. and B.M designed the experiments shown in Fig. 8 and L.L., L.Z. and H.W. performed them. B.M., G.V. and R.R. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Laurie A. Dempsey was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–6, Tables 1 and 2 and Notes 1–4.
Supplementary Dataset 1
List of the bait–prey interactions identified in the present study. In the tab ‘bait–prey interactions’, each line shows an interaction between a bait and a prey and includes the corresponding FDRs, enrichments and stoichiometries before stimulation and at each stimulation time point. Cellular abundances (number of copies per cell) of the listed proteins are also specified. Where applicable, protein–protein interactions identified in public databases are indicated with the corresponding references and detection methods. The lists of the high-confidence bait–prey interactions identified for each bait are also shown in tabs CBL to VAV1.
Supplementary Dataset 2
Proteome of antigen-experienced conventional CD4+ T cells. The proteins identified in CD4+ T cells from wild-type and OST-tagged mice and their cellular abundance (number of copies per cell) are shown (see Methods).
Rights and permissions
About this article
Cite this article
Voisinne, G., Kersse, K., Chaoui, K. et al. Quantitative interactomics in primary T cells unveils TCR signal diversification extent and dynamics. Nat Immunol 20, 1530–1541 (2019). https://doi.org/10.1038/s41590-019-0489-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-019-0489-8
This article is cited by
-
Embracing complexity in sepsis
Critical Care (2023)
-
CD6-mediated inhibition of T cell activation via modulation of Ras
Cell Communication and Signaling (2022)
-
ZAP70 holds the key to kinetic proofreading for TCR ligand discrimination
Nature Immunology (2022)
-
PCDHB14 promotes ferroptosis and is a novel tumor suppressor in hepatocellular carcinoma
Oncogene (2022)
-
Kinetic proofreading through the multi-step activation of the ZAP70 kinase underlies early T cell ligand discrimination
Nature Immunology (2022)