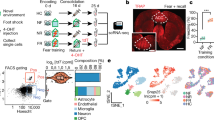
Abstract
The stable formation of remote fear memories is thought to require neuronal gene induction in cortical ensembles that are activated during learning. However, the set of genes expressed specifically in these activated ensembles is not known; knowledge of such transcriptional profiles may offer insights into the molecular program underlying stable memory formation. Here we use RNA-Seq to identify genes whose expression is enriched in activated cortical ensembles labeled during associative fear learning. We first establish that mouse temporal association cortex (TeA) is required for remote recall of auditory fear memories. We then perform RNA-Seq in TeA neurons that are labeled by the activity reporter Arc-dVenus during learning. We identify 944 genes with enriched expression in Arc-dVenus+ neurons. These genes include markers of L2/3, L5b, and L6 excitatory neurons but not glial or inhibitory markers, confirming Arc-dVenus to be an excitatory neuron-specific but non-layer-specific activity reporter. Cross comparisons to other transcriptional profiles show that 125 of the enriched genes are also activity-regulated in vitro or induced by visual stimulus in the visual cortex, suggesting that they may be induced generally in the cortex in an experience-dependent fashion. Prominent among the enriched genes are those encoding potassium channels that down-regulate neuronal activity, suggesting the possibility that part of the molecular program induced by fear conditioning may initiate homeostatic plasticity.
Similar content being viewed by others
Introduction
The recall of remote fear memories–those that persist for days, weeks, or longer–requires the cerebral cortex in addition to the amygdala and other regions1,2. For cued fear memories, the sensory cortex in particular is an appealing region where a remote association between a conditioned sensory cue and an unconditioned stimulus may be formed, producing a memory engram3. Consistent with this idea, sensory cortex is required for recall of remote but not recent associations4, and the activity reporter Egr1 is induced in sensory cortex upon remote but not recent recall5. However, Egr1 is just one member of a larger experience-dependent gene program (including Fos, Npas4, Arc) whose components are induced during learning and thought to be required for long-term memory formation6,7,8,9.
Until recently, limitations in genomic technologies have precluded identification of the most functionally relevant set of fear conditioning-induced genes in the cortex. The cortex contains diverse cell types, and the activity-regulated gene programs differ among them10. Due to this cell-type diversity, detecting gene expression changes in bulk tissue inevitably entails a loss in sensitivity. Although RNA Sequencing (RNA-Seq) is an improvement over microarray methods, technical issues including batch effects and read depth still limit sensitivity11. This limited sensitivity poses a serious challenge for the identification of the neural activity-induced “effector” genes that alter circuit function, as these genes are expressed at lower levels than immediate-early transcription factors7,12. RNA-Seq has now been combined with cell-type specific labeling to profile experience-dependent gene expression13,14, but even these approaches measure gene expression in large populations of cells, only a subset of which is responsive to experience. With the goal of identifying potential effector genes, we performed RNA-Seq specifically in neural ensembles in sensory association cortex that are engaged during fear conditioning.
Results
Our experimental design is outlined in Fig. 1A. In auditory fear conditioning experiments, we habituated mice to handling and the conditioning chamber for 4 or 10 days, without tones (the conditioned stimuli) or shocks (the unconditioned stimuli). We then performed fear conditioning with a series of seven tones and co-terminating footshocks (FC group). In control experiments, we also exposed mice to footshocks without tones (shock only group) or the chamber context only (control group). To confirm that remote memories were acquired, we assessed remote memory recall by re-presenting the tones in a new context after two months (performed in a parallel cohort). In seeking to identify gene expression relevant to remote memory consolidation, we focused our gene expression analysis on the period immediately following learning. This choice may seem counter-intuitive, given that the cortex is not required for recent auditory fear recall4. However, in several cortical areas, gene induction during learning occurs in the same neurons later reactivated during recall15. Given that inducible transcripts likely persist for hours to days, it is the gene induction during learning itself that we hypothesized is the gene induction most relevant to memory consolidation.
