Abstract
Busulfan and cyclophosphamide (B/C)-treated mice exhibited a marked increase in apoptosis and a concomitant decrease in the ovarian weight. Histological and RT-PCR analysis indicate that the period of germ cell depletion in the B/C-treated ovaries coincides with decreased expression of genes Figla, Lhx8, Nobox, c-kit and Sox3. However, depletion of the ovarian germ cells is mediated by autophagy-independent pathways that involve Fas/FasL-, TNF-, and/or p53-signalings. Treatment with B/C resulted in the cease of the reproductive function to produce their offspring during the 15th week post-treatment period in female mice. Furthermore, injection of the 3 × 106 GFP positive primordial follicles into the ovaries of the B/C treated mouse did not show apparent colonization of the transplanted follicles within the recipient ovaries. The present results suggest that B/C treatment is closely associated with an increased risk of premature ovarian failure.
Similar content being viewed by others
Introduction
Chemotherapy with alkylating agents may trigger several deleterious cellular events in both germ cells and somatic cells. Cytotoxic effects are exerted via the transfer of alkyl group(s) to various cellular constituents. Among these, DNA alkylation within the nucleus probably constitutes one of the major provocations that lead to cell death1. In addition, certain class of chemotherapeutic drugs such as busulfan and other alkylating agents were also reported to cause prolonged and sometimes irreversible azoospermia in mice and human2. However, few studies have focused on the cellular and/or molecular mechanisms whereby chemotherapy induces ovarian damage in cancer patients. In clinical circumstances, chemotherapy can induce Fas ligand (FasL) expression in the granulosa cells of developing ovarian follicles3. Recently, apoptosis have been identified as the predominant mechanism responsible for oocyte loss that results from chemotherapy4 and the possible involved pathways have been reported5. However, the underlying cellular and molecular mechanisms by which chemotherapy induces apoptosis of ovarian follicles remain unclear6.
Accumulating evidence suggests that the chemotherapy can cause long-term detrimental effects, including rendering the recipient sterile7,8,9. The transient sterilizing dose of B/C for male subjects is species-specific, with different required doses in mg/kg body weight reported for rodents10,11, pigs11 and coyotes12. Early studies conducted in rats showed that the administration of B/C during pregnancy caused lethal effects on fetal oogonia13,14. Moreover, chronic B/C treatment resulted in widespread apoptosis throughout the entire stages of folliculogenesis15. Recently, Zhang et al.16 and Zou et al.17 reported that they produced mouse female germline stem cell (FGSC)-derived offspring by using intraovarian transplantation of the exogenous FGSC after chemotherapy. This report has received renewed attention since it provided a clue to find an answer for solving the question of “neo-oogenesis”. Therefore it is important to examine the effect of B/C on female murine reproductive function. The aims of the present study were three folds: 1) to define which cell types are the most sensitive to stress induced by B/C treatment; 2) to elucidate the molecular mechanisms of cell death underlying folliculogenesis following B/C treatment; and 3) to examine the recovery status of ovarian function from the exogenous primordial follicles in light of the previous observations in the FGSC transplantation experiment.
Results
General observation in the ovaries of B/C-treated mice
To evaluate germ cell depletion in response to B/C treatment, histological examination at the light microscopic level was performed on the ovarian sections obtained from 28 mice at 5 weeks after B/C treatment. Approximately 46% (13/28) of the mice exhibited complete germ cell depletion at this time period of the post B/C treatment (sTable 1). The remaining 54% (15/28) of the mice exhibited an almost completely depleted pool of primordial and growing follicles, with less than 10% of the follicles remaining in the ovaries. Depletion of germ cells was also observed at 15th weeks after B/C treatment. These results indicate that the single dose of B/C employed in this study was sufficient to sterilize female mice and the resulting germ cell depletion is irreversible.
Consistent with these results, B/C-treated female mice rarely produced litters even after several months of mating with stud male mice. To determine any possible remaining follicles within the ovary and thus to evaluate viable pregnancies if there is any, ovulation was induced with gonadotropins in 22 mice at 15th weeks after B/C treatment and the presence of vaginal plugs was examined the morning after mating. The B/C-treated mice that have vaginal plugs not produced fertilized embryos. Specifically, 17 out of 22 B/C-treated females had plugs but none of them produced pups. In contrast, 13 out of 15 untreated-control females contained plugs and produced 127 pups (Table 1). Thus these results demonstrate that B/C-treatment induces an irreversible germ cell depletion and possibly a defect in the process of follicle development that results in subsequent infertility in mice.
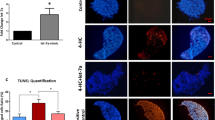
Identification of apoptotic cells by TUNEL assay following B/C treatment
The effects of B/C treatment on the whole ovary and on the developing germ cells were examined in sections of the mouse ovary 1 to 5th weeks following B/C treatment. Both primordial and growing follicles at different stages of development were numerous in controls (Fig. 1a), whereas the number of follicles in ovaries of the B/C-treated mice was dramatically reduced in a time-dependent manner (Fig. 1b–f). TUNEL analysis indicated that 1 week following B/C treatment, apoptosis were confined primarily to the post-meiotic oocytes, with lesser effects on the primordial follicles (Fig. 2a-1, arrow and arrowhead). One to two weeks following B/C treatment, most TUNEL-positive germ cells were identified in the primordial follicles with a few in the growing follicles (Fig. 2a-1 and -2). Ovaries from 3 to 5 week post-treatment showed a massive loss of maturing follicles as well as primordial and primary follicles (Fig. 2a-3 and -4). Furthermore, even at 15th weeks, some primordial, preantral and early antral follicles from B/C-treated animals were still undergoing programmed cell death (Fig. 2a-15). This observation suggests that the functional integrity of the follicles continues to be impaired 15th weeks following B/C treatment, with extensive germ cell apoptosis and/or atresia. In contrast, mature follicles were present in the ovaries of the untreated control animals (Fig. 2a-0 and -0h).
Photomicrographs of cross-sections from mouse ovaries.
(a–f) control (a), 1 (b), 2 (c), 3rd (d), 4th (e) and 5th (f) weeks after B/C treatment. De-waxed paraffin sections were stained with hematoxylin and eosin. After the injection of a single dose of B/C, female germ cells were significantly reduced in number over the duration of the experiment. Although the ovaries of the majority of the B/C-treated mice were depleted of female germ cells, the somatic cells appeared normal in size and shape. PF, PrF, SF and TF indicate primordial, primary, secondary and tertiary follicles, respectively. Magnification: 200×.
Monitoring of female germ cell apoptosis following B/C treatment.
