Abstract
Much like vertebrate hair cells, the chordotonal sensory neurons that mediate hearing in Drosophila are motile and amplify the mechanical input of the ear. Because the neurons bear mechanosensory primary cilia whose microtubule axonemes display dynein arms, we hypothesized that their motility is powered by dyneins. Here, we describe two axonemal dynein proteins that are required for Drosophila auditory neuron function, localize to their primary cilia and differently contribute to mechanical amplification in hearing. Promoter fusions revealed that the two axonemal dynein genes Dmdnah3 (=CG17150) and Dmdnai2 (=CG6053) are expressed in chordotonal neurons, including the auditory ones in the fly’s ear. Null alleles of both dyneins equally abolished electrical auditory neuron responses, yet whereas mutations in Dmdnah3 facilitated mechanical amplification, amplification was abolished by mutations in Dmdnai2. Epistasis analysis revealed that Dmdnah3 acts downstream of Nan-Iav channels in controlling the amplificatory gain. Dmdnai2, in addition to being required for amplification, was essential for outer dynein arms in auditory neuron cilia. This establishes diverse roles of axonemal dyneins in Drosophila auditory neuron function and links auditory neuron motility to primary cilia and axonemal dyneins. Mutant defects in sperm competition suggest that both dyneins also function in sperm motility.
Similar content being viewed by others
Introduction
Cilia can be categorized as motile or primary ones based on the structure of their microtubule axonemes1: Motile cilia display ‘9 + 2’ axonemes, consisting of nine circularly arranged microtubule doublets (‘9’) that surround a central pair of microtubules (‘+2’). Primary cilia, by contrast, present ‘9 + 0’ axonemes that lack the latter central microtubule pair. Primary cilia usually lack axonemal dynein arms and, accordingly, are immotile: instead of generating movements with dynein motors, these cilia serve as chemo- or mechanosensory organelles1. Nevertheless, motile primary cilia that bear axonemal dynein arms exist, such as the vertebrate nodal cilia that promote left-right asymmetry during development2,3. A second example might be found in Drosophila, whose auditory sensory neurons are motile4 and are endowed with primary cilia that present dynein arms5,6. The latter arms are confined to the proximal region of the auditory cilia6 and while they look like dynein arms in electron-micrographs, their molecular composition is unknown7,8.
The motility of Drosophila auditory neurons cannot be accessed directly but is betrayed by the fly’s auditory mechanics. Hearing in Drosophila is mediated by the antenna whose distal part vibrates in response to sound8. This vibration is directly coupled to the ca. 500 chordotonal mechanosensory neurons of Johnston’s organ in the antenna’s proximal part8. Each neuron bears one primary cilium and the neurons actively amplify antennal vibrations on a cycle-by-cycle basis, documenting their motile properties4,9,10. Biophysically, the source of this amplification in hearing might reside in (i) the interplay between force-gated ion channels and associated motor protein, (ii) the collective behavior of cells or motor proteins, or (iii) ionically driven conformational changes of force-gated ion channels or other proteins, without the involvement of ATP-consuming motors11. Modelling studies revealed that the first scenario, the interplay between force-gated channels and motors, might be realized in the Drosophila ear10 and amplification was shown to require the NOMPC (=TRPN1) transient receptor potential (TRP) channel12,13, which localizes to the tips of auditory neuron cilia14,15 and is gated by force16,17. The gain of amplification is negatively controlled by the TRPV channel subunits Nan and Iav12, which form a heteromeric Nan-Iav channel complex in the proximal ciliary region that presents dynein arms18,19. The mere presence of these arms suggests that Drosophila auditory neurons might use axonemal dyneins to drive mechanical amplification, a possibility that seems supported by genetic evidence: both mechanical amplification and the dynein arms are disrupted by mutations in genes that are implicated in axonemal dynein arm assembly, including for example fd3f (Ref. 20), tilB (Refs 4,21), zmynd10 (Refs 22,23), dyx1c1 (Refs 22,24) and hmw (Ref. 25). Although these mutant defects suggest that axonemal dyneins might power mechanical amplification, genetic evidence demonstrating that this amplification involves axonemal dynein genes has hitherto not been reported26.
Axonemal dynein arms can be categorized into outer and inner ones that differ in their molecular composition: each arm represents a multi-protein complex that consists of several axonemal dynein heavy, intermediate and light chain subunits27. The sequenced Drosophila genome includes eleven axonemal dynein heavy chain genes28,29 and twelve genes encoding WD-repeat, dynein intermediate chain proteins (Figs. S2,3). Some proteins of each type seem specific to the sperm flagellum: for instance, three of the axonemal heavy chain genes are on the Y chromosome and so are normally present only in males and several subunits encoded on other chromosomes are expressed at high levels only in testis30. Here we describe two axonemal dynein subunits, an inner arm heavy chain and a WD-repeat intermediate chain, that are expressed in both males and females, in chordotonal sensory neurons including those of Johnston’s organ. Using mutations of these genes, we tested whether and, if so, how axonemal dyneins and ciliary motility contribute to Drosophila auditory neuron function and mechanical amplification in fly hearing.
Results
DmDNAH3 is a monomeric dynein heavy chain
CG17150 is one of eleven axonemal dynein heavy chain genes in Drosophila28,29 (Fig. S1). It falls into the IAD-3 (inner-arm dynein-3) subgroup, described as inner arm-associated and single-headed (monomeric), together with the Drosophila Dhc62B/CG15804 and Dhc36C/CG5526 and human DNAH3 and DNAH7 gene products28,29. Among the human dynein heavy chains, CG17150 is most similar (50% amino acid identity) to DNAH3 and we name the gene Dmdnah3 for this orthology. The IAD-3 heavy chain subgroup has several representatives in each ciliated species, but information about their function is scant. A mutation of Chlamydomonas that deletes most of an IAD-3 heavy chain (DHC9) lacks a dynein inner arm and shows reduced flagellar beat frequency, especially in more viscous media, suggesting that this motor is important for motility under load31.