(A) Experimental design for behavioral testing and RNA sequencing. (B) The extent of lesioning in the two mice with the smallest (dark grey) and largest (gray) lesions of the group. Coronal brain section images adapted from42. S1BF, primary somatosensory cortex barrel; S2, secondary somatosensory cortex; V2L, secondary visual cortex lateral area; AuD, secondary auditory cortex dorsal; AuV, secondary auditory cortex ventral; TeA, temporal association cortex; Ect, ectorhinal cortex; PRh, perirhinal cortex. (C) Remote auditory fear memory in auditory cortex lesioned (n = 8) and sham-operated (n = 8) animals. Fear response was measured as percentage of total freezing both 7 min before (pre tone) and during (test trial) presentation of seven CSs. All values are means +/− SEM. Red lines represent mean freezing across the indicated presentations. **p = 0.0002, unpaired t-test (two-tailed). (D) Auditory cortex lesioned animals were able to reacquire CS-US association. Each data point represents the mean freezing across all seven CS presentations for each animal. *p = 0.0022, Paired t-test (two-tailed); NS, not significant.
For the RNA-Seq experiment, we sought to target a specific area of sensory cortex that is required for remote recall of cued fear memories in mice. We chose mice for these experiments to take advantage of the high quality mouse genome annotations and associated genomic data available12,13,16. We focused on temporal association cortex (area TeA) because it processes auditory information and is rich in projections to the amygdala17,18. To determine whether mouse TeA is required for fear recall, we lesioned TeA after fear conditioning but before testing recall. Our lesions completely covered TeA, extending into ectorhinal and parts of auditory cortex (Au1, AuD, AuV; Fig. 1B). We found that TeA lesions dramatically attenuated remote memory recall, reducing freezing in response to tone presentation two months after training (Fig. 1C). The effect of the lesion cannot be attributed to a defect in hearing, because lesioning did not affect the ability of mice to subsequently re-associate tone and shock (Fig. 1D). These results are in agreement with those obtained from rats4,19,20, consistent with the idea that plasticity in TeA or nearby regions during learning or consolidation may be a substrate for remote associative memory formation.
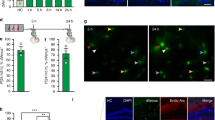
We focused our RNA-Seq efforts on neural ensembles that are activated during fear conditioning through the use of Arc-dVenus activity reporter mice21. In these mice, the activity-regulated Arc promoter drives expression of a destabilized Venus fluorophore, labeling neurons activated within the previous 3–12 hour period. To characterize this reporter, we examined native Arc-dVenus fluorescence in sections from mice sacrificed six hours after fear conditioning. We reasoned that performing RNA-Seq at this timepoint would maximize our ability to detect the activity-regulated effector genes, whose induction is typically slower than that of immediate-early transcription factors. We found that Arc-dVenus is strongly induced in TeA six hours after fear conditioning, primarily in layer 2/3 (L2/3), layer 5 (L5), and layer 6 (L6) (Fig. 2A). The layer-to-layer pattern we observe here is similar to endogenous Arc mRNA expression22, giving us an additional measure of confidence in this established activity reporter21,23.
(A) Representative images and quantification of Arc-dVenus expression in TeA from control, tone-only, shock-only, and fear-conditioned mice. Scale bar, 200 μm. All values are mean +/− SEM. **p < 0.05, unpaired t-test (two-tailed). Unstarred comparisons are not significantly different. N = 3 mice for control and fear conditioned groups and two mice for the tone-only and shock-only groups. (B) Fos in situ hybridization. Representative cropped images and quantification of Fos expression in TeA from control, tone-only, shock-only, and fear-conditioned mice. N = 3 mice for all groups. Scale bar, 200 μm. All values are means +/− SEM. **p < 0.05, unpaired t-test (two-tailed). (C) Heatmap of expression differences between bulk TeA tissue and sorted Arc-dVenus cells. Glial, inhibitory, and excitatory markers26,46 are indicated at left. Each gene’s expression is normalized to its mean across the three bulk-tissue samples. Within excitatory markers, genes are in rough order of specificity for layer 6 (top) to layer 2 (bottom). Markers for non-neural cells include Aqp4 (astrocytes); Pdgfra (oligodendrocyte precursor cells, OPCs); Mog/Opalin (oligodendrocytes); Ctss/Itgam (microglia); Flt1/Xdh (endothelial cells); and Bgn/Myl9 (smooth muscle cells, SMC). Bulk tissue samples are from FC (Tissue 1), shock (Tissue 2–3) mice respectively. Sorted cells are from FC (23 cells), FC (39 cells), FC (40 cells), shock (30 cells), and shock (33 cells) respectively. *Indicates false discovery rate (FDR) <0.05 from an overdispersed Poisson model.