(a) TUNEL analysis was performed using ovary sections from animals sacrificed 1 (b), 2 (c), 3rd (d), 4th (e) and 5th (f) weeks after B/C treatment. Female germ cell apoptosis is indicated by a brown or red color. One week after treatment, apoptotic cells are limited to post-meiotic oocytes, but at 2 to 5th weeks, apoptosis extends to primordial follicles as well as post-meiotic germ cells. (a–g) Control ovary tissue was stained with anti-LHX 8 and the brown color indicates primordial follicles (circle). PF, PrF, SF and TF in the lower panel of a–g) indicate primordial, primary, secondary and antral follicles in control ovaries, respectively. a–h) Tunnel staining of ovary at 15th weeks after B/C treatment. Red color indicates apoptotic subpopulations. Primordial (Pf), primary (PrF), secondary (SF) and tetray follicles (F) in ovary of mice at 15th weeks after B/C treatment are dead or dying (lower panel of A–h). Magnification, 100× for a–f and 200× for g and h. (b) A representative analysis of apoptotic cells in B/C-treated ovaries as detected by Annexin V staining. The distributions of apoptotic subpopulations are shown for cells assayed with secondary IgG alone (negative control), control cells and cells at 1, 3rd, 5th and 8th weeks after B/C treatment. The frequency of Annexin V-positive cells increased 1 week after busulfan treatment, peaked at week 5 and dropped rapidly to week 1 levels by 8th weeks post-treatment. Each value is the mean ± SD of independent triplicate examinations.
The B/C-dependent ovarian apoptosis were also confirmed by the flow cytometry analysis. Exposure to a single dose of B/C caused a significant time-dependent increase in the percentage of apoptotic cells (P < 0.05), as assessed by Annexin V-FITC staining (Fig. 2b). Very few Annexin V-FITC -positive cells were detected in the ovaries of untreated-control mice. The frequency of Annexin V-FITC-positive cells increased markedly 1 week post-treatment and reached to the highest level in the 5th week and declined to the 1 week level at the 8th week after B/C treatment.
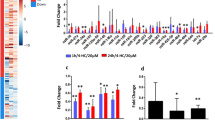
Real-time RT-PCR analysis using follicular marker genes
To determine the cell types that are sensitive to the B/C-induced apoptosis, expression of genes that are known to be specific to certain stage of follicular development29 was examined in the ovaries from imprinting control region (ICR) outbred mice at 1, 2, 3rd, 4th and 5th weeks after B/C treatment (Fig. 3a–q). Expression of genes for both c-kit and SRY-related HMG-box (Sox3) was dramatically down-regulated at 2 weeks post-B/C treatment as compared with ovaries from untreated animals (Fig. 3e and j). However, Sox3 gene expression returned to control levels by 5 weeks after B/C treatment. This may suggest that both the c-kit expressing primary follicles and Sox 3-positive preovulatory follicles are particularly sensitive to the B/C-induced damage and that germ cell apoptosis may be mediated by regulation of follicle-specific gene expression. Notably, the observation that the decrease in c-kit and Sox3 mRNA expression occurred at an earlier time point than those TUNEL-positive cells start to increase suggests that the cellular apoptosis in response to the B/C treatment may be mediated at least in part via the down-regulation of these genes. This result may also suggest that the first wave of B/C-induced apoptosis is limited to the primary and the preovulatory follicles.
Expression of oocyte-specific genes in control and B/C-treated female mouse ovaries.
FIGLA is expressed mainly from progenitor cells to primordial follicles, whereas NOBOX and LHX8 are expressed mainly in primordial follicles. Both FOXO3A and c-kit are expressed in primary follicles and during the transition from primary to secondary follicles. GDF 9 and KITL expression is limited to secondary follicles. DDR2 and AHR are expressed primarily in early antral follicles and PTX3 and IRS2 are expressed primarily in preovulatory follicles. YBX2 and CDK2 are involved in cumulus cell expansion and CDK4 and CDKN1B are involved in ovulation. Quantitative real-time RT-PCR is shown for the relative expression of Figla (a), Nobox (b), Lhx 8 (c), Foxo 3A (d), c-kit (e), Gdf9 (f), Kitl (g), Ddr2 (h), Ahr (i), Sox3 (j), Irs2 (k), Cdk4 (l), TNFAIP6 (m), Ptx3 (n), CDKN1B (o), Nos3 (p) and Ybx2 (q). Data were normalized to GAPDH expression and presented as the mean relative quantity compared to control. Error bars represent standard error of the mean (SEM), in figure a to q groups marked with different letters are significantly different from each other at p < 0.05. In figure a to q groups marked with similar letters are not significantly different from each other. Time points (weeks) after B/C treatment is indicated at the bottom.
The acute toxic effects of B/C treatment also included the follicular degeneration, dramatically reducing its number and leading to infertility. Both large cysts containing more than ten oocytes and the follicles with one or two squamous granulosa cells showed extensive apoptosis (Fig. 1c). In addition, expression of Figla (a germ cell-specific bHLH transcription factor), Nobox (oogenesis homeobox) and Lhx8 (a LIM-homeobox gene 8), all of which are known to be involved in the development of primordial follicles, was significantly down-regulated in a time-dependent manner (Fig. 3a to c). To confirm this, we performed immunohistochemical analysis using Lhx8 antibody. The immunohistochemical analysis using anti-Lhx 8 antibody confirmed presence in the nuclei of primordial (Pr), primary (P), secondary (S), early antrum (eA) and late antrum (lA) follicles of control ovaries (sFig. 2a′), whereas any Lhx 8 positive signal was not detected in 5 weeks (sFig. 2b′) and 15th weeks (sFig. 2c′) ovaries after B/C treatment. These results suggest that progenitor and primordial follicles are sensitive to the B/C treatment, indicate that these follicles after B/C treatment were irreversible. We also screened the expression of several other genes that are important during follicular development beyond the antral stage. During the 3rd and 5th week of post-treatment, expression levels of aryl hydrocarbon receptor (AhR), Sox3, Tumor necrosis factor alpha-induced protein 6 (TNFAIP6), pentraxin-related gene (Ptx3) and Y-box protein 2 (Ybx2) as diplotene stage-specific marker were significantly reduced (Fig. 3i, j, m and n). Whereas, the expression of forkhead box O3A (FOXO3A), Kit ligand (Kitl), discoidin domain receptor tyrosine kinase2 (DDR2) and insulin receptor substrate 2 (IRS2) was not affected by the B/C treatment (Fig. 3d, g, h and k). This may indicate that the B/C treatment also induce follicular cell death beyond the antral stage of the folliculogenesis.
Depletion of female germ cells by a p53- TNF-, Fas/FasL- and autophagy-independent pathway
In order to understand the molecular basis whereby germ cell death occurs, the expression of several genes that have been implicated in germ cell apoptosis in the ovary was examined 1 to 5th weeks after B/C treatment (Fig. 4, 5 and 6). Expression of tumor protein 53 (p53), B-cell lymphoma 2 (Bcl2), bcl2-associated x protein (Bax) and tumor necrosis factor αR55 (TNFRSF1A) was not affected by the B/C treatment (Fig. 4a to d). Even though the mRNA levels for Foxo3A downstream target gene, FasL, in the oocyte of B/C treated mouse ovaries were slightly altered by B/C treatment (Fig. 3d and Fig. 4f), the expression of Fas and Foxo3A mRNAs were not changed (Fig. 4e). This result, coupled with our previous observations18, indicates that B/C-induced germ cell apoptosis in the ovary may not be mediated by a relative concentration of pro- to anti-apoptotic molecules expressed and further suggests that female germ cell apoptosis can occur independently of p53, Fas/FasL and TNF signaling pathways.