DmDNAI2 is an outer arm IC2 protein
CG6053 encodes a WD-repeat, dynein intermediate chain (IC) (Fig. S1) and is one of twelve dynein IC genes in Drosophila (Figs S2–4). When compared to the dynein ICs in Chlamydomonas29, CG6053 protein is most similar to ODA6 (36.7% amino acid identity), placing it with human DNAI2 (44.7% identity) in the IC2 subgroup (Figs. S2–4) and we name it DmDNAI2 for its human ortholog. In both Chlamydomonas flagella and human cilia, loss-of-function mutations in IC2 proteins prevent the assembly of the entire outer arm complex: mutant cilia lack outer arms and their constituent heavy chain proteins32,33, while partial ODA6 revertants have specific effects on flagellar beating34. Structural labeling locates the protein near the base of the outer dynein arm, where it controls the activity of both the outer and inner arm35. IC2 proteins are thus conserved across ciliated eukaryotes and required both for outer dynein arm assembly and for normal motility of the assembled axoneme.
Two other Drosophila IC proteins, encoded by CG1571 and CG10859, also fall within the IC2 subclass, but are not as similar to their algal and human homologs as is CG6053/Dmdnai2. RNA-seq expression data for both genes30 show high transcript levels in testis and little or no expression in females, suggesting that they function in the sperm flagellum. In contrast, CG6053 transcripts, though less abundant, are expressed and more broadly distributed in both males and females30, consistent with a function outside the male germline.
Dmdnah3 and Dmdnai2 are expressed in chordotonal neurons
To characterize the cellular expression patterns of Dmdnah3 and Dmdnai2, we generated fusions between the Dmdnah3 or Dmdnai2 enhancer/promoter regions and the yeast transcription activator GAL4 (see Supplementary Methods). To visualize GAL4 expression, transgenic flies expressing Dmdnah3-GAL4 and Dmdnai2-GAL4 were crossed to flies expressing a green fluorescent protein (GFP) under the control of upstream activating sequence (UAS) elements (UAS-GFP). GFP signals were enhanced with an anti-GFP antibody and neurons were counterstained with the monoclonal anti-Futsch antibody 22c10 (Ref. 36). Labelling induced by the Dmdnah3 and Dmdnai2 enhancer/promoter regions was observed in Johnston’s organ, the chordotonal auditory sensory organ in the fly’s antenna (Fig. 1A). Within this organ, anti-GFP and 22c10 staining superimposed, documenting that virtually all its 500 sensory neurons express Dmdnah3 and Dmdnai2 (Fig. 1A). Apart from Johnston’s organ neurons, expression of Dmdnah3 and Dmdnai2 was also observed in other chordotonal neurons, including those of the femoral chordotonal organ (FCO) in the fly’s leg and those of the larval pentascolopidial organ (lch5) (Fig. 1B). No expression was seen in the central nervous system or ciliated chemoreceptors and mechanosensory bristle neurons whose cilia reportedly lack dynein arms7. Chordotonal sensory neurons thus seem to be the only Drosophila neurons that express Dmdnah3 and Dmdnai2.
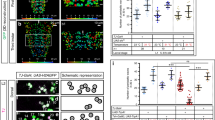
Axonemal dynein expression and localization in chordotonal neurons.
(A,B) Expression of Dmdnah3-GAL4 and Dmdnai2-GAL4 in Johnston’s organ neurons (A) and in chordotonal neurons of the larval pentascolopidial organ (lch5) and the adult femoral chordotonal organ (FCO) (B). Expression was assessed by driving an UAS-GFP reporter via Dmdnah3-GAL4 or Dmdnai2-GAL4. GFP signals were enhanced with an anti-GFP antibody. Johnston’s organ neurons were counterstained with the neuronal antibody 22C10 (A). (C) DmDNAI2 protein localization in Johnston’s organ neurons, revealed by expressing UAS-Dmdnai2-YFP under the control of Dmdnai2-Gal4. YFP signals were enhanced with an anti-GFP antibody and counterstained with an anti-Iav antibody, which recognizes Iav protein in the proximal region of the cilia. Within the cilia, DmDNAI2-YFP signals superimpose with Iav in the proximal ciliary region but do not extend distally in the ciliary tips.
To gain insights into the subcellular localization of axonemal dyneins, we generated transgenic flies carrying a UAS-Dmdnai2-YFP construct, in which DmDNAI2 is tagged with yellow fluorescent protein (YFP). Expression of UAS-Dmdnai2-YFP construct was targeted to Johnston’s organ neurons using Dmdnai2-GAL4 and the subcellular localization of DmDNAI2-YFP protein was assessed after enhancing YFP fluorescence with an anti-GFP antibody that recognizes YFP. DmDNAI2-YFP fluorescence was observed in Johnston’s organ neuron somata and dendrites (Fig. 1C). Within the dendrites, fluorescence signals extended into the mechanosensory cilia, where the signals were confined to the proximal ciliary region that harbors both Nan-Iav TRPV channels and dynein arms6. Counterstaining with an anti-Iav antibody confirmed that DmDNAI2-YFP co-localizes with Iav in the proximal ciliary region but is absent from the distal tips of the cilia that lack Iav protein and dynein arms6. Hence, within Drosophila auditory neuron cilia, at least DmDNAI2 occurs in that region that presents dynein arms.
Mutations in Dmdnah3 and Dmdnai2 affect auditory neuron function and mechanical amplification in the ear
To determine whether axonemal dyneins are required for the motility of Johnston’s organ neurons, we next tested for mutant alterations in mechanical amplification. Flies carrying Minos (Mi) transposon insertions in Dmdnah3 and Dmdnai2 were used as mutants, i.e. Mi{ET1}CG17150MB05004 (hereafter named Dmdnah31) and Mi{ET1}CG6053MB06262 (hereafter named Dmdnai21). PCR confirmed the genomic positions of the two Mi insertions (Fig. S1) and both Dmdnah31 and Dmdnai21 seemed to be null mutations as no transcripts were detected by RT-PCR (Fig. S5). To assess mechanical amplification, we exposed the flies to pure tones at the individual mechanical best frequencies of their antennae and simultaneously monitored the resulting antennal displacement and electrical compound action potentials of Johnston’s organ neurons that were recorded extracellularly from the antennal nerve. Mechanical best frequencies of the antennae were deduced from power spectra of their mechanical free fluctuations in the absence of acoustic stimulation and tone intensities were measured as the sound particle velocity at the position of the flies12.
