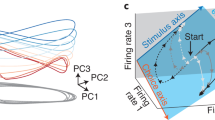
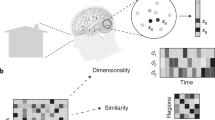
Abstract
Decades of neurobiological research have disclosed the diverse manners in which the response properties of neurons are dynamically modulated to support adaptive cognitive functions. This neuromodulation is achieved through alterations in the biophysical properties of the neuron. However, changes in cognitive function do not arise directly from the modulation of individual neurons, but are mediated by population dynamics in mesoscopic neural ensembles. Understanding this multiscale mapping is an important but nontrivial issue. Here, we bridge these different levels of description by showing how computational models parametrically map classic neuromodulatory processes onto systems-level models of neural activity. The ensuing critical balance of systems-level activity supports perception and action, although our knowledge of this mapping remains incomplete. In this way, quantitative models that link microscale neuronal neuromodulation to systems-level brain function highlight gaps in knowledge and suggest new directions for integrating theoretical and experimental work.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
21 June 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41593-021-00891-9
References
McIntosh, A. R. Contexts and catalysts: a resolution of the localization and integration of function in the brain. Neuroinformatics 2, 175–182 (2004).
Fulcher, B. D. & Fornito, A. A transcriptional signature of hub connectivity in the mouse connectome. Proc. Natl Acad. Sci. USA 113, 1435–1440 (2016).
Bullmore, E. & Sporns, O. The economy of brain network organization. Nat. Rev. Neurosci. 13, 336–349 (2012).
Thompson, E. & Varela, F. J. Radical embodiment: neural dynamics and consciousness. Trends Cogn. Sci. 5, 418–425 (2001).
Mesulam, M. M. From sensation to cognition. Brain 121, 1013–1052 (1998).
Dabney, W. et al. A distributional code for value in dopamine-based reinforcement learning. Nature 577, 671–675 (2020).
Salinas, E. & Sejnowski, T. J. Gain modulation in the central nervous system: where behavior, neurophysiology, and computation meet. Neuroscientist 7, 430–440 (2001).
Ferguson, K. A. & Cardin, J. A. Mechanisms underlying gain modulation in the cortex. Nat. Rev. Neurosci. 21, 80–92 (2020).
Noudoost, B. & Moore, T. The role of neuromodulators in selective attention. Trends Cogn. Sci. 15, 585–591 (2011).
Thiele, A. & Bellgrove, M. Neuromodulation of attention. Neuron 97, 769–785 (2018).
Shu, Y., Hasenstaub, A., Badoual, M., Bal, T. & McCormick, D. A. Barrages of synaptic activity control the gain and sensitivity of cortical neurons. J. Neurosci. 23, 10388–10401 (2003).
Cocchi, L., Gollo, L. L., Zalesky, A. & Breakspear, M. Criticality in the brain: a synthesis of neurobiology, models and cognition. Prog. Neurobiol. 158, 132–152 (2017).
Breakspear, M. Dynamic models of large-scale brain activity. Nat. Neurosci. 20, 340–352 (2017).
Shine, J. M., Aburn, M. J., Breakspear, M. & Poldrack, R. A. The modulation of neural gain facilitates a transition between functional segregation and integration in the brain. eLife 7, e31130 (2018).
Li, M. et al. Transitions in information processing dynamics at the whole-brain network level are driven by alterations in neural gain. PLoS Comput. Biol. 15, e1006957 (2019).
Servan-Schreiber, D., Printz, H. & Cohen, J. A network model of catecholamine effects: gain, signal-to-noise ratio, and behavior. Science 249, 892–895 (1990).
Eldar, E., Cohen, J. D. & Niv, Y. The effects of neural gain on attention and learning. Nat. Neurosci. 16, 1146–1153 (2013).
Warren, C. M., Eldar, E. & van den Brink, R. L. Catecholamine-mediated increases in gain enhance the precision of cortical representations. J. Neurosci. 36, 5699–5708 (2016).