Depletion of female germ cells after B/C treatment is mediated by a FasL/Fas-, TNF- and p53-independent pathway.
The p53 and FasL/Fas systems have been shown to regulate apoptosis in germ cells. To determine whether B/C killed female germ cells through these apoptotic pathways, a real-time RT-PCR analysis was performed. Data are normalized to Gapdh expression and presented as the mean relative quantity compared to control. P53 (a), BCL2 (b), TNFaR55 (c) and Fas (d) expression were unchanged from 1 to 5 weeks after B/C treatment. Bcl2, Bax and FasL (f) expression slightly decreased by five weeks after B/C treatment, indicating that female germ cell apoptosis after B/C treatment does not occur via the p53, TNF, or Fas/FasL pathways. Error bars represent standard error of the mean (SEM), in Figure a to f groups marked with different letters are significantly different from each other at p < 0.05. In figure a to f groups marked with similar letters are not significantly different from each other. Time points (weeks) after B/C treatment is indicated at the bottom.
RT-PCR analysis of c-kit downstream genes in B/C-treated female ovaries.
Time points (weeks) are indicated at the bottom. The expression of MAPK (a), SRC (b) and AKT (c) is significantly reduced, but mTOR (d) is not. Error bars represent standard error of the mean (SEM), in a to d groups marked with different letter are significantly different from each other at p < 0.05. In figure a to d groups marked with similar letter are not significantly different from each other. Time points (weeks) after B/C treatment is indicated at the bottom.
Oocyte autophagy-specific gene expression in different stages of follicles compared with primordial, primary, secondary and antral follicles.
Ovarian ATG4A (a), ATG5 (b), ATG6 (c), LC3 (d) and GABARAP (e) expression is shown for the different follicle stages. Data are normalized to Gapdh expression and presented as the mean relative quantity compared to control. Error bars represent standard error of the mean (SEM), in a to e groups marked with different letters are significantly different from each other at p < 0.05. In a to e groups marked with similar letters are not significantly different from each other. Time points (weeks) after B/C treatment is indicated at the bottom.
C-kit is expressed by oocytes, whereas its ligand, Kitl, is produced by the surrounding follicular somatic cells. Mutations within the sequences of c-kit and Kitl affect germ cell growth, transitional development from primordial to the primary follicle and migration26,27. Although c-kit expression is dramatically down-regulated in a time-dependent manner (Fig. 3e), Kitl expression was not altered in the ovaries of the B/C-treated mice (Fig. 3g). However, the expression levels of dual specificity mitogen-activated protein kinase kinase 1 (Map2k1), v-sarcoma oncogene (Src), the mechanistic target of rapamycin (mTOR) and murine thymoma viral oncogene homolog 1 (Akt1) all of which are located at the downstream of c-kit in the c-kit/SCF signaling pathway were slightly decreased from 1 to 5 th week after B/C treatment (Fig. 5a to d).
The involvement of autophagy in the process of the B/C induced ovarian apoptosis is largely unknown and thus we compared levels of the autophagy-related genes expressed among different stages of developing follicles (Fig. 6) at different time point (Fig. 7) within the ovary after B/C treatment. The mRNAs for autophagy-associated genes (ATGs) were present in all populations of the oocytes analyzed. Expression of the genes ATG4A, ATG5 and microtubule-associated proteins 1A/1B light chain 3A (MAP1LC3A, LC3) is highest in primordial follicle stages, but gradually reduced as follicles develop with the exception of ATG5 which increases again from the primary to the tertiary follicle stages (Fig. 6a, b and d). However, transcript levels of the ATG6 and GABA receptor-associated protein (GABARAP) were significantly (P < 0.05) higher in the primary follicles than in the primordial follicles (Fig. 6c and e).
RT-PCR analysis of autophagy-specific gene expression in the ovaries of B/C-treated female mice from post-treatment week 0 to week 5th.
The expression of autophagy-specific genes is significantly reduced in a time-dependent manner after B/C treatment. Each value is the mean ± D. Ovarian ATG4a (a), ATG4b (b), ATG5 (c), ATG6 (d), LC3 (e) and GABARAP (f) expression at different follicle stages. Data are normalized to Gapdh expression and presented as the mean relative quantity compared to control. Error bars represent standard error of the mean (SEM), in a to e groups marked with different letters are significantly different from each other at p < 0.05. In a to f groups marked with similar letters are not significantly different from each other. Time points (weeks) after B/C treatment is indicated at the bottom.
Overall, the expression of these ATGs within the ovary decreased gradually in a time-dependent manner from 1 to 5th weeks after B/C treatment (Fig. 7a to d). Expression of the autophagic vacuole marker LC3 was also significantly down regulated after B/C treatment in a time-dependent manner (Fig. 7e). Furthermore, expression of genes for the proteins that are involved in the phosphatidylinositol 3-kinases (PI3K)-signaling pathways including mTOR was not elevated but rather decreased (Fig. 5a to d) in the B/C treated ovaries throughout the treatment period, These may suggest that the ovarian germ cell death induced by B/C treatment was not mediated by autophagy and the low expression of ATGs in response to B/C treatment may be the result of ovarian germ cell depletion.
B/C-treated mouse ovary does not support maturation of the exogenous primordial follicles
Recent studies [16, 17] reported that transplantation of FGSCs into the ovaries of B/C treated mice resulted in the successful production of offspring. This means that the ovarian milieu of the B/C induced germ cell depletion may still be favorable for the growth and development of the exogenous germ line stem cells. In an effort to reproduce these findings, we performed intraovarian transplantation experiments employing primordial follicles of green mice expressing EGFP gene which enables to visualize and detect the transplanted germ cell colonies with the aid of UV light (Fig. 8b, c and d). Approximately 3 × 106 GFP-positive primordial follicles derived from postnatal day 5 ovaries, were transplanted at 1 week after B/C treatment into both left and right ovaries of each of the 15 non-transgenic mice (Fig. 8c and d). The immunohistochemical analysis using GFP expression and anti-MVH antibody confirmed presence of the exogenously derived primordial follicles in the recipient ovaries (Fig. 8f). Cells with both GFP expression (green in Fig.8f) and MVH staining (red in Fig.8f) are indicative of deriving from the transplanted primordial follicles and those with either GFP positive only or MVH staining alone indicate the exogenously derived somatic cells (exoS) and the endogenous primordial follicles, respectively. To determine whether these exogenous primordial follicles are settled and maintained within the recipient ovaries following transplantation, mice were sacrificed on days 1, 7 and 35 (n = 5 each) post-transplantation. On day 1 of the post-transplantation, the GFP-positive follicles were observed in many numbers in the injected areas of the recipient ovaries (Fig. 8g). However, the number of GFP-positive follicles significantly reduced on days 7 and 35 following transplantation (Fig. 8h and i). This observation may suggest that survival of the exogenous primordial follicles transplanted within the recipient ovary is influenced by B/C exposure and these cells may undergo apoptosis.