In w1118 genetic background controls, sound particle velocities exceeding ca. 50 μm/s elicited robust electrical compound responses that, increasing sigmoidally with the sound intensity, reached maximum potential amplitudes of ca. 40 μV (Fig. 2A,B). These electrical sound responses of Johnston’s organ neurons were virtually abolished in both Dmdnah31 and Dmdnai21 mutants, whereby the latter mutants retained some residual responses (maximum potential amplitudes around 2 μV) to intense sound stimuli (particle velocities >ca. 5 mm/s) (Fig. 2A,B).
Effects of mutations in Dmdnah3 and Dmdnai2 on hearing.
(A) Pure tone-evoked antennal displacement (top) and normalized amplitude of the associated nerve response (bottom) as a function of the sound particle velocity of the tone. Tone frequencies were adjusted to match the mechanical best frequency of the antenna12 and lines indicate linear antennal mechanics. In w1118 controls, the antenna’s displacement displays a compressive nonlinearity that aligns with the dynamic range of the nerve response and arises from mechanical amplification by JO neurons12 (data from N ≥ 5 flies/antennae per strain). (B) Corresponding sensitivity gain due to mechanical amplification, sound particle velocity thresholds of the nerve responses and maximum amplitudes of the nerve responses extracted from the data in (A) (means ± SD). CS: Canton S wild-type flies. X: not accessible because the nerve response is entirely lost. *significant difference (p < 0.05) from w1118 controls: (Kruskal-Wallis test followed by two-tailed Mann-Whitney U-tests with Bonferroni correction). Nerve responses were measured as compound action potentials (CAP). Mechanical amplification is enhanced in Dmdnah31 mutants, where the nerve response is largely abolished. Both the nerve response and normal amplification are restored by precise excision of the responsible Minos insertion and by a genomic rescue of Dmdnah3. Dmdnai2 mutants lack both the antennal nonlinearity and the nerve response, which are restored by excising the respective Mi insertion, genomic rescue and by targeting the expression of UAS-Dmdnai2-YFP to Johnston’s organ neurons via the chordotonal neuron driver F-GAL4.
The loss of sensitive electrical sound responses in Dmdnai21 mutants was associated with a loss of mechanical amplification: in control flies, antennal displacements showed the characteristic nonlinear intensity scaling (Fig. 2A) that, arising from motile responses of Johnston’s organ neurons10,12, amplified the ear’s mechanical input with a gain of approximately 10 (Fig. 2B). This mechanical amplification was completely abolished in Dmdnai21 mutants (Fig. 2A), as witnessed by amplificatory gains of around one (Fig. 2B). Contrasting with this loss of motility, motility persisted and became excessive in Dmdnah31 mutants (Fig. 2A), with amplification gains around 20 (Fig. 2B). Hence, in addition to affecting electrical auditory neuron responses, Dmdnai21 abolishes active amplification whereas Dmdnah31 facilitates this amplification, documenting that auditory neuron motility requires Dmdnai2 and must be negatively regulated by Dmdnah3.
To determine whether these mutant phenotypes arise from the Mi insertions, we generated precise excisions by mobilizing the respective Mi elements. Both Dmdnai2ex1 and Dmdnah3ex1 revertants displayed normal sound-evoked electrical and mechanical responses that resembled those of the controls (Fig. 2A,B). A full restoration of normal sound-evoked responses was also observed in Dmdnah31 and Dmdnai21 mutants when we expressed respective genomic rescue fragments in the respective mutant backgrounds (Fig. 2A,B). In Dmdnai21 mutants, normal hearing was also restored when we targeted the expression of UAS-Dmdnai2-YFP to chordotonal neurons via F-Gal4 (Fig. 2A), which expresses Gal4 under the control of the nan enhancer/promoter37. Accordingly, the diverse auditory phenotypes observed in Dmdnah31 and Dmdnai21 mutants can all be ascribed to the mutations in the respective dynein genes.
Dmdnah3 regulates motility together with Nan-Iav TRPV channels
Mechanical hyper-amplification and virtual loss of electrical sound responses, as observed in Dmdnah31 mutants, also characterizes flies lacking Nan-Iav TRPV channels (Ref. 12,18). To assess whether Dmdnah3 and Nan-Iav might operate in the same signaling pathway, we generated double mutants carrying Dmdnah31 along with nandy5—a nan null allele that abolishes both Iav and Nan proteins from auditory neuron cilia18. Dmdnah31, nandy5 double mutants entirely lacked sound-evoked electrical potentials, same as single Dmdnah31 and nandy5 mutants (Fig. 3A). Mechanical amplification due to ciliary motility was excessive in the double mutants, with amplification gains of around 25 (Fig. 3B). This gain was substantially lower than that of single nandy5 mutants (gains of around 50) but closely resembled that of single Dmdnah31 mutants (gains of around 20). This establishes an epistatic relation between DmDNAH3 and Nan-Iav, placing DmDNAH3 downstream of Nan-Iav in a regulatory pathway that controls the mechanical amplification gain.
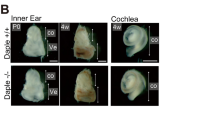
Epistatic relations between axonemal dyneins and Nan-Iav TRP channels and ciliary dynein arm integrity.