Griffiths, T. L., Kemp, C. & Tenenbaum, J. B. in The Cambridge Handbook of Computational Psychology (ed. Sun, R.) 59–100 (Cambridge Univ. Press, 2008).
Moran, R. J. et al. Free energy, precision and learning: the role of cholinergic neuromodulation. J. Neurosci. 33, 8227–8236 (2013).
Sales, A. C., Friston, K. J., Jones, M. W., Pickering, A. E. & Moran, R. J. Locus coeruleus tracking of prediction errors optimises cognitive flexibility: an active inference model. PLoS Comput. Biol. 15, e1006267 (2019).
Yu, A. & Dayan, P. Uncertainty, neuromodulation, and attention. Neuron 46, 681–692 (2005).
Kim, Y. et al. Brain-wide maps reveal stereotyped cell-type-based cortical architecture and subcortical sexual dimorphism. Cell 171, 456–469 (2017).
Fishell, G. & Kepecs, A. Interneuron types as attractors and controllers. Annu. Rev. Neurosci. 43, 1–30 (2020).
Leto, K., Carletti, B., Williams, I. M., Magrassi, L. & Rossi, F. Different types of cerebellar GABAergic interneurons originate from a common pool of multipotent progenitor cells. J. Neurosci. 26, 11682–11694 (2006).
Goldberg, J. H., Farries, M. A. & Fee, M. S. Basal ganglia output to the thalamus: still a paradox. Trends Neurosci. 36, 695–705 (2013).
Halassa, M. M. & Acsády, L. Thalamic inhibition: diverse sources, diverse scales. Trends Neurosci. 39, 680–693 (2016).
Fries, P. Rhythms for cognition: communication through coherence. Neuron 88, 220–235 (2015).
Renart, A. et al. The asynchronous state in cortical circuits. Science 327, 587–590 (2010).
Whittington, M. A., Traub, R. D. & Jefferys, J. G. R. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature 373, 612–615 (1995).
Whittington, M. A., Traub, R. D., Kopell, N., Ermentrout, B. & Buhl, E. H. Inhibition-based rhythms: experimental and mathematical observations on network dynamics. Int. J. Psychophysiol. 38, 315–336 (2000).
Gouaux, E. Structure and function of AMPA receptors: structure and function of AMPA receptors. J. Physiol. 554, 249–253 (2004).
Iacobucci, G. J. & Popescu, G. K. NMDA receptors: linking physiological output to biophysical operation. Nat. Rev. Neurosci. 18, 236–249 (2017).
Gerstner, W. & Kistler, W. M. Spiking Neuron Models: Single Neurons, Populations, Plasticity (Cambridge Univ. Press, 2002).
Friston, K. A theory of cortical responses. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360, 815–836 (2005).
Self, M. W., Kooijmans, R. N., Super, H., Lamme, V. A. & Roelfsema, P. R. Different glutamate receptors convey feedforward and recurrent processing in macaque V1. Proc. Natl Acad. Sci. USA 109, 11031–11036 (2012).
Roth, B. L. Molecular pharmacology of metabotropic receptors targeted by neuropsychiatric drugs. Nat. Struct. Mol. Biol. 26, 535–544 (2019).
Leenders, A. & Sheng, Z. Modulation of neurotransmitter release by the second messenger-activated protein kinases: implications for presynaptic plasticity. Pharmacol. Ther. 105, 69–84 (2005).
Tringham, E. et al. T-type calcium channel blockers that attenuate thalamic burst firing and suppress absence seizures. Sci. Transl. Med. 4, 121ra19 (2012).
Lesage, F. Pharmacology of neuronal background potassium channels. Neuropharmacology 44, 1–7 (2003).
Llinás, R. R. & Steriade, M. Bursting of thalamic neurons and states of vigilance. J. Neurophysiol. 95, 3297–3308 (2006).