Fate of exogenous GFP-positive follicles transplanted into recipient ovaries 1 week after B/C treatment.
(a) GFP expression vector. (b and c) GFP transgenic mice and GFP-positive cells, including primordial follicles, isolated from postnatal day 5 ovaries of GFP transgenic mice. (d) Transplantation of the GFP-positive female germ cells into ovaries at 1 week after B/C treatment. Inset in (d) depicts primordial follicles used for ovarian transplantation. Most of DAPI positive cells showed GFP positive. DAPI staining (e) and localization of exogenous- and endogenous-derived primordial follicles by using GFP expression and MVH staining soon after female germ cell transplantation (f). Both of MVH staining and GFP expression indicate these germ cells are derived from transplanted primordial follicles, whereas MVH staining or GFP expression alone means endogenous primordial follicle or exogenous derived somatic cells (exoS), respectively. Exogenous derived GFP positive primordial follicles were localized along with host follicles containing GFP-negative follicles (MVH positive) in ovaries of all female mice that received GFP-expressing primordial follicles (f). At 1 day post-transplantation, many of the exogenous GFP-positive female germ cells were found along the injection tracts (g). Based on observations from whole-mount preparations, however, some GFP-positive cells were scattered within the ovaries. At 1 week post-transplantation, the number of GFP-positive cells significantly decreased (h) and, by 5th weeks, the GFP-positive cells disappeared (i). These time-lapse experiments showed that after transplantation, transplanted GFP-positive cells did not survive the effects of B/C toxicity. enF and exG indicate endogenous follicles and exogenous GFP-positive cells, respectively.
Discussion
This study examined the deleterious effects of B/C treatment on follicular development in mouse ovaries. Acute treatment of B/C resulted in follicular apoptosis, dramatically reducing their number and leading to infertility. One of the prominent features of this effect is that particularly large cysts that contained more than ten oocytes and follicles with one or two squamous granulosa cells showed an extensive apoptosis. Thus, it is likely that signals that are involved in the differentiation and development of the primordial follicles into the primary follicles might be impaired by B/C treatment. To support this hypothesis, this study examined the expression of some of the genes that were reported to be regulated during early follicular development23,28,29. Expression of genes Figla, Nobox, Lhx8 and Growth differentiation factor 9 (Gdf9) was dramatically down-regulated in a time-dependent manner in response to B/C-treatment as compared to untreated controls (Fig. 3a to c and f). Since Nobox directly regulates downstream genes such as Gdf9 and POU5F1 (POU class 5 homeobox1) through Nobox DNA binding elements, it was also suggested that Nobox deficiency may disrupt the expression of numerous oocyte specific genes14,24. In addition, Lhx8−/− mice are infertile due to the lack of oocytes by postnatal day 7 and the transition of primordial into primary follicles is also impaired. However, unlike Lhx8−/−, Nobox−/− ovaries did not alter expression of genes that are involved in apoptosis signaling pathways24,25. Taken together, these results may support the hypothesis that the early follicular demise caused by B/C treatment is mediated, at least in part, by the down-regulation of the Nobox and/or Lhx8 pathways.
Several lines of evidence suggest that certain genes expressed in developing oocytes and/or their surrounding follicular somatic cells may play an important role(s) in maintaining the integrity of follicles during their development toward ovulation. For example, a previous study demonstrated that Sox3 mRNA was expressed in both oocytes and purified cumulus granulosa cells after induced ovulation although its protein was not detected in ovaries by immunochemistry30. Another study reported that AhR was expressed in the granulose cells, theca cells and oocytes of the rhesus monkey31. In addition, administration of human chorionic gonadotropin (hCG) stimulated expression of TNFAIP6 (tumor necrosis factor alpha induced protein 6) in periantral mural granulosa and cumulus cells of the preovulatory follicles in both mouse and rat32,33. TNFAIP6 has been shown to play important roles for the completion of cumulus cell-oocyte complex (COC) expansion during ovulation because targeted disruption of the TNFAIP6 gene caused a defect in the COC expansion and infertility in mice34. Ptx3 is also reported to play important role in COC expansion. It is produced by mouse cumulus cells during cumulus expansion and is localized in the matrix. Cumuli from Ptx3 (−/−) mice synthesize normal amounts of hyaluronan (HA) but are unable to organize it into a stable matrix35. This study demonstrated that treatment of B/C down-regulated expression of these genes of AhR, Sox3, TNAFIP6 and Ptx3 (Fig 3i, j, m and n). The decreased expression of these genes may then result in an impaired development of cumulus cells and/or oocytes which led to the demise of the developing follicles beyond the antral stage.
One of the apoptotic signaling mechanisms is known to be mediated through the p53 or Fas/FasL signal transduction pathway36,37. In this study, expression of the genes that are involved in the p53 signaling pathway did not appear to change in response to B/C treatment. Also, expression of Fas and Foxo3A mRNAs were relatively constant after germ cell loss (Fig. 5e). However, downstream factors of both Foxo3A and Fas, FasL, was significantly altered by B/C treatment (Fig. 3d and 4f). Therefore, the long-term biological effects of Fas/FasL expression on apoptotic signaling must be further investigated in B/C-treated mouse ovaries. The finding that levels of Bcl2 transcript was not down-regulated in the ovaries of B/C-treated mice may suggest that early follicular apoptosis is not influenced by either the p53 signaling pathway or the apoptotic events that are mediated by a balance between pro- and anti-apoptotic molecules.
Another signal transduction pathway involving a pro-survival factor c-kit was reported to be in part mediated through the activation of mitogen-activated protein kinase (MAPK) and AKT/PI3K pathways and suppression of these survival pathways leads to premature follicle activation and infertility38,39,40,41. Although expression of c-kit was significantly down-regulated in the ovaries of B/C-treated mice, its target gene, Kitl, expression was not altered in the present study. However, MAPK and AKT/PI3K pathways that are involved in c-kit signal transduction were significantly changed in the ovaries of B/C-treated mice (Fig. 5). Taken together, these observations suggest that the follicular apoptosis by B/C in mouse ovaries was caused, at least in part, by the inhibition of AKT/PI3K signaling pathways.
Our previous study reported that busulfan caused demise of developing male germ cells with its effect lasting up to 4 weeks but the spermatogenesis was then resumed at 5th week of post-treatment18,19,20. In contrast, follicular response to B/C appears to be more sensitive than the male counterpart since B/C treatment influenced on the demise of female germ cells for at least 15th weeks (Fig. 2a, 0-h). A possible explanation for this difference is that testis is surrounded by the blood-testis barrier, which may provide protection against direct contact with busulfan toxicity whereas there is no such barrier in the ovary. Regardless of the reason, whereas our observations were in agreement with clearance of busulfan toxicity from mouse testis at 5th week of post-treatment, its toxicity on the ovarian germ cells lasts for up to 15th weeks. Accordingly, it is notable to find that the apoptotic mechanisms in B/C-exposed ovarian germ cells are distinct from those in testicular germ cells. However, it also cannot be ruled out that different apoptotic mechanisms observed between male and female germ cells may be the result of different toxicity between busulfan and B/C that were used for male and female experiments, respectively.