(A) Antennal displacements as functions of the sound particle velocity in single nandy5 and iav1 mutants (left), single Dmdnah31 and Dmdnai21 mutants (middle) and respective double mutants (right). Black lines indicate linearity. Antennal nonlinearity due to auditory neuron motility in the double mutants resembles that of the respective single dynein mutants (data from N = 5 flies/antennae per strain). (B) Respective nonlinear amplification gains (means ± SD). *significant difference (p < 0.05) from w1118 controls: (Kruskal-Wallis test followed by two-tailed Mann-Whitney U-tests with Bonferroni correction). (C) Transmission electron micrographs of cross-sections through the proximal region of the mechanosensory primary cilia of Johnston’s organ neurons, depicting the dynein-arms (arrowheads, top row). Bottom: zoom-ins depicting single microtubule doublets and their dynein arms. The inner (i) and outer (o) arms are present in w1118 genetic background controls and Dmdnah31 mutants, but the outer dynein arms are selectively lost in Dmdnai21 mutants. In the latter mutants, the outer arms are restored upon precise excision of the Mi element in Dmdnai2. m: ciliary membrane. Scale bars: 50 nm (top) and 25 nm (bottom).
We also generated double mutants carrying Dmdnai21 and iav1—an iav null allele that abolishes Nan and Iav from auditory neuron cilia18, same as nandy5. Like single Dmdnai21 and iav1 mutants Dmdnai21, iav1 double mutants lacked sound-evoked nerve responses. Mechanical amplification was excessive in single iav1 mutants, but entirely abolished in Dmdnai21, iav1 double mutants, as in single Dmdnai21 mutants (Fig. 3A,B). Hence, DmDNAI2 is also placed downstream of Nan-Iav in the regulatory pathway that controls amplification, analogous to the respective placement of the NOMPC TRP channel12, which also operates downstream of Nan-Iav in amplificatory gain control12 and is essential for mechanical amplification13.
Axonemal dynein arms in auditory neuron cilia require DmDNAI2
To test whether axonemal dyneins are required for the anatomical integrity of Johnston’s organ neurons, we analyzed their cellular morphologies in Dmdnah31 and Dmdnai21 mutant flies. No gross morphological defects were seen when we visualized the neurons with an anti-horseradish peroxidase (HRP) antibody (Fig. S6A) and antibody staining against NOMPC and Iav documented a normal ciliary localization of these TRPs (Fig. S6B). Inspecting the ciliary axonemes with transmission electron microscopy revealed that the dynein arms in the proximal region of the cilium persist and seem structurally uncompromised in Dmdnah31 mutants, but that the outer arms are selectively lost in Dmdnai21 mutant flies (Fig. 3C). In the latter mutants, the outer dynein arms were restored upon precise excision of the respective Mi element (Fig. 3C), documenting that the outer dynein arms require DmDNAI2.
DmDNAH3 and DmDNAI2 impair sperm competition
In Drosophila, sperm and auditory chordotonal neurons are the only ciliated cells whose cilia generate motility with axonemal dynein arms. Driving UAS-EGFP via Dmdnah3-GAL4 or Dmdnai2-GAL4 revealed that both Dmdnah3 and Dmdnai2 are expressed in sperm (Fig. 4A), corroborating the reported presence of DmDNAH3 in sperm revealed by mass spectrometry38. We thus analyzed male fertility in Dmdnah31 and Dmdnai21 mutants. Homozygous Dmdnah31 mutants were behaviorally able to mate, but no offspring were recovered and testis squashes consistently showed sperm that were not motile. Homozygous Dmdnai21 males, by contrast, if they achieved mating, produced as many males as heterozygous controls and testis squashes from all males showed motile sperm. Offspring was also obtained from Dmdnah31 mutants when the Dmdnah31 mutation was uncovered by the deficiency Df(3L)BSC371, suggesting that the loss of male fertility and sperm motility in the homozygous mutants –but not their hearing defects (Fig. S7)—arise from a secondary mutation. To characterize contributions of DmDNAH3 and DmDNAI2 to the function of sperm, we performed sperm competition assays in which w1118 females were first mated to w; FRT40A, neo FRTG13, w+ males (P1) and then to balanced Dmdnah31/TM3 or Dmdnai21/TM6 flies and mutant Dmdnah31/Df(3L)BSC371 or Dmdnai21/Dmdnai21 males (P2). Scoring the offspring revealed that sperm from control males displaced 80–90% of P1 sperm, but sperm from Dmdnah31/Df(3L)BSC371 males were almost completely unable to compete and Dmdnah21 homozygotes showed a highly significant reduction in sperm competition (Fig. 4B). This extends the roles of axonemal dyneins in sperm competition39 to DmDNAH3 and DmDNAI2 and shows that these two proteins, in addition to their auditory requirements, contribute to sperm function and motility.
Dmdnah3 and Dmdnai2 are expressed in sperm and impair sperm competition.
(A) Regulatory sequences of Dmdnah3 (left) and Dmdnai2 (right) fused to Gal4 can both drive expression of fluorescent markers in sperm. Expression is seen both in developing sperm tails in the testis (t) as well as in mature sperm in the seminal vesicles (sv). (B) Mutations in Dmdnah3 and Dmdnai2 impair sperm competition. Dmdnah31and Dmdnai21 mutant males (black bars) and their heterozygous controls (white bars) were tested for their ability to displace sperm in previously mated females. Sperm displacement was measured as the proportion of offspring from the second male (P2/P1 + P2). Bars represent means, error bars represent SEM. Number of males measured for each genotype is shown in or above the bar. **p < 0.01; ****p < 0.0001 (Kruskal-Wallis test followed by two-tailed Mann-Whitney U tests). Data were included only if at least one offspring from the second male was recovered. This conservative approach was to eliminate any possible effect of the mutations on the ability of males to achieve copulation. The numbers of males that were thus not taken into consideration were 0 for Dmdnah31/TM3 but 23 for Dmdnah31/Df and 1 for Dmdnai21/TM6 male but 4 for Dmdnai21/Dmdnai21.
Discussion
Much like vertebrate hair cells, Drosophila auditory neurons are motile and mechanically amplify the vibrations they transduce4,10. Whereas mechanical amplification by hair cells involves prestin molecules and, presumably, myosin motors40,41, amplification by the fly’s auditory chordotonal neurons is now shown to involve dyneins: according to our results, axonemal dyneins are expressed in Drosophila chordotonal neurons and are required for their mechanosensory function and mechanical amplification in the ear. DmDNAH3 controls the mechanical amplification gain together with Nan-Iav TRPV channels and DmDNAI2 seems to be an important constituent of outer dynein arms in auditory neuron primary cilia that is essential for mechanical amplification. That both DmDNAH3 and DmDNAI2 participate in ciliary motility is supported by mutant defects in sperm competition. This establishes the presence of specific axonemal dynein proteins in insect chordotonal neuron cilia and links mechanical amplification by Drosophila auditory neurons to primary cilium motility and axonemal dynein motor components.