To investigate the potential relationship between Arc-dVenus+ neurons and an associative memory trace, we asked which specific elements of associative learning lead to Arc-dVenus induction in TeA: tone (CS), shock (US), or coincident tone-shock association. We found that expression of Arc-dVenus is induced by shock alone but not tone alone (Fig. 2A). Moreover, Arc-dVenus expression is indistinguishable in shock-only and fear conditioning conditions, suggesting that it is dependent on US rather than coincident CS/US detection. To address whether this US-dependence is specific to this particular reporter or to the Arc gene, we examined the mRNA expression of another well-studied immediate early gene, Fos, and found it also to be US- but not CS-dependent (Fig. 2B). Despite the tone having no apparent influence on Arc-dVenus expression, these results are consistent with the idea that an associative memory trace could be contained within a subset of Arc-dVenus neurons that coincidently detect tone and shock. Whether the specific Arc-dVenus+ neural ensemble activated in TeA during fear conditioning is reactivated during recall has not been established, although ensemble reactivation has been observed in other cortical areas15.
To obtain RNA from Arc-dVenus+ neurons, we performed cell dissociation and manual sorting24, isolating between 23 and 39 Arc-dVenus+, TeA cells per animal. The manual sorting approach produces results similar to Fluorescent-Activated Cell Sorting but is advantageous when labeled cells are sparse or tissue amount is limiting25. From the RNA isolated from sorted cells, we made five sequencing libraries: three from fear conditioned mice and two from shock-only control mice. We did not sequence Arc-dVenus+ cells from context-only controls because these mice have very few activated Arc-dVenus+ cells (Fig. 2A). To provide a reference point for calculating the enrichment of gene expression in Arc-dVenus+ cells, we sequenced three TeA bulk-tissue samples. To maximize their value as reference points, we processed these bulk-tissue samples in exactly the same way as the tissue sections that we used for cell sorting (i.e., both were protease-treated identically). Two were from shock-only mice, and one was from a fear-conditioned mouse. We used both conditions for the purpose of comparing bulk-tissue to sorted Arc-dVenus+ neurons. In support of this approach, bulk-tissue samples were highly similar to each other and distinct from Arc-dVenus+ sorted cells, regardless of condition (Supplementary Fig. S1). However, our study was not designed or powered to address gene expression differences between bulk-tissue conditions. Instead, by comparing mRNAs enrichment in Arc-dVenus+ cells compared to bulk-tissue samples, we aimed to identify those genes that are enriched in ensembles of neurons activated during learning.
Analysis of Arc-dVenus+ ensembles enables detection of gene expression only in the specific cell types in which Arc-dVenus is expressed. To identify cell types that express the Arc-dVenus reporter after fear conditioning, we compared RNA-Seq data for Arc-dVenus+ cells and bulk-tissue controls. We found that non-neuronal markers are de-enriched in Arc-dVenus+ cells, including greater than 100-fold de-enrichments of the astrocyte marker Gfap, the microglial marker Ctss, and the oligodendrocyte precursor marker Pdgfra (p < 10−5, Fig. 2C). Markers for GABAergic inhibitory neurons are also de-enriched, including the pan-inhibitory marker Gad1 and markers for three major classes of inhibitory neurons, Vip, Sst, and Pvalb (each >100-fold de-enriched, p < 10−13). In contrast, the pan-excitatory marker Slc17a7 (Vglut1) is enriched 11-fold (p < 10−6). Thus, analysis of the Arc-dVenus+ transcriptome does not permit detection of inhibitory neuron-specific gene expression but does enable detection of excitatory neuron-specific gene expression. Since principal neurons in the cortex are excitatory, Arc-dVenus reporter mice are well-suited for identifying genes enriched in activated principal neurons.