Since FGSC finding by Tilly group in 200442, a number of FGSCs have been reported by different laboratories16,17,43,44,45,46,47,48,49. One of the verifications to identify functional FGSCs is that these FGSC should be able to produce mature oocytes and fertilized eggs are capable of generating offspring in vivo. Recently, Wu group16,17 reported that transplantation of FGSCs into the ovaries of B/C pretreated recipient mice resulted in the successful production of offspring. This report has received renewed attention since it provided a possibility for “neo-oogenesis”. Apart from establishing FGSC, we have focused our studies on the recipient environment for germ cell transplantation after B/C treatment. Provided that Wu group's observation16,17 is correct, B/C treated female mice should provide an environment for supporting survival of exogenously transplanted germ cells and also restore their reproductive function after B/C is cleared from the tissue. In an effort to reproduce these findings, a series of experiments was performed with the procedures as previously described16,17. First, we examined the effect of B/C on female germ cells at different time points post-treatment. At 1 to 2 weeks after a single intraperitoneal injection of B/C, most TUNEL-positive germ cells were identified in the primordial follicles and a few were identified in the growing follicles. However, the numbers of germ cells within the ovary reached to near obsolete at 5th week. Specifically, 46% of the B/C treated mice had a complete loss of endogenous germ cell and 53% retained only a few of primordial and growing follicles (sTable 1). Furthermore, the toxic effect of B/C on the endogenous follicles and germ cells persisted for at least 15th weeks. Consistent with this observation, a previous study2 showed that female mice administered a median lethal dose of busulfan (20 mg/kg) and cyclophosphamide (200 mg/kg) gave birth to offspring after the first mating, but none of them had a successful second pregnancy over the 7 month period of mating trials. This result can be explained by the possibility that the first successful pregnancy may have been obtained from mature oocytes which remained intact until shortly after B/C treatment. Whereas, the lack of the second pregnancy may suggest that the immature developing follicles were exposed to the chemotherapeutic toxic as seen in the present study. Taken together, we propose that B/C treatment induces long-term detrimental effects on the ovary leading to the loss of functional folliculogenesis.
Second, in order to examine the B/C exposed ovarian environment for supporting exogenous follicles we performed an intraovarian transplantation in which GFP-positive primordial follicles were injected into the ovaries of non-transgenic B/C-treated mice (Fig. 8d). Observation of the GFP-positive cells indicate that whereas a number of follicles showed GFP-positive near the injected area of the recipient ovaries on day 1 (Fig. 8g), its number significantly decreased on days 7 and 35 of post-transplantation (Fig. 8h and i). It is suggested that the exogenous primordial follicles possibly underwent through apoptosis under the conditions described here. Furthermore, B/C treatment resulted in a significant down-regulation of receptor tyrosine kinase, which promotes primordial germ cell migration, from 2 to 5 weeks after the initial treatment. Thus, it is difficult at the present time to understand and reproduce the previous claim by the Wu group16,17 that not only can exogenously derive FGSCs survive in the recipient ovaries, but also that they can produce offspring. Here we report that the primordial follicles transplanted into the B/C-treated ovaries could not give rise to progeny and suggest that standardization and optimization of transplantation protocols for FGSCs need to be established in future studies.
Since tubules or the efferent ducts of testes provide a highly efficient route to introduce immature male germ cells, B/C is commonly used to prepare a recipient testis for male germ cell transplantation50,51. In contrast, it is generally difficult to perform intraovarian transplantation because the ovaries are not only filled with follicles and connective tissues but also bleed easily after puncture52. In addition, orthotopic transplantation of female germ cells into the ovaries is generally unattainable because they are rapidly eliminated from the host tissues after physical damage or other unknown causes. To overcome this problem, most studies focus on ectopic transplantation such as kidney capsules of ovariectomized female. Previously, intraovarian transplantation of female germ cells has been developed by Wu groups16,17. Although this study did not use FGSC for transplantation, our results indicate that B/C treated ovary does not support an environment for proliferating a small number of primordial follicles, indicating that FGSCs transplanted into the ovaries of recipient mice that were pretreated with a high dose of B/C cannot develop into fertilizable oocytes.
Methods
Animal ethics and treatments with busulfan plus cyclophosphamide (B/C)
All animal experiments were approved and performed in accordance with the guidelines of the Konkuk University Animal Care and Experimentation Committee (IACUC approval number: KU11035). Eight week-old ICR female mice received a single intraperitoneal injection of busulfan (30 mg/kg) plus cyclophosphamide (B/C) (120 mg/kg) as described previously11,19,20,21. Among 487 B/C treated mice, 450 mice (n = 30 for 1 to 15th wks) were subjected to histological or, TUNEL, qRT-PCR, western blotting and flow cytometry analyses, whereas 22 and 15 mice were used for a pregnancy test or as recipients for germ cell transplantation, respectively. Also, 120 mice from 8th to 12-week-old age were served as internal controls for experiments such as RT-PCR or flow cytometry, western analysis and etc. The mice were housed in wire cages at 22 ± 1°C under a 12 h light–12 h dark cycle with 70% humidity. Mice were fed a standard diet and water ad libitum.
TUNEL assay (TdT-mediated dUTP-X Nick End labeling)
Bilateral ovarian tissues were collected immediately after the mice were sacrificed at 1~15th weeks after B/C treatment. For histological studies, the tissues were fixed in 4% (w/v) paraformaldehyde in 0.01 M phosphate buffered saline (PBS) (pH 7.4), washed in PBS, dehydrated in ascending concentrations of ethanol (70, 90 and 100%) and embedded in paraffin wax. Tissue sections (5 μm in thickness) were collected, rehydrated in a series of xylene (5 min) and descending concentrations of ethanol treatments (100%, 95%, 70%, 2 min each) and washed in distilled water prior to TUNEL staining18. Tissue sections were incubated for 15 min with proteinase K (20 μg/mL) at room temperature and washed in PBS. Endogenous peroxidase activity was then blocked by incubation with 2% H2O2 for 5 min, washed three times with PBS and incubated at 37°C for 60 min in a moist chamber with the TUNEL solution (0.3 U/μL calf thymus terminal deoxynucleotidyl transferase, 7 pmol/μL biotin dUTP, 1 mM CoCl2 in distilled water). After washing four times with PBS for 5 min each at room temperature, the sections were incubated in 2% bovine serum albumin (BSA) for 10 min at room temperature to block non-specific binding sites of antibody and followed by treatment for 30 min at 37°C in a moist chamber with a 1:20 dilution of Extra Avidin peroxidase antibody. Tissue sections were washed three times in PBS and incubated with the substrate solution [1.24 mg diaminobenzidine (DAB), 25 μL 3% NiCl2, 152 μL 1 M Tris-HCl (pH 7.5) in 2 ml distilled water] for the development of the colorimetric signal, washed in PBS and mounted on microscope slides with a crystal mounting medium (Biomeda, Foster City, CA).