Judging from the present and previous10,13,16,17 findings, it now seems that mechanical amplification by Drosophila auditory neurons might arise from the interplay between the force-gating of NOMPC channels and associated active movements of axonemal dynein motors. If so, NOMPC and axonemal dyneins would have to signal along the cilium because they reside in distinct ciliary compartments6,11, an indirect interaction that might be mediated by the microtubule axoneme: both NOMPC and axonemal dyneins bind to microtubules42,43,44,45 and can be activated by force16,45. Being mechanically coupled through the axoneme, gating movements of the channels thus might directly trigger motor movements and vice versa, allowing for the fast channel-motor interactions that are required to explain the cycle-by-cycle amplification of vibrations in the Drosophila ear, which operates at frequencies above 100 Hz10.
The above scenario seems consistent with an amplification model in which the interplay between motors and force-gated channels drives amplification10. According to this model, motor movements power amplification by promoting transducer adaptation, whereby the motors actively reclose the mechanotransduction channels when forcing is maintained10. Besides NOMPC, Nan-Iav has been surmised to mediate transduction in JO neurons26,46, yet recent evidence suggests that Nan-Iav cannot be a mechanotransduction channel because it is not mechanosensitive19. Correlates of NOMPC-dependent force-gating and channel adaptation have been observed in the fly’s antennal mechanics10,17,47, suggesting that dyneins might drive amplification by promoting adaptation of NOMPC. We now found that these correlates are entirely abolished from the antennal mechanics of Dmdnai21 mutants (Fig. S8), documenting that forces no longer gate the channels, possibly because the channels can no longer adapt and, thus, maintain mechanosensitivity. Recent work has shown that NOMPC requires direct interactions with microtubules for mechanogating44. Future work will be needed to test whether NOMPC adaptation requires microtubule-bound dyneins.
The different effects on dynein arm morphology and amplification resulting from loss of the DmDNAI2 and DmDNAH3 proteins are consistent with the different functions of their homologs in other species. The IC2 intermediate chain protein encoded by DmDNAI2 is not a force-generating subunit, but IC2 proteins in Chlamydomonas and in humans also function in assembly of the outer arms32,33,48,49, which are required for flagellar and ciliary motility. In contrast, monomeric inner arm dynein proteins such as DmDNAH3 are not absolutely required for motility: Chlamydomonas mutants lacking one such protein show reduced flagellar beating only under viscous load31, consistent with our finding that DmDNAH3 mutant sperm function defects are only revealed in competition with normal sperm flagella. The Drosophila motor that includes DmDNAH3 may modulate antennal motility in response to TRPV channel activity, mechanical loading, or both. Candidates for the force-generating proteins that drive auditory motility in Drosophila are the outer arm heavy chains encoded by CG9492 and Dhc93AB, which both have been detected in the fly’s auditory organ22. Rigorously testing their roles in amplification must involve genetic rescue experiments as presented here for Dmdnai2 and Dmdnah3 and demonstrating that they drive amplification and ion channel adaptation will require targeted manipulations of their active motor properties50,51.
Methods
Nomenclature
A convention to date has been to identify autosomal Drosophila dynein heavy chain (Dhc) genes by their chromosomal location. CG17150, however, is located in the same cytogenetic interval (64C) as the cytoplasmic dynein gene Dhc64C, so following convention in this case could cause confusion between these distinct dynein species.
Flies
w1118 was used as genetic background control and Canton-S as wild-type strain. w1118; Mi{ET1}CG17150MB05004/TM6C, Sb1, w1118; Df(3L)BSC371/TM6C, Sb1 cu1, w1118; Mi{ET1}CG6053MB06262, SM6a-Trans(MiT)hs24/snaSco, nandy5, iav1, F-GAL4, and UAS-GFP-T2, strains were obtained from Bloomington Drosophila Stock Center. w; FRT40A, neo FRTG13, w+ were previously described52. Flies were raised on standard cornmeal medium under a 12 hr light-dark cycle at 25 °C temperature and 75% humidity. Heat-shock inducible Minos transposase, SM6a-Trans(MiT24)hs24, was used to excise the Minos transposon from Mi{ET1}CG17150MB05004 and Mi{ET1}CG6053MB06262 strains. To generate excisions, SM6a-Trans(MiT24)hs24 flies were crossed with both Mi{ET1}CG17150MB05004 and Mi{ET1}CG6053MB06262 flies and their progeny was heat-shocked daily for one hour at 37 °C during pupariation.
Promoter fusions and reporter constructs
Genomic DNA was extracted from WT-flies using Qiagen DNeasy Blood and Tissue kit. To generate Dmdnai2-GAL4 promoter fusions, the putative promoter gene sequences were amplified using the primers 5′-CGAATTCAAATCAAACCAGCTCTTGTAGTTACC-3′ (forward) and 5′-CGGATCCGAGTTCTCGGTGAACACCACCT-3′ (reverse) and inserted into pPTGAL vector53. To generate UAS-Dmdnai2 rescue constructs, a coding sequence (cds) clone of CG6053 (IP13643, DGRC, Vienna) was used to amplify the CG6053 cDNA region using the 5′-CCGAATTCAAATATTTTGCTAAGTTTCCGATTGAAATGGAA-3′ (forward) and 5′-GATCTAGACAGCCTCCTCCGCATCCTCTAC-3′ (reverse) primers. The amplicon was inserted into an UAS-attP vector obtained from the Konrad Bassler lab at the University of Zurich. The same construct was tagged with YFP to generate the UAS-Dmdnai2-YFP reporters. To generate a genomic rescue construct for Dmdnai2, the BACPAC clone pBAC70G22 (P(acman) resource centre, http://www.pacmanfly.org/), which contains a 20 kb region spanning 11683101 to 11705106 of chromosome arm 3L that includes the complete genomic region of CG6053 (which spans from 11690438 to 11692363), was inserted into the w1118 background. Microinjections were performed by BestGene Inc (http://www.thebestgene.com/).
