Abstract
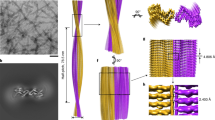
Self-templating assemblies of the human prion protein are clinically associated with transmissible spongiform encephalopathies. Here we present the cryo-EM structure of a denaturant- and protease-resistant fibril formed in vitro spontaneously by a 9.7-kDa unglycosylated fragment of the human prion protein. This human prion fibril contains two protofilaments intertwined with screw symmetry and linked by a tightly packed hydrophobic interface. Each protofilament consists of an extended beta arch formed by residues 106 to 145 of the prion protein, a hydrophobic and highly fibrillogenic disease-associated segment. Such structures of prion polymorphs serve as blueprints on which to evaluate the potential impact of sequence variants on prion disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Prusiner, S. B. Prions. Proc. Natl Acad. Sci. USA 95, 13363–13383 (1998).
Wasmer, C. et al. Amyloid fibrils of the HET-s(218–289) prion form a β solenoid with a triangular hydrophobic core. Science 319, 1523–1526 (2008).
Yuan, A. H. & Hochschild, A. A bacterial global regulator forms a prion. Science 355, 198–201 (2017).
Cobb, N. J. & Surewicz, W. K. Prion diseases and their biochemical mechanisms. Biochemistry 48, 2574–2585 (2009).
Riek, R. et al. NMR structure of the mouse prion protein domain PrP(121–231). Nature 382, 180–182 (1996).
Diaz-Espinoza, R. & Soto, C. High-resolution structure of infectious prion protein: the final frontier. Nat. Struct. Mol. Biol. 19, 370–377 (2012).
Wille, H. & Requena, J. R. The structure of PrPSc prions. Pathogens 7, E20 (2018).
Surewicz, W. K. & Apostol, M. I. in Prion Proteins (ed. Tatzelt, J.) 135–167 (Springer Berlin Heidelberg, 2011).
Caughey, B. & Lansbury, P. T. Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu. Rev. Neurosci. 26, 267–298 (2003).
Rodriguez, J. A., Jiang, L. & Eisenberg, D. S. Toward the atomic structure of PrPSc. Cold Spring Harb. Perspect. Biol. 9, a031336 (2017).
Wille, H. et al. Structural studies of the scrapie prion protein by electron crystallography. Proc. Natl Acad. Sci. USA 99, 3563–3568 (2002).
Prusiner, S. B., Scott, M. R., DeArmond, S. J. & Cohen, F. E. Prion protein biology. Cell 93, 337–348 (1998).
Collins, S. J., Lawson, V. A. & Masters, C. L. Transmissible spongiform encephalopathies. Lancet 363, 51–61 (2004).
Prusiner, S. B., Groth, D., Serban, A., Stahl, N. & Gabizon, R. Attempts to restore scrapie prion infectivity after exposure to protein denaturants. Proc. Natl Acad. Sci. USA 90, 2793–2797 (1993).
Caughey, B., Raymond, G. J., Kocisko, D. A. & Lansbury, P. T. Scrapie infectivity correlates with converting activity, protease resistance, and aggregation of scrapie-associated prion protein in guanidine denaturation studies. J. Virol. 71, 4107–4110 (1997).
McKinley, M. P., Bolton, D. C. & Prusiner, S. B. A protease-resistant protein is a structural component of the scrapie prion. Cell 35, 57–62 (1983).
Rubenstein, R. et al. Detection of scrapie-associated fibril (SAF) proteins using anti-SAF antibody in non-purified tissue preparations. J. Gen. Virol. 67(Pt.4), 671–681 (1986).
Silveira, J. R. et al. The most infectious prion protein particles. Nature 437, 257–261 (2005).
Bolton, D. C., Meyer, R. K. & Prusiner, S. B. Scrapie PrP 27-30 is a sialoglycoprotein. J. Virol. 53, 596–606 (1985).
Pan, K. M. et al. Conversion of α-helices into β-sheets features in the formation of the scrapie prion proteins. Proc. Natl Acad. Sci. 90, 10962–10966 (1993).
Nguyen, J. T. et al. X-ray diffraction of scrapie prion rods and PrP peptides. J. Mol. Biol. 252, 412–422 (1995).
Vázquez-Fernández, E. et al. The structural architecture of an infectious mammalian prion using electron cryomicroscopy. PLoS Pathog. 12, e1005835 (2016).
Gallagher-Jones, M. et al. Sub-ångström cryo-EM structure of a prion protofibril reveals a polar clasp. Nat. Struct. Mol. Biol. 25, 131–134 (2018).