To identify subtypes of excitatory neurons that express Arc-dVenus, we examined the Arc-dVenus enrichment of 28 markers that distinguish subtypes of cortical excitatory neurons26 (Fig. 2C). The layer 2/3 (L2/3) markers Ptgs2 and Inhba are greater than 300-fold enriched (p < 10−16). However, the L4-specific Scnn1a and Arf5 are not significantly enriched (<1.5-fold, p > 0.93). The L5b markers Cdh13, Tcerg1l, and Qrfpr are expressed in Arc-dVenus+ neurons and trend toward positive enrichment (33-fold, 14-fold, 1.8-fold; p = 0.002, 0.06, 0.86). The L6a markers Car12 and Myl4 also have positive enrichment (>9-fold, p = 0.02 and 0.52). The prominence of L2/3, L5, and L6 markers is consistent with the abundance of Arc-dVenus fluorescence in these layers (Fig. 2A) and suggests that our approach is well-powered to detect fear conditioned-induced genes in the excitatory neurons in these layers.
We asked whether global gene expression in activated neurons differs following fear conditioning versus shock presentation alone. We found the Arc-dVenus-sorted expression profiles from fear conditioning and shock to be indistinguishable (Supplementary Fig. S2A,B). One possible explanation would be that most of the genes enriched in Arc-dVenus+ neurons are cell-type markers rather than neuronal activity-regulated genes. However, we found that the expression of a smaller set of bona fide activity-regulated genes is not differentially expressed in Arc-dVenus+ neurons between fear conditioning and shock conditions (Supplementary Fig. S2C). Our experiments are not sufficiently powered to rule out the possibility of potential gene expression differences between fear conditioning and shock. However, a comparison to KCl-induced gene expression in primary neurons12 suggests that our experiments here are adequately powered to detect a systematic difference in expression of activity-regulated genes between FC and shock conditions (Supplementary Fig. S2D,E). The lack of such systematic bias suggests that global TeA gene induction during auditory fear conditioning may not require coincident detection of a tone and shock. This idea is consistent both with similar results in the auditory cortex27 and with the idea that disinhibition of L2/3 neurons in sensory cortex is shock-dependent but not auditory cue-dependent28. In light of the indistinguishability of gene expression from shock and fear conditioning conditions, we subsequently combined data from these conditions to identify with maximal statistical power the genes whose expression is elevated in Arc-dVenus+ neurons compared to bulk-tissue. Our decision to pool all sorted samples together is supported by our analysis showing that gene expression differs much more between sorted neurons and bulk-tissue than between sorted conditions (Supplementary Fig. S1).
We identified 944 Arc-dVenus-enriched genes, which have >2-fold enrichment in Arc-dVenus+ neurons (over bulk-tissue) and a false discovery rate of less than 5%. To identify the subset of these genes likely to be induced by fear conditioning, we compared them to genes previously shown to be either activity-regulated in vitro12 or induced upon first exposure to visual stimulus in the visual cortex13. We found 125 activity-regulated Arc-dVenus-enriched genes, which are Arc-dVenus-enriched and regulated by neuronal activity or visual stimulus (Fig. 3A,B, Supplementary Table S1). The overlap of Arc-dVenus-enriched genes with activity-regulated and visual stimulus-induced genes is highly significant (each p < 10−20, hypergeometric test) (Fig. 3C,D). In addition, most activity regulated genes, including the generally cell type non-specific early genes Fos, Fosb, and Junb, have higher expression in Arc-dVenus neurons (p = 2*10−5 from permutation, Supplementary Fig. 3 and Supplementary Table 1). Moreover, 116 Arc-dVenus-enriched genes are also enriched in Fos+ neurons in the hippocampus29 (p = 10−23, hypergeometric test). These results do not provide direct evidence that genes enriched in Arc-dVenus+ neurons are induced during fear learning or even that Arc-dVenus+ cells exhibit more learning-associated gene induction than Arc-dVenus- cells. However, the highly significant overlap of Arc-dVenus-enriched with experience-regulated genes suggests that this overlapping set of genes may be induced during fear learning in Arc-dVenus+ neurons.