RNA extraction and cDNA preparation
RNA was extracted from whole flies using the ZR Tissue & Insect RNA Microprep Kit (Zymo Research, Irvine, CA) following the manufacturer’s instructions and cDNA was synthetized using the QuantiTect Reverse Transcription Kit (Qiagen, Venlo, Netherlands).
Sound responses and correlates of mechanotransduction
The methods to assess sound-evoked antennal displacements and nerve responses as well as mechanical correlates of force-gating and adaptation have been described17,47,54. To compare auditory sensitivities across flies, nerve response amplitudes were plotted against the sound particle velocity and the increasing slope was fitted with a Hill equation. The sound particle velocity corresponding to 10% of the maximum amplitude assumed by the Hill fit was defined as the threshold intensity22.
Immunohistochemistry
The following primary antibodies were used: mouse anti-Futsch (22c10) (Developmental Studies Hybridoma Bank, http://dshb.biology.uiowa.edu/), rabbit anti-GFP (Abcam, Cambridge, UK), anti-IAV (gift from Changsoo Kim, Seoul), rabbit anti-HRP (Invitrogen, Carlsbad, CA), mouse anti-NOMPC (gift from Jonathan Howard, Yale) and Alexa Fluor 647 conjugated anti-phalloidin (Molecular Probes, Eugene, OR). The secondary antibodies used are Alexa Fluor anti-rabbit 488, Alexa Fluor anti-mouse 488, Alexa Fluor anti-mouse 546 and Alexa Fluor anti-rat 633 (Invitrogen).
For antibody staining, fly heads (Johnston’s organ) were washed with 0.1% PBT (0.2% bovine serum albumin, 0.1% Triton-X in phosphate buffered saline) and fixed in 4% paraformaldehyde (PFA) for one hour. Heads were embedded in gelatin-albumin and sectioned at 40 to 50 μm with a vibratome (Leica, Oberkochen, Germany). Expression in the pentascolopidial organ was examined in larval fillets that were fixed for one hour in 4% PFA.
Upon sample preparation, samples were washed three times for 15 minutes in 1% PBT and blocked in 0.25% bovine serum albumin (BSA) and 10% normal goat serum (NGS) for one hour at room temperature. Antibodies (1%) were added to the samples and were incubated at 4 °C temperature overnight. The samples were washed three times for 5 minutes per wash in PBT and treated with anti-rabbit and -mouse fluorophore conjugated secondary antibodies at a 1:300 concentration in PBS-T for 3 hours at room temperature. After washing the samples three times for 15 minutes, they were mounted in DABCO (Sigma-Aldrich, St. Lewis, MI). Staining was visualized with a Leica SP8 confocal microscope and images were analyzed with ImageJ.
Transmission electron microscopy
To maintain the structural integrity of the samples, we used high pressure freezing. A 200 μm deep aluminium specimen carrier (Leica, 16770141 Type A) was filled with 20% Polyvinylpyrrolidone (Sigma-Aldrich) in fly ringer (70 mM NaCl, 5 mM KCl, 10 mM NaHCO3, 1,5 mM CaCl2 (*2H2O), 4 mM MgCl2, 5 mM trehalose, 115 mM saccharose, 5 mM HEPES). Dissected antennae were transferred into the platelet, closed with a second specimen carrier (16770142 Type B) and immediately frozen using a Leica EM HPM 100. Freeze substitution was carried out in a Leica EM AFS at −90 °C for 100 h in 0.1% tannic acid and another 40 h in 2% OsO4 (each mass/volume (w/v) in dry acetone + 1% water) while slowly increasing temperature55. Antennae were then infiltrated with Durcupan (Fluka, 30% for 3 h, 70% for 3 h, 90% overnight, 2 × 100% for 3 h and thin embedded between two glass slides for 48 h at 70 °C. For microscopic examination, 70 nm sections were cut using a Reichert Ultracut E ultramicrotome and transferred onto Formvar-coated copper mesh grids (PLANO G2405C). Sections were post-stained for 40 min with 4% (w/v) uranyl acetate in water and for 2 min with lead citrate56. Micrographs were taken in a JEOL electron microscope (JEM 1011, JEOL, Eching, Germany) with a Gatan Orius 1200A camera (Gatan, Munich, Germany).
Sperm competition
Females (w1118) were crossed to w; FRT40A,neo FRTG13 w+ males in groups of 10 pairs per vial. These first males were removed after 48 hrs. After an additional 48 hrs, the females were crossed individually to second males of the following four genotypes: Dmdnah31/TM3 and their sibs Dmdnah31/Df(3L)BSC371, Dmdnai21/TM6C and their sibs Dmdnai21/Dmdnai21. After 60 hrs, the second males were discarded and the females were transferred to fresh vials for 5 days and subsequently discarded as well. All offspring from both vials for each second male were scored for white (P2) or orange (P1) eyes. For each second male, sperm displacement was calculated as P2/(P1 + P2). Statistical significance was determined by Mann-Whitney U tests.
Additional Information
How to cite this article: Karak, S. et al. Diverse Roles of Axonemal Dyneins in Drosophila Auditory Neuron Function and Mechanical Amplification in Hearing. Sci. Rep. 5, 17085; doi: 10.1038/srep17085 (2015).
References
Davenport, J. R. & Yoder, B. K. An incredible decade for the primary cilium: a look at a once-forgotten organelle. Am. J. Physiol. Renal Physiol. 289, F1159–F1169 (2005).
Hirokawa, N., Tanaka, Y., Okada, Y. & Takeda, S. Nodal flow and the generation of left-right asymmetry. Cell 125, 33–45 (2006).