Steriade, M., McCormick, D. & Sejnowski, T. Thalamocortical oscillations in the sleeping and aroused brain. Science 262, 679–685 (1993).
Saper, C. B., Fuller, P. M., Pedersen, N. P., Lu, J. & Scammell, T. E. Sleep state switching. Neuron 68, 1023–1042 (2010).
Brown, E. N., Purdon, P. L. & Van Dort, C. J. General anesthesia and altered states of arousal: a systems neuroscience analysis. Annu. Rev. Neurosci. 34, 601–628 (2011).
Destexhe, A., Rudolph, M. & Paré, D. The high-conductance state of neocortical neurons in vivo. Nat. Rev. Neurosci. 4, 739–751 (2003).
McCormick, D. A. Cholinergic and noradrenergic modulation of thalamocortical processing. Trends Neurosci. 12, 215–221 (1989).
Jones, E. G. The thalamic matrix and thalamocortical synchrony. Trends Neurosci. 24, 595–601 (2001).
Pollard, T. D. in Cell Biology (eds Pollard, T. D. et al.) 443–462 (Elsevier, 2017).
Aston-Jones, G. & Cohen, J. D. An integrative theory of locus coeruleus–norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 28, 403–450 (2005).
Armstrong, C. M. & Hille, B. Voltage-gated ion channels and electrical excitability. Neuron 20, 371–380 (1998).
Ramos, B. P. & Arnsten, A. F. T. Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacol. Ther. 113, 523–536 (2007).
McGinley, M. J. et al. Waking state: rapid variations modulate neural and behavioral responses. Neuron 87, 1143–1161 (2015).
Reimer, J. et al. Pupil fluctuations track rapid changes in adrenergic and cholinergic activity in cortex. Nat. Commun. 7, 13289 (2016).
Phillips, W. A., Larkum, M. E., Harley, C. W. & Silverstein, S. M. The effects of arousal on apical amplification and conscious state. Neurosci. Conscious. 2016, niw015 (2016).
Harnett, M. T., Magee, J. C. & Williams, S. R. Distribution and function of HCN channels in the apical dendritic tuft of neocortical pyramidal neurons. J. Neurosci. 35, 1024–1037 (2015).
Larkum, M. E., Nevian, T., Sandler, M., Polsky, A. & Schiller, J. Synaptic integration in tuft dendrites of layer 5 pyramidal neurons: a new unifying principle. Science 325, 756–760 (2009).
Shah, M. M. Cortical HCN channels: function, trafficking and plasticity. J. Physiol. 592, 2711–2719 (2014).
Labarrera, C. et al. Adrenergic modulation regulates the dendritic excitability of layer 5 pyramidal neurons in vivo. Cell Rep. 23, 1034–1044 (2018).
Wang, M. et al. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell 129, 397–410 (2007).
Shai, A. S., Anastassiou, C. A., Larkum, M. E. & Koch, C. Physiology of layer 5 pyramidal neurons in mouse primary visual cortex: coincidence detection through bursting. PLoS Comput. Biol. 11, e1004090 (2015).
Mehaffey, W. H. Deterministic multiplicative gain control with active dendrites. J. Neurosci. 25, 9968–9977 (2005).
Palomero-Gallagher, N. & Zilles, K. Cortical layers: cyto-, myelo-, receptor- and synaptic architecture in human cortical areas. Neuroimage 197, 716–741 (2019).
Palomero-Gallagher, N. & Zilles, K. Cyto- and receptor architectonic mapping of the human brain. Handb. Clin. Neurol. 150, 355–387 (2018).
Zilles, K. & Palomero-Gallagher, N. Multiple transmitter receptors in regions and layers of the human cerebral cortex. Front. Neuroanat. 11, 78 (2017).
Hedrick, T. & Waters, J. Acetylcholine excites neocortical pyramidal neurons via nicotinic receptors. J. Neurophysiol. 113, 2195–2209 (2015).
Dembrow, N. & Johnston, D. Subcircuit-specific neuromodulation in the prefrontal cortex. Front. Neural Circuits 8, 54 (2014).