(A) Venn diagram showing overlap between Arc-dVenus-enriched genes, genes regulated by activity in vitro12, visual stimulus-regulated genes13, and genes enriched in Fos+ hippocampal neurons29. Arc-dVenus-enriched genes are defined by FDR <0.05 and fold-enrichment of >2 in Arc-dVenus+ neurons (n = 5) compared to bulk-tissue controls (n = 3). (B) Heatmap of expression in Arc-dVenus+ neurons and bulk-tissue for genes that are both Arc-dVenus-enriched and activity- or visual stimulus-regulated. Each gene’s expression is normalized to its mean across the three bulk-tissue samples. (C,D) The expected versus actual numbers of Arc-dVenus-enriched genes that are also visual stimulus-induced (C) or activity-induced (D) (p < 10−20, hypergeometric tests). Distributions of expected numbers from the hypergeometric distribution are in black; actual numbers are in red.
The overlap of Arc-dVenus-enriched genes with visual stimulus-induced genes suggests that the plasticity that occurs during remote memory formation could be related to visual stimulus-dependent homeostatic plasticity30. We therefore asked whether activity-regulated Arc-dVenus-enriched genes are enriched for particular functions. Analysis for gene category enrichment revealed enrichment for genes whose products target the plasma membrane (p < 0.05 with Benjamini correction). Interestingly, activity-regulated Arc-dVenus-enriched genes are significantly enriched for potassium channels (Kcna4, Kcnc3, Kcnj2, and Kcnj4, p < 0.0002, hypergeometric test). Reinforcing this finding, several additional activity-regulated potassium channel genes trend toward enrichment in Arc-dVenus+ neurons but narrowly miss our FDR or fold-change thresholds (Hcn1, Kcnj3, Kcnh7; all 2-fold enriched with FDR <10%). Moreover, three of the activity-regulated, Arc-dVenus-enriched potassium channels (Kcnc3, Kcnj2, Kcnj4) are also enriched in Fos+ neurons in the hippocampus29. The enrichment of potassium channel transcripts among activity-regulated Arc-dVenus-enriched genes suggests that fear conditioning may lead to homeostatic decreases in the excitability of activated neural ensembles.
Discussion
In this study, we set out to profile gene expression in neural ensembles that are activated during fear learning. We used the well-tested auditory fear conditioning paradigm in mice. We first established that the temporal association cortex (TeA) is necessary for recall of remote auditory fear memories. Next, using Arc-dVenus reporter mice, we sequenced RNA specifically from neural ensembles in TeA that are activated during fear conditioning. These ensembles are composed of excitatory neurons, as they are both enriched for layer-specific excitatory neuronal markers as well as de-enriched for inhibitory neuronal and glial markers. We found that the genes expressed in these ensembles overlap with those induced in vitro by depolarization and in the visual cortex by visual stimuli, suggesting that these genes may contribute to activity-dependent plasticity. Among these genes are multiple potassium channel-encoding transcripts, suggesting that fear conditioning may initiate a homeostatic response that dampens the activity of the cortical ensembles that it most strongly activates.
Cortical neurons are known to exhibit non synapse-specific, homeostatic decreases in activity that can be transcription-dependent. These include not only Arc-dependent plasticity of synaptic strength31,32, but also plasticity of intrinsic excitability33, which is dynamically regulated in visual cortex pyramidal neurons via regulation of potassium conductance34. Furthermore, the specific neural ensembles defined by Arc+ or Fos+ expression have lower intrinsic excitability35,36 and fewer spines37 than their neighbors, consistent with the idea that they may be undergoing homeostatic downregulation. Although such downregulation could function exclusively to preserve homeostasis, plasticity of intrinsic excitability is also a fundamental mechanism of encoding information in circuits38. One possibility is that decreased intrinsic excitability could desensitize the activity of footshock-responsive cortical ensembles to subsequent footshock presentations, thereby protecting against chronic pain or stress. In contrast to these ideas, the activity-regulated gene program has also long been suggested to be required for Hebbian increases in synaptic strength39,40. Yet it has not been clear whether Hebbian plasticity, homeostatic plasticity, or both modes of plasticity are engaged in the specific neural ensembles activated during fear conditioning. Our RNA-sequencing analyses provide fresh support for the importance of transcription-dependent homeostatic responses in these activated ensembles.
