Abstract
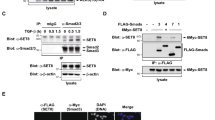
Serum response factor (SRF) is a widely expressed transcription factor involved in immediate-early and tissue-specific gene expression, cell proliferation and differentiation. We defined a new role of SRF as a nuclear repressor of the tumor growth factor β1 (TGF-β1) growth-inhibitory signal during cell proliferation. We show that SRF significantly inhibits the TGF-β1/Smad-dependent transcription by associating with Smad3. SRF causes resistance to the TGF-β1 cytostatic response by directly repressing the Smad transcriptional activity and Smad binding to DNA. Furthermore, we demonstrated that overexpression of SRF markedly decreases the level of Smad3 complex binding to the promoters of Smad3 target genes, p15INK4b and p21Cip1. This leads to the inhibition of expression of TGF-β1-responsive genes. SRF therefore acts as a nuclear repressor of Smad3-mediated TGF-β1 signaling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Abraham SE, Carter MC, Moran E . (1992). Mol Biol Cell 3: 655–665.
Alexandrow MG, Kawabata M, Aakre M, Moses HL . (1995). Proc Natl Acad Sci USA 92: 3239–3243.
Carcamo J, Zentella A, Massague J . (1995). Mol Cell Biol 15: 1573–1581.
Chai J, Tarnawski AS . (2002). J Physiol Pharmacol 53: 147–157.
Claassen GF, Hann SR . (2000). Proc Natl Acad Sci USA 97: 9498–9503.
Danielpour D, Sporn MB . (1990). J Biol Chem 265: 6973–6977.
Datto MB, Li Y, Panus JF, Howe DJ, Xiong Y, Wang X-F . (1995). Proc Natl Acad Sci USA 92: 5545–5549.
de Caestecker MP, Piek E, Roberts AB . (2000). J Natl Cancer Inst 92: 1388–1402.
Depoortere F, Pirson I, Bartek J, Dumont JE, Roger PP . (2000). Mol Biol Cell 11: 1061–1076.
Derynck R, Zhang Y, Feng XH . (1998). Cell 95: 737–740.
Ewen ME, Sluss HK, Whitehouse LL, Livingston DM . (1993). Cell 74: 1009–1020.
Feng X-H, Lin X, Derynck R . (2000). EMBO J 19: 5178–5193.
Feng XH, Liang YY, Liang M, Zhai W, Lin X . (2002). Mol Cell 9: 133–143.
Garrigue-Antar L, Barbieux I, Lieubeau B, Boisteau O, Gregoire M . (1995). J Immunol Methods 186: 267–274.
Geng Y, Weinberg RA . (1993). Proc Natl Acad Sci USA 90: 10315–10319.
Hannon GJ, Beach D . (1994). Nature 371: 257–261.
Iavarone A, Massague J . (1997). Nature 387: 417–422.
Iyer D, Belaguli N, Fluck M, Rowan BG, Wei L, Weigel NL et al. (2003). Biochemistry 42: 7477–7486.
Iyer D, Chang D, Marx J, Wei L, Olson EN, Parmacek MS et al. (2006). Proc Natl Acad Sci USA 103: 4516–4521.
Janknecht R, Hipskind RA, Houthaeve T, Nordheim A, Stunnenberg HG . (1992). EMBO J 11: 1045–1054.
Johansen F, Prywes R . (1993). Mol Cell Biol 13: 4640–4647.
Kang SH, Bang YJ, Jong HS, Seo JY, Kim NK, Kim S-J . (1999). Br J Cancer 80: 1144–1149.
Kim BC, Lee HJ, Park SH, Lee SR, Karpova TS, McNally JG et al. (2004). Mol Cell Biol 24: 2251–2262.
Kim JH, Johansen FE, Robertson N, Catino JJ, Prywes R, Kumar CC . (1994). J Biol Chem 269: 13740–13743.