Real-time RT-PCR analysis
Total RNA was extracted from ovaries or oocytes using a Micro-to-Midi Total RNA Purification System (Life Technologies, Inc. Grand Island. NY). Real-time RT-PCR was conducted using a DNA Engine OPTICON2 system (MJ Research, Reno, NV) and SYBR Green as the double-stranded DNA-specific fluorescent dye (SYBR Green qPCR premix; Finnzymes Oy. Espoo, Finland). Target gene expression levels were normalized to GAPDH gene expression, which was unaffected by B/C treatment. The RT-PCR primer sets are shown in supplementary Table 2. Real-time RT-PCR was performed independently in triplicate for each of the different samples and the data are presented as the mean values of gene expression measured in B/C-treated sample verses control.
Apoptosis assay by flow cytometry
The detection of apoptotic cells was based on Annexin V binding to the translocated plasma membrane phosphatidylserine (PS), as described previously18. Briefly, cells were washed, trypsinized, pelleted by centrifugation, resuspended in staining buffer (10 mM HEPES/NaOH, pH 7.4, 140 mM NaCl, 5 mM CaCl2) containing 1 μg/ml PI and 5 μl Annexin V-FITC (BD Biosciences PharMingen, San Diego, CA) and incubated for 15 min. The cell mixture was then subjected to analysis with a flow cytometer (FACS Calibur, Becton Dickinson, Franklin Lakes, NJ). A minimum of 3 × 104 events was collected for each sample and analysis of the multivariate data was performed with CELLQuest software (Becton Dickinson, Bioscience, San José, CA).
Immunohistochemical analysis
Immunohistochemical staining was conducted as previously described20. Briefly, paraffin sections of ovaries were cleared in histoclear for approximately 10 min and dehydrated in ascending concentrations of ethanol. Immunohistochemistry was performed with an ABC Kit (Oncogene Science, MA, USA), according to the manufacturer's instructions. Sections were placed in 3% peroxide in pure methanol and then in 0.1% pepsin in 0.05 N HCl (pH 2.25) for 30 min each to reduce background staining. Sections were washed twice (5 min each) in TBS (0.05 M Tris-HCl, pH 7.4 and 0.85% NaCl), blocked with normal horse serum diluted in TBS (1:5; NHS-TBS) and incubated overnight with either an anti-LHX 8 polyclonal antibody (a gift from Dr. Y Choi, CHA University, Seongnam, Gyeonggi-do, Korea) or an anti-MVH polyclonal antibody (Abcam Sys-tem, UK). A normal horse serum from the ABC Kit was used as a negative control. Excess antibody was removed by washing twice for 5 min each with TBS. Tissue sections were then incubated with the biotinylated secondary IgG for 30 min, rinsed with three changes of TBS for 5 min each and followed by incubation with ABC reagent for 30 min for signal amplification. Sections were washed extensively with TBS and rinsed with 1% Triton-X in PBS for 30 sec. The colorimetric reaction was developed with a solution of 0.5% DAB in 0.05 M Tris-HCl (pH 7.6) containing 0.01% hydrogen peroxide. For microscopic analysis, sections were washed in water, dehydrated and mounted with a cover glass slide using mounting medium.
Construction of the pEF1α-eGFP expression vector and generation of transgenic mice
To generate the transgenic plasmid construct of the pEF1α-eGFP, 1186 bp of human elongation factor 1 alpha (hEF1α) promoter DNA was cloned into the pEGFP-N1 expression vector (Clontech, Mountain View, CA). The hEF1α promoter DNA was provided by Dr. Kim (CHA University, Seongnam, Gyeonggi-do, Korea). Restriction endonuclease sites of AseI and BamHI in both hEF1α promoter DNA and pEGFP-N1 were completely digested and then ligated together to generate pEF1α-eGFP expression vector under the control of 1186 bp hEF1 α promoter (Fig. 8, panel A).
Transgenic mice were generated according to our previous procedure22. Briefly, fertilized one-cell embryos were obtained from BDF1 (C57BL/6 × DBA hybrid) females. A DNA expression cassette was microinjected into the pronuclei of the fertilized embryos and the embryos were cultured for 20 hrs before being transferred for implantation to a pseudopregnant ICR female mouse. All mice were maintained under specific-pathogen-free conditions. Genomic DNA was obtained from the tails and the transgene was identified by PCR analysis. The specific forward and backward primers used for GFP were 5-CGACGTAAACGGCCACAAGTTCA-3′ and 5′-GAAGTCGTGCTGCTTCATGTG-3′ respectively. Transgenic mice screened by PCR analysis were also confirmed with GFP expression analysis.
Isolation and transplantation ovarian primordial follicle
Ovaries from the 1 week-old transgenic mice expressing green fluorescent protein (GFP) under the control of the EF-1 promoter were minced with scissors and dissociated in 0.2% collagenase (Invitrogen, Carlsbad, CA, USA) in PBS for 1 h at 37.5°C. The resulting ovarian follicle/cell suspension was filtered through a 60 μm mesh to remove large debris, washed with PBS and resuspended in 30 μl PBS. The number of GFP-positive primordial follicles was counted by fluorescence microscopy (Olympus, BH-2, Japan) using a hemocytometer. All of the cell counts are reported as the mean ± SD.
Similar to the published methods for transplantation of mouse FGSCs into the recipient ovaries16,17, the primordial follicles were directly transplanted into the ovary at both sides at 1 week after semi-sterilization with B/C treatment. Trypan blue was added to the suspensions to monitor successful transplantation of the donor follicles into each ovary. To transplant the follicles, mice were anesthetized, placed in dorsal recumbency and viewed with a stereomicroscope (Olympus SZX12, Lake Success, NY). The ovaries were exteriorized and microinjected with approximately 10 μl of the follicular suspension per ovary using a 20 μl glass micro-pipette. The mice were treated post operatively with antibiotics for 3 days. At 1, 7 and 35 days post-transplantation, ovaries were examined by fluorescence microscopy to identify the GFP-positive female germ cells.
Preparation of frozen tissue sections
Transplanted female mice (Konkuk University Animal Breeding Center, Seoul, Korea) were housed in a temperature-controlled environment under a 14 h light–12 h dark regime with food and water ad libitum. The ovaries were harvested on days 1, 7 and 35 post-transplantation, fixed in cold 4% neutral-buffered paraformaldehyde for 48 h and then cryoprotected by immersion in 20% sucrose in PBS for 24 hours at 4°C. Samples were frozen in O.C.T. compound (A.O. Co., USA) and 10 μm thick coronal sections were prepared (Leica Cryostat CM 3050C, Germany). Tissue sections were thaw-mounted on probe-on plus charged slides (Fisher Scientific, Pitts- burgh, PA) at room temperature and stored at -70°C until further use. The slides were examined with a laser-scanning confocal microscopy using a Bio-Rad MRC 1024 equipped with a krypton-argon ion laser.