Shine, J. M. et al. Human cognition involves the dynamic integration of neural activity and neuromodulatory systems. Nat. Neurosci. 22, 289–296 (2019).
Gryglewski, G. et al. Spatial analysis and high resolution mapping of the human whole-brain transcriptome for integrative analysis in neuroimaging. Neuroimage 176, 259–267 (2018).
Meador-Woodruff, J. Dopamine receptor mRNA expression in human striatum and neocortex. Neuropsychopharmacology 15, 17–29 (1996).
Katz, R. J., Turner, B. B., Roth, K. A. & Carroll, B. J. in Catecholamines: Basic and Clinical Frontiers (eds Usdin, E. et al.) (Elsevier, 1979).
Miller, P. Dynamical systems, attractors, and neural circuits. F1000Res. 5, 992 (2016).
Tan, Z., Hu, H., Huang, Z. J. & Agmon, A. Robust but delayed thalamocortical activation of dendritic-targeting inhibitory interneurons. Proc. Natl Acad. Sci. USA 105, 2187–2192 (2008).
Haken, H. Synergetics: Introduction and Advanced Topics (Springer, 2013).
Izhikevich, E. M. Spike-timing dynamics of neuronal groups. Cereb. Cortex 14, 933–944 (2004).
Freeman, W. J. Mass Action in the Nervous System: Examination of the Neurophysiological Basis of Adaptive Behavior through the EEG (Academic Press, 1975).
Deco, G., Jirsa, V. K., Robinson, P. A., Breakspear, M. & Friston, K. The dynamic brain: from spiking neurons to neural masses and cortical fields. PLoS Comput. Biol. 4, e1000092 (2008).
Carlu, M. et al. A mean-field approach to the dynamics of networks of complex neurons, from nonlinear integrate-and-fire to Hodgkin–Huxley models. J. Neurophysiol. 123, 1042–1051 (2020).
Breakspear, M., Williams, L. M. & Stam, C. J. A novel method for the topographic analysis of neural activity reveals formation and dissolution of ‘dynamic cell assemblies’. J. Comput. Neurosci. 16, 49–68 (2004).
Jirsa, V. K. & Haken, H. Field theory of electromagnetic brain activity. Phys. Rev. Lett. 77, 960–963 (1996).
Robinson, P. A., Rennie, C. J. & Wright, J. J. Propagation and stability of waves of electrical activity in the cerebral cortex. Phys. Rev. E 56, 826–840 (1997).
Bastos, A. M. et al. Canonical microcircuits for predictive coding. Neuron 76, 695–711 (2012).
Zerlaut, Y. et al. Firing rate response of neocortical neurons in the fluctuation-driven regime. BMC Neurosci. 16, P59 (2015).
Robinson, P. A., Rennie, C. J., Rowe, D. L. & O’Connor, S. C. Estimation of multiscale neurophysiologic parameters by electroencephalographic means. Hum. Brain Mapp. 23, 53–72 (2004).
Berger, T., Senn, W. & Lüscher, H.-R. Hyperpolarization-activated current Ih disconnects somatic and dendritic spike initiation zones in layer V pyramidal neurons. J. Neurophysiol. 90, 2428–2437 (2003).
Shine, J. M. Neuromodulatory influences on integration and segregation in the brain. Trends Cogn. Sci. 23, 572–583 (2019).
Jirsa, V. K., Stacey, W. C., Quilichini, P. P., Ivanov, A. I. & Bernard, C. On the nature of seizure dynamics. Brain 137, 2210–2230 (2014).
Edelman, G. M. Neural Darwinism: selection and reentrant signaling in higher brain function. Neuron 10, 115–125 (1993).
Briggman, K. L. Optical imaging of neuronal populations during decision-making. Science 307, 896–901 (2005).
Churchland, M. M. et al. Neural population dynamics during reaching. Nature 487, 51–56 (2012).