Supp, D. M., Witte, D. P., Potter, S. S. & Brueckner, M. Mutation of an axonemal dynein affects left–right asymmetry in inversus viscerum mice. Nature 389, 963–966 (1997).
Göpfert, M. C. & Robert, D. Motion generation by Drosophila mechanosensory neurons. Proc. Natl. Acad. Sci. USA 100, 5514–5519 (2003).
Sarpal, R. et al. Drosophila KAP interacts with the kinesin II motor subunit KLP64D to assemble chordotonal sensory cilia, but not sperm tails. Curr. Biol. 13, 1687–1996 (2003).
Lee, E., Sivan-Loukianova, E., Eberl, D. F. & Kernan, M. J. An IFT-A protein is required to delimit functionally distinct zones in mechanosensory cilia. Curr. Biol. 18, 1899–1906 (2008).
Keil, T. A. Sensory cilia in arthropods. Arthropod. Struct. Dev. 41, 515–534 (2012).
Boekhoff-Falk, G. & Eberl, D. F. The Drosophila auditory system. Wiley Interdiscip. Rev. Dev. Biol. 3, 179–191 (2013).
Göpfert, M. C., Humphris, A. D., Albert, J. T., Robert, D. & Hendrich, O. Power gain exhibited by motile mechanosensory neurons in Drosophila ears. Proc. Natl. Acad. Sci. USA 102, 325–330 (2005).
Nadrowski, B., Albert, J. T. & Göpfert, M. C. Transducer-based force generation explains active process in Drosophila hearing. Curr. Biol. 18, 1365–1372 (2008).
Mhatre, N. Active amplification in insect ears: mechanics, models and molecules. J. Comp. Physiol. A 201, 19–37 (2015).
Göpfert, M. C., Albert, J. T., Nadrowski, B. & Kamikouchi, A. Specification of auditory sensitivity by Drosophila TRP channels. Nat. Neurosci. 9, 999–1000 (2006).
Effertz, T., Wiek, R. & Göpfert, M. C. NompC TRP channel is essential for Drosophila sound receptor function. Curr. Biol. 21, 592–597 (2011).
Lee, J., Moon, S., Cha, Y. & Chung, Y. D. Drosophila TRPN (=NOMPC) channel localizes to the distal end of mechanosensory cilia. PLoS One 5: e11012 (2010).
Liang, X., Madrid, J., Saleh, H. S. & Howard, J. NOMPC, a member of the TRP channel family, localizes to the tubular body and distal cilium of Drosophila campaniform and chordotonal receptor cells. Cytoskeleton 68, 1–7 (2011).
Yan, Z. et al. Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature 493, 221–225 (2013).
Effertz, T. et al. Direct gating and mechanical integrity of Drosophila auditory transducers require TRPN1. Nat Neurosci 15, 1198–2000 (2012).
Gong, Z. et al. Two interdependent TRPV channel subunits, Inactive and Nanchung, mediate hearing in Drosophila. J. Neurosci. 24, 9059–9066 (2004).
Nesterov, A. et al. TRP Channels in insect stretch receptors as insecticide targets. Neuron 86, 665–671 (2015).
Newton, F. G. et al. Forkhead transcription factor Fd3F cooperates with Rfx to regulate a gene expression program for mechanosensory cilia specialization. Dev. Cell 22, 1221–1233 (2012).
Kavlie, R. G., Kernan, M. J. & Eberl, D. F. Hearing in Drosophila requires TilB, a conserved protein associated with ciliary motility. Genetics 185, 177–188 (2010).
Senthilan, P. R. et al. Drosophila auditory organ genes and genetic hearing defects. Cell 150, 1042–1054 (2012).
Moore, D. J. et al. Mutations in ZMYND10, a gene essential for proper axonemal assembly of inner and outer dynein arms in humans and flies, cause primary ciliary dyskinesia. Am. J. Hum. Genet. 93, 346–356 (2013).
Tarkar, A. et al. DYX1C1 is required for axonemal dynein assembly and ciliary motility. Nat. Genet. 45, 995–1003 (2013).
Soulavie, F. et al. hemingway is required for axonemal stability and ciliary motility in Drosophila. Mol. Biol. Cell 25, 1276–1286 (2014).
Albert, J. T. & Göpfert, M. C. Hearing in Drosophila. Curr. Opin. Neurobiol. 34C, 79–85 (2015).
King, S. M. Dyneins: structure, biology and disease. Academic press, London (2011).
Wickstead, B. & Gull, K. Dyneins across eukaryotes: a comparative genomic analysis. Traffic 8, 1708–1721 (2007).
Hom, E. F. et al. A unified taxonomy for ciliary dyneins. Cytoskeleton 68, 555–565 (2011).
Gelbart, W. M. & Emmert, D. B. FlyBase: high throughput expression pattern data. Accessible online at http://flybase.org/ (2013). Date of access: 25/08/2015.
Yagi, T. et al. An axonemal dynein particularly important for flagellar movement at high viscosity. Implications from a new Chlamydomonas mutant deficient in the dynein heavy chain gene DHC9. J. Biol. Chem. 280, 41412–41420 (2005).
Mitchell, D. R. & Kang, Y. Identification of oda6 as a Chlamydomonas dynein mutant by rescue with the wild-type gene. J. Cell. Biol. 113, 835–842 (1991).
Loges, N. T. et al. DNAI2 mutations cause primary ciliary dyskinesia with defects in the outer dynein arm. Am. J. Hum. Genet. 83, 547–58 (2008).
Mitchell, D. R. & Kang, Y. Reversion analysis of dynein intermediate chain function. J. Cell Sci. 105, 1069–1078 (1993).
Oda, T., Yagi, T., Yanagisawa, H. & Kikkawa, M. Identification of the outer-inner dynein linker as a hub controller for axonemal dynein activities. Curr. Biol. 23, 656–664 (2013).
Fujita, S. C. et al. Monoclonal antibodies against the Drosophila nervous system. Proc. Natl. Acad. Sci .USA 79, 7929–7933 (1982).
Liu, L. et al. Drosophila hygrosensation requires the TRP channels water witch and nanchung. Nature 450, 294–298 (2007).