Data analysis
All experimental data are presented as the mean ± SD. Each experiment was performed at least three times and subjected to statistical analysis. Results from the three representative experiments are presented in the Figures. For statistical analysis, one-way analysis of variance (ANOVA) was first performed to determine whether there were differences among groups (P < 0.05). Fisher′s post-test was then performed to determine significant differences between pairs. A value of P < 0.05 was considered significant. Statistical tests were performed using Stat View software version 5.0 (SAS Institute Inc., Cary, NC).
References
Shiraishi, A., Sakum, K. & Sekiguchi, M. Increased susceptibility to chemotherapeutic alkylating agents of mice deficient in DNA repair methyltransferase. Carcinogenesis 21, 1879–1883 (2000).
Lee, H. J., Selesniemi, K., Niikura, Y., Niikura, T. & Klein et al. Bone marrow transplantation generates immature oocytes and rescues long-term fertility in a preclinical mouse model of chemotherapy-induced premature ovarian failure. J. Clin. Oncol. 25, 3198–3204 (2007).
Utsunomiya, T., Tanaka, T., Utsunomiya, H. & Umesaki, N. A novel molecular mechanism for anticancer drug-induced ovarian failure: irinotecan HCl, an anticancer topoisomerase I inhibitor, induces specific FasL expression in granulosa cells of large ovarian follicles to enhance follicular apoptosis. Int. J. Oncol. 32, 991–1000 (2008).
Morita, Y. & Tilly, J. L. Oocyte apoptosis: like sand through an hourglass. Dev. Biol. 213, 1–17 (1999).
Perez, G. I., Knudson, C. M., Leykin, L., Korsmerer, S. J. & Tilly, J. L. Apoptosis-associated signaling pathways are required for chemotherapy-mediated female germ cell destruction. Nat Med. 3, 1228–1232 (1997)
Morgan, S., Anderson, R. A., Gourley, C., Wallace, W. H. & Spears, N. How do chemotherapeutic agents damage the ovary? Human Reprod Update 18, 525–535 (2012).
Teinturier, C., Hartmann, O., Valteau-Couanet, D., Benhamou, E. & Bougneres, P. F. Ovarian function after autologous bone marrow transplantation in childhood: high-dose busulfan is a major cause of ovarian failure. Bone Marrow Transplant. 22, 989–94 (1998).
Meistrich, M. L. Hormonal stimulation of the recovery of spermatogenesis following chemo- or radiotherapy. APMIS. 106, 37–45 (1998).
Sakurada, Y., Kudo, S., Iwasaki, S., Miyata, Y. & Nishi et al. Collaborative work on evaluation of ovarian toxicity. 5) Two- or four-week repeated-dose studies and fertility study of busulfan in female rats. J. Toxicol Sci. 34 Suppl 1, SP65–72 (2009).
Bucci, L. R. & Meistrich, M. L. Effects of busulfan on murine spermatogenesis: cytotoxicity, sterility, sperm abnormalities and dominant lethal mutations. Mutat. Res. 176, 259–268 (1987).
Kim, J. H., Jung-Ha, H. S., Lee, H. T. & Chung, K. S. Development of a positive method for male stem cell-mediated gene transfer in mouse and pig. Mol. Reprod. Dev. 46, 515–526 (1997).
Stellflug, J. N., Green, J. S. & Leathers, C. W. Anti fertility effect of busulfan and procarbazine in male and female coyotes. Biol. Reprod. 33, 1237–1243 (1985).
Hilscher, W. & Hilscher, B. Comparative studies on oogenesis and prespermatogenesis in the wistar rat under normal and pathological conditions. Ann. Biol. Anim. Biochim. Biophys. 13, 127–136 (1973).
Shirota, M., Soda, S., Katoh, C., Asai, S. & Sato et al. Effects of reduction of the number of primordial follicles on follicular development to achieve puberty in female rats. Reproduction 125, 85–94 (2003).
Burkl, W. & Schiechl, H. The growth of follicles in the rat ovary under the influence of busulphan and endoxan. Cell Tissue Res. 186, 351–359 (1978).
Zhang, Y., Yang, Z., Yang, Y., Wang, S. & Shi et al. Production of transgenic mice by random recombination of targeted genes in female germline stem cells. J. Mol. Cell Biol. 3, 131–142 (2011).
Zou, K., Yuan, Z., Yang, Z., Luo, H. & Sun et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat. Cell Biol. 11, 631–636 (2009).
Choi, Y. J., Ok, D., Kwon, D. N., Chung, J. I. & Kim et al. Murine male germ cell apoptosis induced by busulfan treatment correlates with loss of c-kit-expression in a Fas/FasL- and p53-independent manner. FEBS Lett. 575, 41–51 (2004).
Choi, Y. J., Park, J. K., Lee, M. S., Ahn, J. D. & Hwang et al. Long-term follow-up of porcine male germ cells transplanted into mouse testes. Zygote 15, 325–335 (2007).
Hwang, K. C., Ok, D. W., Hong, J. C., Kim, M. O. & Kim, J. H. Cloning, sequencing and characterization of the murine nm23-M5 gene during mouse spermatogenesis and spermiogenesis. Biochem. Biophys. Res. Commun. 306, 198–207 (2003).
Choi, Y. J., Song, H., Kwon, D. N., Cho, S. K. & Kang et al. Significant IgG-immunoreactivity of the spermatogonia of the germ cell-depleted testis after busulfan treatment. Anim. Reprod. Sci. 91, 317–335 (2006).
Kwon, D. N., Choi, Y. J., Park, J. Y., Cho, S. K. & Kim et al. Cloning and molecular dissection of the 8.8 kb pig uroplakin II promoter using transgenic mice and RT4 cells. J. Cell Biochem. 99, 462–477 (2006).
Joshi, S., Davies, H., Sims, L. P., Levy, S. E. & Dean, J. Ovarian gene expression in the absence of FIGLA, an oocyte-specific transcription factor. BMC Dev. Biol. 7, 67 (2007).
Rajkovic, A., Pangas, S. A., Ballow, D., Suzumori, N. & Matzuk, M. M. NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science 305, 1157–1159 (2004).
Choi, Y., Ballow, D. J., Xin, Y. & Rajkovic, A. Lim Homeobox Gene, Lhx8, Is Essential for Mouse Oocyte Differentiation and Survival. Biol. Reprod. 79, 442–449 (2008).
Dolci, S., Williams, D. E., Ernst, M. K., Resnick, J. L. & Brannan et al. Requirement for mast cell growth factor for primordial germ cell survival in culture. Nature 352, 809–811 (1991).
Matsui, Y., Zsebo, K. M. & Hogan, B. L. Embryonic expression of a haematopoietic growth factor encoded by the Sl locus and the ligand for c-kit. Nature 347, 667–669 (1990).