Sawaya, M. R. et al. Atomic structures of amyloid cross-β spines reveal varied steric zippers. Nature 447, 453–457 (2007).
Wan, W. et al. Structural studies of truncated forms of the prion protein PrP. Biophys. J. 108, 1548–1554 (2015).
Kocisko, D. A. et al. Cell-free formation of protease-resistant prion protein. Nature 370, 471 (1994).
Legname, G. et al. Synthetic mammalian prions. Science 305, 673–676 (2004).
Choi, J.-K. et al. Amyloid fibrils from the N-terminal prion protein fragment are infectious. Proc. Natl Acad. Sci. USA 113, 13851–13856 (2016).
Taraboulos, A. et al. Acquisition of protease resistance by prion proteins in scrapie-infected cells does not require asparagine-linked glycosylation. Proc. Natl Acad. Sci. USA 87, 8262–8266 (1990).
Zhang, Z. et al. De novo generation of infectious prions with bacterially expressed recombinant prion protein. FASEB J. 27, 4768–4775 (2013).
Wille, H. et al. Natural and synthetic prion structure from X-ray fiber diffraction. Proc. Natl Acad. Sci. USA 106, 16990–16995 (2009).
Theint, T. et al. Species-dependent structural polymorphism of Y145Stop prion protein amyloid revealed by solid-state NMR spectroscopy. Nat. Commun. 8, 753 (2017).
Theint, T. et al. Structural studies of amyloid fibrils by paramagnetic solid-state nuclear magnetic resonance spectroscopy. J. Am. Chem. Soc. 140, 13161–13166 (2018).
Terry, C. et al. Structural features distinguishing infectious ex vivo mammalian prions from non-infectious fibrillar assemblies generated in vitro. Sci. Rep. 9, 376 (2019).
Terry, C. et al. Ex vivo mammalian prions are formed of paired double helical prion protein fibrils. Open Biol. 6, 160035 (2016).
Prusiner, S. B., Groth, D. F., Bolton, D. C., Kent, S. B. & Hood, L. E. Purification and structural studies of a major scrapie prion protein. Cell 38, 127–134 (1984).
Stahl, N. et al. Structural studies of the scrapie prion protein using mass spectrometry and amino acid sequencing. Biochemistry 32, 1991–2002 (1993).
Walsh, P., Simonetti, K. & Sharpe, S. Core structure of amyloid fibrils formed by residues 106–126 of the human prion protein. Structure 17, 417–426 (2009).
Jobling, M. F. et al. The hydrophobic core sequence modulates the neurotoxic and secondary structure properties of the prion peptide 106–126. J. Neurochem. 73, 1557–1565 (1999).
Biasini, E. et al. The hydrophobic core region governs mutant prion protein aggregation and intracellular retention. Biochem. J. 430, 477–486 (2010).
Norstrom, E. M. & Mastrianni, J. A. The AGAAAAGA palindrome in PrP is required to generate a productive PrPSc-PrPC complex that leads to prion propagation. J. Biol. Chem. 280, 27236–27243 (2005).
Aucoin, D. et al. Protein-solvent interfaces in human Y145Stop prion protein amyloid fibrils probed by paramagnetic solid-state NMR spectroscopy. J. Struct. Biol. 206, 36–42 (2019).
Asante, E. A. et al. Inherited prion disease A117V is not simply a proteinopathy but produces prions transmissible to transgenic mice expressing homologous prion protein. PLoS Pathog. 9, e1003643 (2013).
Rodriguez, M.-M. et al. A novel mutation (G114V) in the prion protein gene in a family with inherited prion disease. Neurology 64, 1455–1457 (2005).
Collinge, J., Palmer, M. S. & Dryden, A. J. Genetic predisposition to iatrogenic Creutzfeldt–Jakob disease. Lancet 337, 1441–1442 (1991).
Fitzpatrick, A. W. P. et al. Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature 547, 185–190 (2017).
Falcon, B. et al. Novel tau filament fold in chronic traumatic encephalopathy encloses hydrophobic molecules. Nature 568, 420–423 (2019).
Asante, E. A. et al. A naturally occurring variant of the human prion protein completely prevents prion disease. Nature 522, 478–481 (2015).
Zheng, Z. et al. Structural basis for the complete resistance of the human prion protein mutant G127V to prion disease. Sci. Rep. 8, 13211 (2018).
Zhou, S., Shi, D., Liu, X., Liu, H. & Yao, X. Protective V127 prion variant prevents prion disease by interrupting the formation of dimer and fibril from molecular dynamics simulations. Sci. Rep. 6, 21804 (2016).