Dorus, S., Busby, S. A., Gerike, U., Shabanowitz, J., Hunt, D. F. & Karr, T. L. Genomic and functional evolution of the Drosophila melanogaster sperm proteome. Nat. Genet. 38, 1440–1445 (2006).
Yeh, S. D. et al. Functional evidence that a recently evolved Drosophila sperm-specific gene boosts sperm competition. Proc. Natl. Acad. Sci. USA 109, 2043–8 (2012).
Hudspeth, A. J. Making an effort to listen: mechanical amplification in the ear. Neuron 59, 530–545 (2008).
Kazmierczak, P. & Müller, U. Sensing sound: molecules that orchestrate mechanotransduction by hair cells. Trends Neurosci. 35, 220–9 (2012).
Cheng, L. E., Song, W., Looger, L. L., Jan, L. Y. & Jan, Y. N. The role of the TRP channel NompC in Drosophila larval and adult locomotion. Neuron 67, 373–380 (2010).
Liang, X. et al. A NOMPC-dependent membrane-microtubule connector is a candidate for the gating spring in fly mechanoreceptors. Curr. Biol. 23, 755–763 (2013).
Zhang, W. et al. Ankyrin repeats convey force to gate the NOMPC mechanotransduction channel. Cell 162, 1391–1403 (2015).
King, S. M. Sensing the mechanical state of the axoneme and integration of Ca2+ signaling by outer arm dynein. Cytoskeleton 67, 207–213 (2010).
Lehnert, B. P., Baker, A. E., Gaudry, Q., Chiang, A. S. & Wilson, R. I. Distinct roles of TRP channels in auditory transduction and amplification in Drosophila. Neuron 77, 115–128 (2013).
Albert, J. T., Nadrowski, B. & Göpfert, M. C. Mechanical signatures of transducer gating in the Drosophila ear. Curr. Biol. 17, 1000–1006 (2007)
Dean, A. B. & Mitchell, D. R. Chlamydomonas ODA10 is a conserved axonemal protein that plays a unique role in outer dynein arm assembly. Mol. Biol. Cell 24, 3689–3696 (2013).
Desai, P. B., Freshour, J. R. & Mitchell, D. R. Chlamydomonas axonemal dynein assembly locus ODA8 encodes a conserved flagellar protein needed for cytoplasmic maturation of outer dynein arm complexes. Cytoskeleton 72, 16–28. (2015).
Holt, J. R. et al. A chemical-genetic strategy implicates myosin-1c in adaptation by hair cells. Cell 108, 371–381 (2002).
Jacobs, J. S., Hong, X. & Eberl, D. F. A. “mesmer“izing new approach to site-directed mutagenesis in large transformation-ready constructs: mutagenesis via serial small mismatch recombineering. Fly 4, 162–169 (2011).
Eberl, D. F., Duyk, G. M. & Perrimon, N. A genetic screen for mutations that disrupt an auditory response in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 94, 14837–14842 (1997).
Sharma, Y., Cheung, U., Larsen, E. W., & Eberl, D. F. (2002). pPTGAL, a convenient Gal4 P-element vector for testing expression of enhancer fragments in Drosophila. Genesis, 34, 115–118.
Albert, J. T., Nadrowksi, B., Kamikouchi, A. & Göpfert, M. C. Mechanical tracing of protein function in the Drosophila ear. Nat Protoc 2006.346 (2006). (Accessible at http://www.nature.com/protocolexchange/protocols/58). Date of access: 25/08/2015.
Rostaing, P. et al. Anaylsis of synaptic ultrastructure without fixative using high-pressure freezing and tomography. Eur. J. Neurosci. 24, 3463–3474 (2006).
Reynolds, E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 17, 208–212 (1963).
Acknowledgements
We thank Changsoo Kim and Jonathon Howard for anti-Iav and anti-NOMPC antibodies, Carolin Wichmann and Wiebke Möbius for support for electron microscopy, Damiano Zanini for help with bioinformatics and Margret Winkler and Stephanie Pauls for technical assistance. This work was supported by the Iowa Center for Molecular Auditory Neuroscience, a stipend from the Neurosenses Program of the State of Lower Saxony, Germany (to S.K.), the US National Institutes of Health (DC011357 to M.J.K. and NIH P30 core grant DC010362 to Steven Green (D.F.E.)), the German Science Foundation (GO 1092/2, SPP 1680, SFB 889 A1 and INST 186/1081-1) and the BMBF Göttingen Bernstein Centre (BCCN-2, 01GQ1005A, to M.C.G.).
Author information
Authors and Affiliations
Contributions
M.C.G., D.F.E. and M.J.K. designed research. S.K. and J.S.J. generated transgenic flies and analyzed expression patterns. S.K. accessed auditory phenotypes with the help of C.S. and R.K. M.K. performed electron microscopy. Male fertility and sperm competition were assayed by J.S.J., M.A.S., E.S.-L. and D.F.E. and sequence comparisons were made by M.J.K. The manuscript was written by M.C.G., S.K., D.F.E., M.J.K. and M.K.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Karak, S., Jacobs, J., Kittelmann, M. et al. Diverse Roles of Axonemal Dyneins in Drosophila Auditory Neuron Function and Mechanical Amplification in Hearing. Sci Rep 5, 17085 (2015). https://doi.org/10.1038/srep17085
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep17085
This article is cited by
-
Single-cell profiling of Anopheles gambiae spermatogenesis defines the onset of meiotic silencing and premeiotic overexpression of the X chromosome
Communications Biology (2023)
-
The dynamics of protein localisation to restricted zones within Drosophila mechanosensory cilia
Scientific Reports (2022)
-
How to wake up the electric synapse coupling between neurons?
Nonlinear Dynamics (2022)
-
Comparative transcriptomics between Drosophila mojavensis and D. arizonae reveals transgressive gene expression and underexpression of spermatogenesis-related genes in hybrid testes
Scientific Reports (2021)
-
Biophysical mechanism of signal encoding in an auditory neuron
Nonlinear Dynamics (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.