Tomas, F. H. & Vanderhydern, B. C. Oocyte-granulosa cell interactions during mouse follicular development: regulation of kit ligand expression and its role in oocyte growth. Reprod Biol Endocrinol. 12, 4–19 (2006).
Martin, M. M. & Dolores, J. L. The biology of infertility: research advances. and clinical challenges. Nature medicine 14, 1197–1210 (2008)
Weiss, J., Meeks, J. J., Hurley, L., Raverot, G. & Frassetto et al. Sox3 is required for gonadal function, but not sex determination, in males and females. Mol. Cell Biol. 23, 8084–8091 (2003).
Baldridge, M. G. & Hutz, R. J. Autoradiographic localization of aromatic hydrocarbon receptor (AHR) in rhesus monkey ovary. Am. J. Primatol. 69, 681–691 (2007).
Fulop, C., Kamath, R. V., Li, Y., Otto, J. M. & Salustri et al. Coding sequence, exon–intron structure and chromosomal localization of murine TNF-stimulated gene 6 that is specifically expressed by expanding cumulus cell-oocyte complexes. Gene 202, 95–102 (1997).
Yoshioka, S., Ochsner, S., Russell, D. L., Ujioka, T. & Fujii et al. Expression of tumor necrosis factor-stimulated gene-6 in the rat ovary in response to an ovulatory dose of gonadotropin. Endocrinology 141, 4114–4119 (2000).
Fulop, C., Szanto, S., Mukhopadhyay, D., Bardos, T. & Kamath et al. Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice. Development 130, 2253–2261 (2003).
Salustri, A., Garlanda, C., De Hirsch, E., Acetis, M. & Maccagno et al. PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development 131, 1577–1586 (2004).
Sjoblom, T. & Lahdetie, J. Expression of p53 in normal and gamma-irradiated rat testis suggests a role for p53 in meiotic recombination and repair. Oncogene 12, 2499–505 (1996).
Embree-Ku, M., Venturini, D. & Boekelheide, K. Fas is involved in the p53-dependent apoptotic response to ionizing radiation in mouse testis. Biol Reprod. 66, 1456–61 (2002).
Adhikari, D., Flohr, G., Gorre, N., Shen, Y. & Yang et al. Disruption of Tsc2 in oocytes leads to overactivation of the entire pool of primordial follicles. Mol. Hum. Reprod. 15, 765–770 (2009).
Adhikari, D., Zheng, W., Shen, Y., Gorre, N. & Hämäläinen et al. Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum. Mol. Genet. 19, 397–410 (2010).
Reddy, P., Adhikari, D., Zheng, W., Liang, S. & Hämäläinen et al. PDK1 signaling in oocytes controls reproductive aging and lifespan by manipulating the survival of primordial follicles. Hum. Mol. Genet. 18, 2813–2824 (2009).
Reddy, P., Liu, L., Adhikari, D., Jagarlamudi, K. & Rajareddy et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science 319, 611–613 (2008).
Johnson, J., Canning, J., Kaneko, T., Pru, J. K. & Tilly, J. L. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature 428, 145–150 (2004).
Pacchiarotti, J., Maki, C., Ramos, T., Marh, J. & Howerton et al. Differentiation potential of germ line stem cells derived from the postnatal mouse ovary. Differentiation 79, 159–170 (2010).
Parte, S., Bhartiya, D., Telang, J., Daithankar, V. & Salvi et al. Detection, characterization and spontaneous differentiation in vitro of very small embryonic-like putative stem cells in adult mammalian ovary. Stem Cells Dev. 20, 1451–1464 (2011).
Virant-Klun, I., Zech, N., Rozman, P., Vogler, A. & Cvjeticanin et al. Putative stem cells with an embryonic character isolated from the ovarian surface epithelium of women with no naturally present follicles and oocytes. Differentiation 76, 843–856 (2008).
White, Y. A., Woods, D. C., Takai, Y., Ishihara, O. & Seki et al. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med. 18, 413–421 (2012)
Zhang, D., Fouad, H., Zoma, W. D., Salama, . A. & Wentz et al. Expression of stem and germ cell markers within nonfollicle structures in adult mouse ovary. Reprod Sci. 15, 139–146 (2008)
Abban, G. & Johnson, J. Stem cell support of oogenesis in the human. Hum Reprod. 24, 2974–2978 (2009).
De Felici, M. Germ Stem cells in the mammalian adult ovary: consideration by a fan of the primordial germ cells. Mol Hum Reprod. 16, 632–636 (2010).
Brinster, R. L. & Avarbock, M. R. Germline transmission of donor haplotype following spermatogonial transplantation. Proc. Natl Acad. Sci. U S A. 91, 11303–11307 (1994).
Brinster, R. L. & Zimmermann, J. W. Spermatogenesis following male germ-cell transplantation. Proc. Natl Acad. Sci. U S A. 91, 11298–11302 (1994).
Matoba, S. & Ogura, A. Generation of functional oocytes and spermatids from fetal primordial germ cells after ectopic transplantation in adult mice. Biol. Reprod. 84, 631–638 (2011).
Acknowledgements
This work was supported by Woo Jang-Choon project (PJ007849) and next generation of Biogreen 21 (PJ009107) from the Rural Development Administration (RDA), Republic of Korea.
Author information
Authors and Affiliations
Contributions
M.R. Park conceived and designed the generation of transgenic mice and performed cell culture, busulfan treatment and ovarian transplantation. Y.J. Choi, H.T. Bui, D.N. Kwon, S.-G. Cho, C. Park and H. Song performed procedures for paraffin embedding, haematoxylin/eosin, immunohistochemistry staining, RT-PCR and western blotting analysis. Y.J. Choi, H.-G. Seo and S. Gurunathan performed vector construction for the transgenic production experiments, interpretation of manuscript. G.-Min and J.H. Kim designed, supervised the project and wrote manuscript. All authors contributed to sections of the manuscript and approved it.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
supplementary data
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareALike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Park, MR., Choi, YJ., Kwon, DN. et al. Intraovarian transplantation of primordial follicles fails to rescue chemotherapy injured ovaries. Sci Rep 3, 1384 (2013). https://doi.org/10.1038/srep01384
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep01384
This article is cited by
-
Candidate genes for infertility: an in-silico study based on cytogenetic analysis
BMC Medical Genomics (2022)
-
The process of ovarian aging: it is not just about oocytes and granulosa cells
Journal of Assisted Reproduction and Genetics (2022)
-
hUMSCs regulate the differentiation of ovarian stromal cells via TGF-β1/Smad3 signaling pathway to inhibit ovarian fibrosis to repair ovarian function in POI rats
Stem Cell Research & Therapy (2020)
-
Effects of low-intensity pulsed ultrasound (LIPUS)-pretreated human amnion-derived mesenchymal stem cell (hAD-MSC) transplantation on primary ovarian insufficiency in rats
Stem Cell Research & Therapy (2017)
-
Reproductive issues in patients undergoing Hematopoietic Stem Cell Transplantation: an update
Journal of Ovarian Research (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.