Abstract
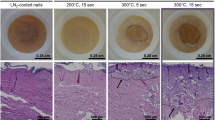
Host defense peptides (HDP) are naturally occurring effector molecules of the innate immune system, which might be an alternative to currently used antibiotics. The objective of this study was to investigate the efficiency of transient cutaneous adenoviral transfection with human cathelicidin hCAP-18/LL-37 in infected burn wounds. Specific transgene expression was analyzed in vitro on mRNA and protein level using real-time PCR and Western-blot. Male Sprague–Dawley rats (n=40) received a second degree scald burn on both flanks (5% BSA), which were inoculated with 108 colony-forming units (CFU) Pseudomonas aeruginosa. Two days later, rats were randomized into the following groups: (1) adenoviral delivery of LL-37 (Ad5-hCAP-18, n=10), (2) synthetic host defense peptide LL-37 (1 mg; n=10), (3) carrier control (PBS, n=10) and (4) empty-virus control (Ad5-LacZ, n=10). Agents were injected intradermally and subcutaneously into both flanks. After either 2 or 7 days, skin samples were harvested and homogenized. CFU per gram tissue were determined. The hCAP-18/LL-37 expression was confirmed by real-time PCR and localized using in situ hybridization. In vitro transfection of cutaneous cells delivered a specific response on mRNA production. Western blot analysis revealed protein expression of hCAP-18/LL-37 in conditioned medium and cell pellet. The host defense peptide LL-37 was detectable after cleavage of the inactive pro-form hCAP-18/LL-37 with human elastase. Ad5-hCAP-18 showed a significant bacterial inhibition of approximately 10 000 fold compared to the control group (P<0.001) and 1000-fold (P<0.001) compared to the synthetic HDP LL-37 7 post-transfection. No inhibition was observed for the carrier or empty-virus control. Real-time PCR and in situ hybridization confirmed expression of hCAP-18/LL-37. In conclusion, transient cutaneous adenoviral delivery of the host defense peptide hCAP-18/LL-37 is significantly more effective than administration of synthetic host defense peptides and might be a potential adjunct for wound treatment in the near future.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Pruitt Jr BA, McManus AT, Kim SH, Goodwin CW . Burn wound infections: current status. World J Surg 1998; 22: 135–145.
Estahbanati HK, Kashani PP, Ghanaatpisheh F . Frequency of Pseudomonas aeruginosa serotypes in burn wound infections and their resistance to antibiotics. Burns 2002; 28: 340–348.
Alekseev AA, Iakovlev VP, Fedorov VD . Infection in burn patients: the problems of pathogenesis, prevention and treatment. Khirurgiia (Mosk) 1999; 6: 4–9.
Lindberg RB et al. The successful control of burn wound sepsis. J Trauma 1965; 5: 601–616.
Stone HH . Review of Pseudomonas sepsis in thermal burns: verdoglobin determination and gentamicin therapy. Ann Surg 1966; 163: 297–305.
Fox Jr CL, Rappole BW, Stanford W . Control of Pseudomonas infection in burns by silver sulfadiazine. Surg Gynecol Obstet 1969; 128: 1021–1026.
Hanberger H et al. Antibiotic susceptibility among aerobic Gram-negative bacilli in intensive care units in 5 European countries. French and Portuguese ICU Study Groups. JAMA 1999; 281: 67–71.
Lehrer RI, Ganz T . Antimicrobial peptides in mammalian and insect host defence. Curr Opin Immunol 1999; 11: 23–27.
Zanetti M, Gennaro R, Romeo D . Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett 1995; 374: 1–5.
Bals R, Wang X, Zasloff M, Wilson JM . The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci USA 1998; 95: 9541–9546.
Frohm M et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem 1997; 272: 15258–15263.
Ganz T, Weiss J . Antimicrobial peptides of phagocytes and epithelia. Semin Hematol 1997; 34: 343–354.
Frohm M et al. Biochemical and antibacterial analysis of human wound and blister fluid. Eur J Biochem 1996; 237: 86–92.
Bals R . Epithelial antimicrobial peptides in host defense against infection. Respir Res 2000; 1: 141–150.
Koczulla R et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest 2003; 111: 1665–1672.
Lawrence JC . Burn bacteriology during the last 50 years. Burns 1992; 18 (Suppl 2): S23–S29.
Milner SM, Ortega MR . Reduced antimicrobial peptide expression in human burn wounds. Burns 1999; 25: 411–413.
Ortega MR, Ganz T, Milner SM . Human beta defensin is absent in burn blister fluid. Burns 2000; 26: 724–726.
Almeida MS et al. Solution structure of Pisum sativum defensin 1 by high resolution NMR: plant defensins, identical backbone with different mechanisms of action. J Mol Biol 2002; 315: 749–757.
Campain JA et al. Lipid- and adenoviral-mediated gene transfer into AIDS-Kaposi's sarcoma cell lines. Cancer Gene Ther 1998; 5: 131–143.
Campbell C et al. Green fluorescent protein-adenoviral construct as a model for transient gene therapy for human cultured keratinocytes in an athymic mouse model. J Trauma 2003; 54: 72–79; discussion 79–80.
Domashenko A, Gupta S, Cotsarelis G . Efficient delivery of transgenes to human hair follicle progenitor cells using topical lipoplex. Nat Biotechnol 2000; 18: 420–423.