Sabareesan, A. T. & Udgaonkar, J. B. The G126V mutation in the mouse prion protein hinders nucleation-dependent fibril formation by slowing initial fibril growth and by increasing the critical concentration. Biochemistry 56, 5931–5942 (2017).
Morales, R. Prion strains in mammals: different conformations leading to disease. PLoS Pathog. 13, e1006323 (2017).
Tattum, M. H. et al. Elongated oligomers assemble into mammalian PrP amyloid fibrils. J. Mol. Biol. 357, 975–985 (2006).
Ghetti, B. et al. Vascular variant of prion protein cerebral amyloidosis with tau-positive neurofibrillary tangles: the phenotype of the stop codon 145 mutation in PRNP. Proc. Natl Acad. Sci. USA 93, 744–748 (1996).
Piccardo, P. et al. Prion proteins with different conformations accumulate in Gerstmann-Sträussler-Scheinker disease caused by A117V and F198S mutations. Am. J. Pathol. 158, 2201–2207 (2001).
Sim, V. L. & Caughey, B. Ultrastructures and strain comparison of under-glycosylated scrapie prion fibrils. Neurobiol. Aging 30, 2031–2042 (2009).
Li, Q. et al. Structural attributes of mammalian prion infectivity: insights from studies with synthetic prions. J. Biol. Chem. 293, 18494–18503 (2018).
Prusiner, S. B. Novel proteinaceous infectious particles cause scrapie. Science 216, 136–144 (1982).
Gao, Y., Tran, P., Petkovic-Duran, K., Swallow, T. & Zhu, Y. Acoustic micromixing increases antibody-antigen binding in immunoassays. Biomed. Microdevices 17, 79 (2015).
Nagapudi, K., Umanzor, E. Y. & Masui, C. High-throughput screening and scale-up of cocrystals using resonant acoustic mixing. Int. J. Pharm. 521, 337–345 (2017).
Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
Otwinowski, Z., Minor, W., Borek, D. & Cymborowski, M. in International Tables for Crystallography Volume F. Crystallography of Biological Macromolecules 2nd edn. (eds. Rossman, M. G. et al.) Ch. 11.4 (Wiley, 2012).
Leslie, A. G. W. & Powell, H. R. in Evolving Methods for Macromolecular Crystallography (eds. Read, R. J. & Sussman, J. L.) 41–51 (Springer Netherlands, 2007).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Murshudov, G. N. et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. D Biol. Crystallogr. 67, 355–367 (2011).
Hilz, H., Wiegers, U. & Adamietz, P. Stimulation of proteinase K action by denaturing agents: application to the isolation of nucleic acids and the degradation of ‘masked’ proteins. Eur. J. Biochem. 56, 103–108 (1975).
Carragher, B. et al. Leginon: an automated system for acquisition of images from vitreous ice specimens. J. Struct. Biol. 132, 33–45 (2000).
Li, X. et al. Electron counting and beam-induced motion correction enable near-atomic-resolution single-particle cryo-EM. Nat. Methods 10, 584–590 (2013).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. Elife 7, e42166 (2018).
Zhang, K. Gctf: real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
He, S. Helical Reconstruction in RELION. Doctoral thesis, Univ. of Cambridge (2018).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Liberta, F. et al. Cryo-EM fibril structures from systemic AA amyloidosis reveal the species complementarity of pathological amyloids. Nat. Commun. 10, 1104 (2019).
Delano, W. The PyMOL Molecular Graphics System (Schrödinger LLC).
Chaudhury, S., Lyskov, S. & Gray, J. J. PyRosetta: a script-based interface for implementing molecular modeling algorithms using Rosetta. Bioinforma. Oxf. Engl 26, 689–691 (2010).
Eisenberg, D. & McLachlan, A. D. Solvation energy in protein folding and binding. Nature 319, 199–203 (1986).
Eisenberg, D., Wesson, M. & Yamashita, M. Interpretation of protein folding and binding with atomic solvation parameters. Chemica Scr. 29A, 217–221 (1989).
Koehl, P. & Delarue, M. Application of a self-consistent mean field theory to predict protein side-chains conformation and estimate their conformational entropy. J. Mol. Biol. 239, 249–275 (1994).