Cisek, P. Making decisions through a distributed consensus. Curr. Opin. Neurobiol. 22, 927–936 (2012).
Tononi, G., Sporns, O. & Edelman, G. M. Measures of degeneracy and redundancy in biological networks. Proc. Natl Acad. Sci. USA 96, 3257–3262 (1999).
García-Cabezas, M. Á., Zikopoulos, B. & Barbas, H. The structural model: a theory linking connections, plasticity, pathology, development and evolution of the cerebral cortex. Brain Struct. Funct. 224, 985–1008 (2019).
Harris, K. D. & Shepherd, G. M. G. The neocortical circuit: themes and variations. Nat. Neurosci. 18, 170–181 (2015).
Rao, R. P. N. & Ballard, D. H. Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects. Nat. Neurosci. 2, 79–87 (1999).
Robinson, P. A. et al. Eigenmodes of brain activity: neural field theory predictions and comparison with experiment. Neuroimage 142, 79–98 (2016).
Hasselmo, M. E. & McGaughy, J. High acetylcholine levels set circuit dynamics for attention and encoding and low acetylcholine levels set dynamics for consolidation. Prog. Brain Res. 145, 207–231 (2004).
Kawai, H., Lazar, R. & Metherate, R. Nicotinic control of axon excitability regulates thalamocortical transmission. Nat. Neurosci. 10, 1168–1175 (2007).
Poorthuis, R. B. et al. Layer-specific modulation of the prefrontal cortex by nicotinic acetylcholine receptors. Cereb. Cortex 23, 148–161 (2013).
Tikhonova, T. B., Miyamae, T., Gulchina, Y., Lewis, D. A. & Gonzalez-Burgos, G. Cell type- and layer-specific muscarinic potentiation of excitatory synaptic drive onto parvalbumin neurons in mouse prefrontal cortex. eNeuro 5, ENEURO.0208-18.2018 (2018).
Tiesinga, P. & Sejnowski, T. J. Cortical enlightenment: are attentional gamma oscillations driven by ING or PING? Neuron 63, 727–732 (2009).
Owen, S. F., Berke, J. D. & Kreitzer, A. C. Fast-spiking interneurons supply feedforward control of bursting, calcium, and plasticity for efficient learning. Cell 172, 683–695 (2018).
Tremblay, R., Lee, S. & Rudy, B. GABAergic interneurons in the neocortex: from cellular properties to circuits. Neuron 91, 260–292 (2016).
Cardin, J. A. et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459, 663–667 (2009).
Pafundo, D. E., Miyamae, T., Lewis, D. A. & Gonzalez-Burgos, G. Cholinergic modulation of neuronal excitability and recurrent excitation-inhibition in prefrontal cortex circuits: implications for gamma oscillations: cholinergic modulation of mPFC local circuits. J. Physiol. 591, 4725–4748 (2013).
Disney, A. A., Aoki, C. & Hawken, M. J. Gain modulation by nicotine in macaque V1. Neuron 56, 701–713 (2007).
Lu, Y., Sarter, M., Zochowski, M. & Booth, V. Phasic cholinergic signaling promotes emergence of local gamma rhythms in excitatory-inhibitory networks. Eur. J. Neurosci. https://doi.org/10.1111/ejn.14744 (2020).
Schmitz, T. W. & Duncan, J. Normalization and the cholinergic microcircuit: a unified basis for attention. Trends Cogn. Sci. 22, 422–437 (2018).
Reynolds, J. H. & Heeger, D. J. The normalization model of attention. Neuron 61, 168–185 (2009).
Takahashi, N., Oertner, T. G., Hegemann, P. & Larkum, M. E. Active cortical dendrites modulate perception. Science 354, 1587–1590 (2016).
Suzuki, M. & Larkum, M. E. General anesthesia decouples cortical pyramidal neurons. Cell 180, 666–676 (2020).
Carhart-Harris, R. L. et al. The entropic brain: a theory of conscious states informed by neuroimaging research with psychedelic drugs. Front. Hum. Neurosci. 8, 20 (2014).