Laiho M, DeCaprio JA, Ludlow JW, Livingston DM, Massague J . (1990). Cell 62: 175–185.
Lee DK, Kim BC, Kim IY, Cho EA, Satterwhite DJ, Kim S-J . (2002). J Biol Chem 277: 38557–38564.
Lee HJ, Lee JK, Miyake S, Kim S-J . (2004). J Biol Chem 279: 2666–2672.
Mack CP, Owens GK . (1999). Circ Res 84: 852–861.
Lee MH, Yang HY . (2003). Cancer Metast Rev 22: 435–449.
Liu SH, Ma JT, Yueh AY, Lees-Miller SP, Anderson CW, Ng SY . (1993). J Biol Chem 268: 21147–21154.
Manak JR, Prywes R . (1991). Mol Cell Biol 11: 3652–3659.
Massague J, Chen YG . (2000). Genes Dev 14: 627–644.
Miano JM. . (2003). J Mol Cell Cardiol 35: 577–593.
Moustakas A, Kardassis D . (1998). Proc Natl Acad Sci USA 95: 6733–6738.
Norman C, Runswick M, Pollock R, Treisman R . (1988). Cell 55: 989–1003.
Paradis P, MacLellan WR, Belaguli NS, Schwartz RJ, Schneider MD . (1996). J Biol Chem 271: 10827–10833.
Pardali K, Kurisaki A, Moren A, ten Dijke P, Kardassis D, Moustakas A . (2000). J Biol Chem 275: 29244–29256.
Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, Roberts JM et al. (1994). Genes Dev 8: 9–22.
Psichari E, Balmain A, Plows D, Zoumpourlis V, Pintzas A . (2002). J Biol Chem 277: 29490–29495.
Rivera VM, Miranti CK, Misra RP, Ginty DD, Chen R-H, Blenis J et al. (1993). Mol Cell Biol 13: 6260–6273.
Qiu P, Feng X-H, Li L . (2003). J Mol Cell Cardiol 35: 1407–1420.
Schratt G, Weinhold B, Lundberg A, Schuck S, Berger J, Schwarz H et al. (2001). Mol Cell Biol 21: 2933–2943.
Shi Y, Massagué J . (2003). Cell 113: 685–700.
Sotiropoulos A, Gineitis D, Copeland J, Treisman R . (1999). Cell 98: 159–169.
Treisman R . (1992). Trends Biochem Sci 17: 423–426.
Treisman R . (1994). Curr Opin Genet Dev 4: 96–101.
Treisman R . (1995). EMBO J 14: 4905–4913.
Warner BJ, Blain SW, Seoane J, Massague J . (1999). Mol Cell Biol 19: 5913–5922.
Wrana JL, Attisano L . (2000). Cytokine Growth Factor Rev 11: 5–13.
Wrana JL, Attisano L, Carcamo J, Zentella A, Doody J, Laiho M et al. (1992). Cell 71: 1003–1014.
Zawel L, Dai JL, Buckhaults P, Zhou S, Kinzler KW, Vogelstein B et al. (1998). Mol Cell 1: 611–617.
Acknowledgements
This work was supported by funds from the intramural program of National Cancer Institute. We thank J Massaguè for 3TP-Lux, S Kern for SBE4-luc, JH Kim for SRF constructs and AB Roberts for the critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Lee, HJ., Yun, CH., Lim, S. et al. SRF is a nuclear repressor of Smad3-mediated TGF-β signaling. Oncogene 26, 173–185 (2007). https://doi.org/10.1038/sj.onc.1209774
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1209774
Keywords
This article is cited by
-
Transcriptional profiling revealed the anti-proliferative effect of MFN2 deficiency and identified risk factors in lung adenocarcinoma
Tumor Biology (2016)
-
GREAT improves functional interpretation of cis-regulatory regions
Nature Biotechnology (2010)
-
Predicting cancer involvement of genes from heterogeneous data
BMC Bioinformatics (2008)