Acknowledgements
We thank D. Cascio (UCLA), H. McFarlane (UCLA) and C. Sigurdson (UCSD). This work is supported by National Science Foundation (NSF) Grants DMR-1548924 and DBI-1338135, DOE Grant DE-FC02-02ER63421 and National Institutes of Health (NIH) grants R35 GM128867, AG054022 and 1U24GM116792, as well as NIH instrumentation grants 1S10OD016387-01, 1S10RR23057 and 1S10OD018111, which support our use of instruments at the Electron Imaging Center for NanoMachines and CNSI at UCLA. C.G. was funded by the Ruth L. Kirschstein NRSA GM007185 (NIH T32 Cellular and Molecular Biology Training Grant, UCLA) and is now funded by the Ruth L. Kirschstein Predoctoral Individual NRSA, 1F31 AI143368. J.A.R. is supported as a Searle Scholar, a Pew Scholar and a Beckman Young Investigator. D.S.E is supported by the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
C.G. and R.B. produced, evaluated and optimized recombinant PrP fibril preparations. C.G. and P.G. performed electron microscopy. M.A. and M.R.S. performed X-ray structure determination of PrP segments. C.G. and C.W.S. selected particles for analysis. C.G., P.G. and M.G.J. performed fibril reconstruction. C.G. and M.R.S. built the fibril model. C.G., P.G., M.R.S., M.G.J., C.W.S., R.B., M.A., Z.H.Z., D.S.E. and J.A.R. critically analyzed and provided feedback on data. C.G. and J.A.R. wrote the manuscript, with input from all authors.
Corresponding author
Ethics declarations
Competing interests
D.S.E. is SAB chair and an equity holder in ADRx.
Additional information
Peer review information Inês Chen was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Summary sentence: The cryo-EM structure of a denaturant- and protease-resistant human prion fibril shows a parallel, in-register assembly with a hydrophobic core.
Extended data
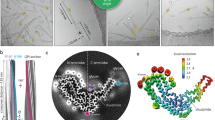
Extended Data Fig. 1 Production, isolation and characterization of rPrP94–178 fibrils.
a, Purification of untagged human PrP94–178. Samples collected during size exclusion chromatography of PrP94–178 were run on a 4–12 % SDS-PAGE gel corresponding to labeled peaks b, Gel lanes left to right: 1. Pre-stained protein ladder (Thermo), 2. Post-induction fraction, 3. Solubilized material from inclusion bodies, 4. Void fraction (A) from (a), 5. Peak (B) from (a), 6. Peak (C) from (a) containing 9.7 kDa monomeric rPrP94–178, 7–9. Peaks D-F from (a) containing excess guanidine or other UV active small eluates. c, Mass spectrum showing most abundant peaks correspond to ions with an extracted molecular weight that matches rPrP94–178. d, Representative micrographs of a heterogeneous mix of untreated fibrils (left) in Growth Buffer; promising filaments (black arrows) and disordered, clumped, or amorphous material (blue arrows). Adjacent images are of filaments treated with 1:10 molar ratio proteinase K:rPrP94–178 monomer and bath sonicated for 10 minutes. Proteinase K-treated and sonicated filaments exchanged into water in a frozen-hydrated state (third column, top) or 2 % SDS (third column, bottom). Representative image of Proteinase K, sonication, and SDS treated filaments used for high-resolution imaging and reconstruction (right). Scale bars, 200 nm. Scale bar for high-resolution image (right) 50 nm.
Extended Data Fig. 2 Partial protease digestion of rPrP94–178 fibrils.
a, Plots of incubation time versus nephelometry units as a measure of insoluble character in fibril suspensions treated with proteinase K compared to proteinase K only and buffer controls. b, Representative electron micrographs of each sample in panel (a) before proteinase K digestion at the start of the incubation period. This image of fibrils is the same as that shown in Extended Data Fig. 1d. c, Representative micrographs of each sample in Panel (a) after the 24-hour incubation period. All scale bars are 200 nm.
Extended Data Fig. 3 Classification of major species of protease-resistant rPrP94–178 fibrils.
a, Final 2D class averages from the major species comprising 71 % of defined segments using a 535 Å box with a 530 Å mask (left) or 428 Å (400 pixel) box with a 400 Å mask (right). b, A composite image (left) formed by stitching of 2D class averages with the small box shown in (a), agrees with a composite image (right) formed by class averages obtained using a box size encompassing a full crossover distance in (a). The crossover distance and full pitch are both marked. c, Comparison between a 2D class (enlarged view of boxed class in (a)), the map backprojection, and model backprojection with accompanying Fourier transforms below. d, Slice through the 3D density with dimensions of the ordered region and surrounding diffuse density noted.
