Abstract
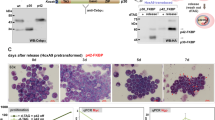
HoxB8 was the first homeobox gene identified as a cause of leukemia. In murine WEHI3B acute myeloid leukemia (AML) cells, proviral integration leads to the expression of both HoxB8 and Interleukin (IL-3). Enforced expression of HoxB8 blocks differentiation of factor-dependent myeloid progenitors, while IL-3 co-expression induces autocrine proliferation and overt leukemogenicity. Previously, we demonstrated that HoxB8 binds DNA cooperatively with members of the Pbx family of transcription factors, and that HoxB8 makes contact with the Pbx homeodomain through a hexameric sequence designated the Pbx-interaction motif (PIM). E2a-Pbx1, an oncogenic derivative of Pbx1, both retains its ability to heterodimerize with Hox proteins and arrest myeloid differentiation. This observation prompts the question of whether E2a-Pbx1 and Hox oncoproteins use endogenous Hox and Pbx proteins, respectively, to target a common set of cellular genes. Here, we use four different models of neutrophil and macrophage differentiation to determine whether HoxB8 needs to bind DNA or Pbx cofactors in order to arrest myeloid differentiation. The ability of HoxB8 to bind DNA or to bind Pbx was essential (1) to block differentiation of factor-dependent myeloid progenitors from primary marrow; (2) to block IL-6-induced monocytic differentiation of M1-AML cells; and (3) to block granulocytic differentiation of GM-CSF-dependent ECoM-G cells. However, while DNA-binding was required, the HoxB8 Pbx-interaction motif was unnecessary for preventing macrophage differentiation of ECoM-M cells. We conclude that HoxB8 prevents differentiation by directly influencing cellular gene expression, and that the genetic context within a cell dictates whether the effect of HoxB8 is dependent on a physical interaction with Pbx proteins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Blatt C, Aberdam D, Schwartz R, Sachs L . 1988 EMBO J. 7: 4283–4290
Borrow J, Shearman AM, Stanton VP, Becher R, Collins T, Williams AJ, Dube I, Katz F, Kwong YL, Morris C, Ohyashiki K, Toyama K, Rowley J, Housman DE . 1996 Nat. Genet. 12: 159–167
Calvo KR, Knoepfler P, McGrath S, Kamps MP . 1999 Oncogene 18: 8033–8043
Calvo KR, Sykes D, Pasillas M, Kamps MP . 2000 Mol. Cell. Biol. 20: 3274–3285
Care A, Valtieri M, Mattia G, Meccia E, Masella B, Luchetti L, Felicetti F, Colombo MP, Peschle C . 1999 Oncogene 18: 1993–2001
Chang CP, Brocchieri L, Shen WF, Largman C, Cleary ML . 1996 Mol. Cell. Biol. 16: 1734–1745
Chang CP, Jacobs Y, Nakamura T, Jenkins NA, Copeland NG, Cleary ML . 1997 Mol. Cell. Biol. 17: 5679–5687
Chen J, Ruley HE . 1998 J. Biol. Chem. 273: 24670–24675
Dedera DA, Waller EK, Lebrun DP, Sen-Majumdar A, Stevens ME, Barsh GS, Cleary ML . 1993 Cell 74: 833–843
Di Rocco G, Mavilio F, Zappavigna V . 1997 EMBO J. 16: 3644–3654
Downing JR . 1999 Br. J. Haematol. 106: 296–308
Golub TT, Slonim DK, Tamayo P, Huard C, Gaasenbeek M, Mesirov JP, Coller H, Loh ML, Downing JR, Caligiuri MA, Bloomfield CD, Lander ES . 1999 Science 286: 531–537
Goudet G, Delhalle S, Biemar F, Martial JA, Peers B . 1999 J. Biol. Chem. 274: 4067–4073
Hatano M, Roberts CW, Minden M, Crist WM, Korsmeyer SJ . 1991 Science 253: 79–82
Hawley RG, Fong AZ, Lu M, Hawley TS . 1994 Oncogene 9: 1–12
Izon DJ, Rozenfeld S, Fong ST, Komuves L, Largman C, Lawrence HJ . 1998 Blood 92: 383–393
Jacobs Y, Schnabel CA, Cleary ML . 1999 Mol. Cell. Biol. 19: 5134–5142
Kamps MP, Baltimore D . 1993 Mol. Cell. Biol. 13: 351–357