Benn SI et al. Particle-mediated gene transfer with transforming growth factor-beta1 cDNAs enhances wound repair in rat skin. J Clin Invest 1996; 98: 2894–2902.
Davidson JM et al. Gene therapy of wounds with growth factors. Curr Top Pathol 1999; 93: 111–121.
Fallaux FJ et al. Characterization of 911: a new helper cell line for the titration and propagation of early region 1-deleted adenoviral vectors. Hum Gene Ther 1996; 7: 215–222.
Steinstraesser L et al. Activity of novispirin G10 against Pseudomonas aeruginosa in vitro and in infected burns. Antimicrob Agents Chemother 2002; 46: 1837–1844.
Nizet V et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 2001; 414: 454–457.
Steinstraesser L, Oezdogan Y, Wang SC, Steinau HU . Host defense peptides in burns. Burns 2004; 30: 619–627.
Chung WO, Dale BA . Innate immune response of oral and foreskin keratinocytes: utilization of different signaling pathways by various bacterial species. Infect Immun 2004; 72: 352–358.
Sorensen OE et al. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood 2001; 97: 3951–3959.
Cole AM et al. Inhibition of neutrophil elastase prevents cathelicidin activation and impairs clearance of bacteria from wounds. Blood 2001; 97: 297–304.
Zasloff M . Antimicrobial peptides of multicellular organisms. Nature 2002; 415: 389–395.
Agerberth B et al. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood 2000; 96: 3086–3093.
Nagaoka I et al. Augmentation of the lipopolysaccharide-neutralizing activities of human cathelicidin CAP18/LL-37-derived antimicrobial peptides by replacement with hydrophobic and cationic amino acid residues. Clin Diagn Lab Immunol 2002; 9: 972–982.
Hancock RE . Peptide antibiotics. Lancet 1997; 349: 418–422.
Shafer WM, Qu X, Waring AJ, Lehrer RI . Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc Natl Acad Sci USA 1998; 95: 1829–1833.
Breithaupt H . The new antibiotics. Nat Biotechnol 1999; 17: 1165–1169.
Latham PW . Therapeutic peptides revisited. Nat Biotechnol 1999; 17: 755–757.
Sylvester KG et al. Adenoviral-mediated gene transfer in wound healing: acute inflammatory response in human skin in the SCID mouse model. Wound Repair Regen 2000; 8: 36–44.
Schiff GD et al. Improving inpatient antibiotic prescribing: insights from participation in a national collaborative. Jt Comm J Qual Improv 2001; 27: 387–402.
Bals R et al. Mouse beta-defensin 3 is an inducible antimicrobial peptide expressed in the epithelia of multiple organs. Infect Immun 1999; 67: 3542–3547.
Christensen R, Jensen UB, Jensen TG . Cutaneous gene therapy—an update. Histochem Cell Biol 2001; 115: 73–82.
Koczulla AR, Bals R . Antimicrobial peptides: current status and therapeutic potential. Drugs 2003; 63: 389–406.
Blau HM, Springer ML . Gene therapy—a novel form of drug delivery. N Engl J Med 1995; 333: 1204–1207.
Leiden JM . Gene therapy—promise, pitfalls, and prognosis. N Engl J Med 1995; 333: 871–873.
Grayson LS et al. Quantitation of cytokine levels in skin graft donor site wound fluid. Burns 1993; 19: 401–405.
Harvey BG et al. Cellular immune responses of healthy individuals to intradermal administration of an E1–E3-adenovirus gene transfer vector. Hum Gene Ther 1999; 10: 2823–2837.
St George JA . Gene therapy progress and prospects: adenoviral vectors. Gene Therapy 2003; 10: 1135–1141.
Somia N, Verma IM . Gene therapy: trials and tribulations. Nat Rev Genet 2000; 1: 91–99.
Heilborn JD et al. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J Invest Dermatol 2003; 120: 379–389.
Yang Y, Li Q, Ertl HC, Wilson JM . Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol 1995; 69: 2004–2015.
Goosney DL, Nemerow GR . Adenovirus infection: taking the back roads to viral entry. Curr Biol 2003; 13: R99–R100.
Acknowledgements
We sincerely thank Janine Mertens and Sabine Böhm for expert technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft (DFG, Ste1099).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jacobsen, F., Mittler, D., Hirsch, T. et al. Transient cutaneous adenoviral gene therapy with human host defense peptide hCAP-18/LL-37 is effective for the treatment of burn wound infections. Gene Ther 12, 1494–1502 (2005). https://doi.org/10.1038/sj.gt.3302568
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302568
Keywords
This article is cited by
-
Efficacy of LL-37 cream in enhancing healing of diabetic foot ulcer: a randomized double-blind controlled trial
Archives of Dermatological Research (2023)
-
Basic concepts, current evidence, and future potential for gene therapy in managing cutaneous wounds
Biotechnology Letters (2019)
-
Komodo dragon-inspired synthetic peptide DRGN-1 promotes wound-healing of a mixed-biofilm infected wound
npj Biofilms and Microbiomes (2017)
-
Intratympanic Gene Delivery of Antimicrobial Molecules in Otitis Media
Current Allergy and Asthma Reports (2015)
-
Skin Electroporation of a Plasmid Encoding hCAP-18/LL-37 Host Defense Peptide Promotes Wound Healing
Molecular Therapy (2014)