Extended Data Fig. 4 2D classification of minor morphological populations formed by rPrP94–178.
a, Stain-embedded, Proteinase K and sonication treated fibrils show several minor populations of ribbon-like polymorphs (white arrows), including wide filaments with regions that abruptly stop (red arrows). These morphologies remain a minor species after vitrification in 2 % SDS alongside the major twisted species (single example black arrow) (b) A 2D classification round selecting for minor species results in 2D classes that make up 29 % (75,860 segments) of total particles sorted into defined classes (c) with 2.1% of the total segments across major and minor populations (~6000 particles) being sorted into classes that resemble thick ribbons with columns of alternating electron dense and poor material (stars). Additional 2D classification of segments that did not sort into thick ribbon classes (d) revealed several classes containing high-resolution information, in some classes even revealing 4.8 Å strand separation. The 16 best looking classes are shown and make up 62 % (47,479 segments) of the particles shown in (c). 3D reconstruction of these filaments was unsuccessful. A magnified view of select classes from (c) and (d) show the range of fibril widths observed (e) Scalebar in (a) 200 nm, in (b) 50 nm.
Extended Data Fig. 5 Agreement of rPrP106–145 model with core density in rPrPRes.
a, Fourier Shell Correlation (FSC) between half-maps (blue) and the map and model (green) with resolutions at FSC = 0.5 (black dotted line) and 0.143 (gray dotted line) noted. b, Agreement of turn region with density looking down the fibril axis and (c) a side view of the same region shows clear strand separation. d, Overall fit of model into density. e, Magnified view of protofilament interface showing spacing between backbones at the center of the interface is at the most tightly spaced region between G114 of one chain and G119 of its mate. f, Magnified view of linchpin region with inferred salt bridge between residues H111 and D144 that seal off the interior of each chain and a side view (g) of the same region showing 4.8 Å separation between strands.
Extended Data Fig. 6 Structural agreement of hexapeptide prion zipper structures to rPrPRes.
a, ZipperDB26 profile of the segment encoding for rPrPRes. Bars extending below the line represent hexapeptides predicted to form steric zippers. Peptides with crystal structures aligned to the fibril model are boxed. Two of these were previously published27,28. Alignment of crystal structures 119GAVVGG124 (b) 113AGAAAA118 (c) 127GYMLGS132 (e) and 138IIHFGS143 (f) with rPrPRes (d) Symmetry mates in plane, all atom, and backbone (parentheses) RMSD values against rPrPRes are shown (g) Sequence alignment for species of common interest to prion disease. Darker blues correlates with more variability, loosely defined as more residue mismatches among the species compared, and mapping of these residues onto rPrPRes h, Hexapeptide structure for muPrP137–142 (mouse numbering) is compatible with rPrPRes (i) while shPrP138–143 is not (j).
Extended Data Fig. 7 Molecular contacts at the core of rPrPRes.
a, Cartoon representation of one protofilament with three stacked chains highlighting outer (yellow) and inner (blue) sheets. Stick representation of stacked inner (b) and outer (c) sheets with inferred hydrogen bonding networks based on refinement giving weight to standard beta sheet geometry, favored Ramachandran angles, and map to model fit. Residues where geometry deviated during refinement to break backbone hydrogen bonds are noted in blue text. d, Side view highlighting a favorable salt bridge between H111 and D144 and the same region rotated to show the interaction looking down the fiber axis (e).
Extended Data Fig. 8 Alignment of fast-relaxed mutant sequences to rPrPRes.
a, Left: Alignment of eight chains of the model (gray) to the same number of chains of a fast-relaxed model produced in PyRosetta (green) along with magnified views with sidechains in the turn region (center) and the protofilament interface (right). b, Eight chains of fast relaxed G114V mutant (left) along with magnified view of the protofilament interface near the mutation site (red arrow). The same set of three views are also shown for A117V (c) G127V (d) and M129V (e) mutants or polymorphisms.
Supplementary information
Supplementary Information
Supplementary Tables 1–3
Rights and permissions
About this article
Cite this article
Glynn, C., Sawaya, M.R., Ge, P. et al. Cryo-EM structure of a human prion fibril with a hydrophobic, protease-resistant core. Nat Struct Mol Biol 27, 417–423 (2020). https://doi.org/10.1038/s41594-020-0403-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-020-0403-y
This article is cited by
-
Misfolded protein oligomers: mechanisms of formation, cytotoxic effects, and pharmacological approaches against protein misfolding diseases
Molecular Neurodegeneration (2024)
-
Synthetic β-sheets mimicking fibrillar and oligomeric structures for evaluation of spectral X-ray scattering technique for biomarker quantification
Cell & Bioscience (2024)
-
Prion protein amino acid sequence influences formation of authentic synthetic PrPSc
Scientific Reports (2023)
-
Therapeutic strategies for identifying small molecules against prion diseases
Cell and Tissue Research (2023)
-
Prion strains viewed through the lens of cryo-EM
Cell and Tissue Research (2023)