Kamps MP, Look T, Baltimore D . 1991 Genes Dev. 5: 358–368
Kamps MP, Wright DD . 1994 Oncogene 9: 3159–3166
Kasper LH, Brindle PK, Schnabel CA, Pritchard CE, Cleary ML, Van Deursen JM . 1999 Mol. Cell. Biol. 19: 764–776
Knoepfler PS, Calvo KR, Chen H, Antonarakis SE, Kamps MP . 1997 Proc. Natl. Acad. Sci. USA 94: 14553–14558
Knoepfler PS, Kamps MP . 1995 Mol. Cell. Biol. 15: 5811–5819
Knoepfler PS, Lu Q, Kamps MP . 1996 Nucleic Acids Res. 24: 2288–2294
Kroon E, Krosl J, Thorsteinsdottir U, Baban S, Buchberg AM, Sauvageau G . 1998 EMBO J. 17: 3714–3725
Krosl J, Baban S, Krosl G, Rozenfeld S, Largman C, Sauvageau G . 1998 Oncogene 16: 3403–3412
Krumlauf R . 1994 Cell 78: 191–201
Lawrence HJ, Helgason CD, Sauvageau G, Fong S, Izon DJ, Humphries RK, Largman C . 1997 Blood 89: 1922–1930
Lawrence HF, Sauvageau G, Humphries RK, Largman C . 1996 Stem Cells 14: 281–291
Lewis EB . 1978 Nature 276: 565–570
Lu Q, Kamps MP . 1996 Mol. Cell. Biol. 16: 1632–1640
Lu Q, Kamps MP . 1997 Oncogene 14: 75–83
McGinnis W, Krumlauf R . 1992 Cell 68: 283–302
Nakamura T, Largaespada DA, Lee MP, Johnson LA, Ohyashiki K, Toyama K, Chen SJ, Willman CL, Chen IM, Feinberg AP, Jenkins NA, Copeland NG, Shaughnessy JD . 1996 Nat. Genet. 12: 154–158
Nakamura T, Largaespada DA, Shaughnessy JD, Jenkins NA, Copeland NG . 1996 Nat. Genet. 12: 149–153
Passner JM, Ryoo HD, Shen L, Mann RS, Aggarwal AK . 1999 Nature 397: 714–719
Peers B, Sharma S, Johnson T, Kamps MP, Montminy M . 1995 Mol. Cell. Biol. 15: 7091–7097
Perkins A, Kongsuwan K, Visvader J, Adams JM, Cory S . 1990 Proc. Natl. Acad. Sci. USA 87: 8398–8402
Piper DE, Batchelor AH, Chang C, Cleary ML, Wolberger C . 1999 Cell 96: 587–597
Popperl H, Bienz M, Studer M, Chan SK, Aparicio S, Brenner S, Mann RS, Krumlauf R . 1995 Cell 81: 1031–1042
Sauvageau G, Thorsteinsdottir U, Eaves CJ, Lawrence HF, Largman C, Lansdorp PM, Humphries RK . 1995 Genes Dev. 9: 1753–1765
Sauvageau G, Thorsteinsdottir U, Hough MR, Hugo P, Lawrence HJ, Largman C, Humphries RK . 1997 Immunity 6: 13–22
Sykes DB, Kamps MK . 2001 Blood in press
Tora L, Mullick A, Metzger D, Ponglikitmongkol M, Park I, Chambon P . 1989 EMBO J. 8: 1981–1986
Thorsteinsdottir U, Sauvageau G, Hough MR, Dragowska W, Lansdorp PM, Lawrence HJ, Largmen C, Humphries RK . 1997 Mol. Cell. Biol. 17: 495–505
Vogt PK, Bos TJ . 1990 Adv. Cancer Res. 55: 1–35
Yaron Y, McAdara JK, Lynch M, Hughes E, Gasson JC . 2001 J. Immunol. 166: 5058–5067
Acknowledgements
This work was supported by Public Health Services grant CA56876.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Knoepfler, P., Sykes, D., Pasillas, M. et al. HoxB8 requires its Pbx-interaction motif to block differentiation of primary myeloid progenitors and of most cell line models of myeloid differentiation. Oncogene 20, 5440–5448 (2001). https://doi.org/10.1038/sj.onc.1204710
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1204710
Keywords
This article is cited by
-
Oncogenic HOXB8 is driven by MYC-regulated super-enhancer and potentiates colorectal cancer invasiveness via BACH1
Oncogene (2020)
-
A 92-gene cancer classifier predicts the site of origin for neuroendocrine tumors
Modern Pathology (2014)
-
Hoxb8 regulates expression of microRNAs to control cell death and differentiation
Cell Death & Differentiation (2013)
-
Pbx1 is a downstream target of Evi-1 in hematopoietic stem/progenitors and leukemic cells
Oncogene (2009)
-
New insight into the role of miRNAs in leukemia
Science in China Series C: Life Sciences (2009)