Williams, S. R. & Fletcher, L. N. A dendritic substrate for the cholinergic control of neocortical output neurons. Neuron 101, 486–499 (2019).
Hearne, L. J., Cocchi, L., Zalesky, A. & Mattingley, J. B. Reconfiguration of brain network architectures between resting-state and complexity-dependent cognitive reasoning. J. Neurosci. 37, 8399–8411 (2017).
Cohen, J. R. & D’Esposito, M. The segregation and integration of distinct brain networks and their relationship to cognition. J. Neurosci. 36, 12083–12094 (2016).
Cruzat, J. et al. The dynamics of human cognition: increasing global integration coupled with decreasing segregation found using iEEG. Neuroimage 172, 492–505 (2018).
Shine, J. M. et al. The dynamics of functional brain networks: integrated network states during cognitive task performance. Neuron 92, 544–554 (2016).
Zerbi, V. et al. Rapid reconfiguration of the functional connectome after chemogenetic locus coeruleus activation. Neuron 103, 702–718 (2019).
Sejnowski, T. J. The unreasonable effectiveness of deep learning in artificial intelligence. Proc. Natl Acad. Sci. USA 117, 30033–30038 (2020).
Richards, B. A. et al. A deep learning framework for neuroscience. Nat. Neurosci. 22, 1761–1770 (2019).
Singer, Y. et al. Sensory cortex is optimized for prediction of future input. eLife 7, e31557 (2018).
Nadim, F. & Bucher, D. Neuromodulation of neurons and synapses. Curr. Opin. Neurobiol. 29, 48–56 (2014).
O’Donnell, J., Zeppenfeld, D., McConnell, E., Pena, S. & Nedergaard, M. Norepinephrine: a neuromodulator that boosts the function of multiple cell types to optimize CNS Performance. Neurochem. Res. 37, 2496–2512 (2012).
Theriault, J. E., Young, L. & Barrett, L. F. The sense of should: a biologically-based framework for modeling social pressure. Phys. Life Rev. 36, 100–136 (2021).
Daw, N. D., O’Doherty, J. P., Dayan, P., Seymour, B. & Dolan, R. J. Cortical substrates for exploratory decisions in humans. Nature 441, 876–879 (2006).
Freyer, F., Roberts, J. A., Ritter, P. & Breakspear, M. A canonical model of multistability and scale-invariance in biological systems. PLoS Comput. Biol. 8, e1002634 (2012).
van den Brink, R. L., Pfeffer, T. & Donner, T. H. Brainstem modulation of large-scale intrinsic cortical activity correlations. Front. Hum. Neurosci. 13, 340 (2019).
Margulies, D. S. et al. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc. Natl Acad. Sci. USA 113, 12574–12579 (2016).
Freyer, F., Aquino, K., Robinson, P. A., Ritter, P. & Breakspear, M. Bistability and non-Gaussian fluctuations in spontaneous cortical activity. J. Neurosci. 29, 8512–8524 (2009).
Deco, G. & Jirsa, V. K. Ongoing cortical activity at rest: criticality, multistability, and ghost attractors. J. Neurosci. 32, 3366–3375 (2012).
Freyer, F. et al. Biophysical mechanisms of multistability in resting-state cortical rhythms. J. Neurosci. 31, 6353–6361 (2011).
Lord, L.-D. et al. Dynamical exploration of the repertoire of brain networks at rest is modulated by psilocybin. Neuroimage 199, 127–142 (2019).
Deco, G. et al. Whole-brain multimodal neuroimaging model using serotonin receptor maps explains non-linear functional effects of LSD. Curr. Biol. 28, 3065–3074 (2018).
Hauser, T. U. et al. Noradrenaline blockade specifically enhances metacognitive performance. eLife 6, e24901 (2017).
Marreiros, A. C., Kiebel, S. J., Daunizeau, J., Harrison, L. M. & Friston, K. J. Population dynamics under the Laplace assumption. Neuroimage 44, 701–714 (2009).
Busse, L., Wade, A. R. & Carandini, M. Representation of concurrent stimuli by population activity in visual cortex. Neuron 64, 931–942 (2009).
Zemel, R. S., Dayan, P. & Pouget, A. Probabilistic interpretation of population codes. Neural Comput. 10, 403–430 (1998).
Roberts, J. A., Friston, K. J. & Breakspear, M. Clinical applications of stochastic dynamic models of the brain, part I: a primer. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2, 216–224 (2017).
Beck, J. M. et al. Probabilistic population codes for Bayesian decision making. Neuron 60, 1142–1152 (2008).
Pouget, A., Dayan, P. & Zemel, R. S. Inference and computation with population codes. Annu. Rev. Neurosci. 26, 381–410 (2003).
Spratling, M. W. A review of predictive coding algorithms. Brain Cogn. 112, 92–97 (2017).
Friston, K., Mattout, J. & Kilner, J. Action understanding and active inference. Biol. Cybern. 104, 137–160 (2011).
Friston, K. J. et al. Dopamine, affordance and active inference. PLoS Comput. Biol. 8, e1002327 (2012).
Iglesias, S. et al. Hierarchical prediction errors in midbrain and basal forebrain during sensory learning. Neuron 101, 1196–1201 (2019).
Niv, Y., Daw, N. D., Joel, D. & Dayan, P. Tonic dopamine: opportunity costs and the control of response vigor. Psychopharmacology 191, 507–520 (2007).
Bayer, H. M. & Glimcher, P. W. Midbrain dopamine neurons encode a quantitative reward prediction error signal. Neuron 47, 129–141 (2005).
Jahn, C. I. et al. Dual contributions of noradrenaline to behavioural flexibility and motivation. Psychopharmacology 235, 3081–3081 (2018).
Sara, S. J. & Bouret, S. Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron 76, 130–141 (2012).
Mather, M., Clewett, D., Sakaki, M. & Harley, C. W. GANEing traction: the broad applicability of NE hotspots to diverse cognitive and arousal phenomena. Behav. Brain Sci. 39, e228 (2016).
Miyazaki, K., Miyazaki, K. W. & Doya, K. The role of serotonin in the regulation of patience and impulsivity. Mol. Neurobiol. 45, 213–224 (2012).
Parr, T. & Friston, K. J. Uncertainty, epistemics and active interference. J. Royal Soc. Interface 14, 20170376 (2017).
Acknowledgements
We thank C. Whyte and G. Wainstein for their thoughtful comments on our manuscript. We acknowledge funding from the NHMRC (GNT1118153 (M.B.), GNT1095227 (M.B.), GNT1193857 (J.M.S.)), The University of Sydney (J.M.S.) and the Portuguese Foundation for Science and Technology projects (UIDB/50026/2020, UIDP/50026/2020 and CEECIND/03325/2017 (J.C.)).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Neuroscience thanks Anita Disney and Eran Eldar for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shine, J.M., Müller, E.J., Munn, B. et al. Computational models link cellular mechanisms of neuromodulation to large-scale neural dynamics. Nat Neurosci 24, 765–776 (2021). https://doi.org/10.1038/s41593-021-00824-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-021-00824-6
This article is cited by
-
Semantic representation of neural circuit knowledge in Caenorhabditis elegans
Brain Informatics (2023)
-
Modulating arousal to overcome gait impairments in Parkinson’s disease: how the noradrenergic system may act as a double-edged sword
Translational Neurodegeneration (2023)
-
Connectome-based modelling of neurodegenerative diseases: towards precision medicine and mechanistic insight
Nature Reviews Neuroscience (2023)
-
Towards a biologically annotated brain connectome
Nature Reviews Neuroscience (2023)
-
A comparative analysis of 2D and 3D experimental data for the identification of the parameters of computational models
Scientific Reports (2023)