Method and Materials
Animals
All animal studies were approved by and performed in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) at Harvard University. P60 to P90 male wild-type C57BL/6 mice (Charles River) were used for excitotoxic NMDA lesions, remote memory recall tests, and Fos Fluorescent in situ hybridization; For RNA sequencing. P60 to P90 Arc-dVenus transgenic male C57BL/6 mice were used21. All animals were housed individually for 4 days prior to all experiments. Behavioral studies were conducted between 1:30 and 2:00 pm, except for the RNA sequencing group, which was conducted between 9:00 and 10 am. All experiments were performed during the light-phase of the mice’s daily cycle.
Behavioral procedures
Fear conditioning
The mice were conditioned in standard operant conditioning chamber apparatus (Med Associates) located in the NeuroBehavior Laboratory at Harvard Medical School. For the NMDA lesion study, individually housed mice were habituated to the conditioning chambers for 20 min for 4 consecutive days. For the RNA sequencing study, the mice were habituated to the conditioning chambers for 10 consecutive days prior to experiment, to ensure low background gene expression. For the NMDA lesion study (n = 16), fear conditioning (FC) consisted of presenting 7 repetitions of a CS tone (70 +/− 5 dB, 6 kHZ) terminating with the US (1-s footshock, 0.5 mA DC; inter-trial interval: 20–180 s). The animals were returned to their home cages right after the FC.
For the RNA sequencing study, the Arc-dVenus transgenic mice were divided into three different groups. One group (FC, n = 3) went through the same FC protocol as the NMDA lesion group and received 7 CS and US pairing. Another group (Shock, n = 2) received 7 repetitions of US (1-s footshock, 0.5 mA DC; inter-trial interval: 20–180 s) without any CSs. The bulk-tissue controls included two mice that were shocked without tones, and one mouse that was fear conditioned. All Arc-dVenus transgenic mice were sacrificed 6 hrs after the behavioral procedures.
For Fos expression analysis, the mice were conditioned in custom operant conditioning chambers described in a previous study41. The mice were divided into four different groups. The first group (FC, n = 3) went through the same FC protocol as the NMDA lesion group and received 7 CS and US pairings. The second group (Tone, n = 3) received 7 repetitions of CS (70 +/− 5 dB, 6 kHZ; inter-trial interval: 20–180 s) without any USs. The third group (Shock, n = 3) received 7 repetitions of US (1-s footshock, 0.5 mA DC; inter-trial interval: 20–180 s) without any CSs. The last group (Control, n = 3) was put into the conditioning chamber and did not receive any CS nor US. All mice were sacrificed 1 hr after the behavioral procedures.
Remote memory test
On the 60th day after FC (10 days after NMDA lesion surgery), auditory remote fear memory was assessed by placing the animals in a new environment and measuring the freezing response to 7 presentations of CS+. Freezing measurements were automated using the FreezeScan system (Clever sys.). The animals were returned to their home cages right after the recall test.
Retrained and recent memory test
Two days after assessing their remote memory, the animals were retrained to the same CS (70 +/− 5 dB, 6 kHZ) using the same FC protocol (above), and 1 day later, their recent memory was assessed by measuring the freezing response to the same 7 presentation of CS+. After the recall test, the mice were sacrificed to assess the size of the lesions.
Excitotoxic NMDA lesion surgery
50 days after the FC, excitotoxic lesion of TeA was performed on 8 mice which were randomly chosen from 16 animals that were fear conditioned. Mice were anaesthetized with isoflurane (5% for induction, 1–1.5% for maintenance) in a Kopf stereotaxic apparatus. In order to lesion the entire TeA, the mice (n = 8) received two injections per hemisphere using an infusion needle (33 gauge) connected to a microsyringe pump (UMP3;WPI) and controller (Micro4;WPI) to deliver 20 μg/μl NMDA (0.3 μl/site;Sigma), dissolved in phosphate-buffered saline (PBS) at a rate of 0.1 μl/min. After the injection, the needle was left for 5 min to ensure diffusion of NMDA into the target structure and was then slowly retracted. Infusion sites were as follows (in mm): AP = −2.06 and −3.80 (from bregma), DV = −0.68 (from surface of the cortex) and ML = +/−3.75 mm42. The sham-operated controls (n = 4) were infused with PBS alone instead of NMDA at the same coordinates, and control mice (n = 4) only received surgery consisting of a craniotomy and no injections. These two groups were pulled into one sham group because the freezing behaviors were indistinguishable between the two (unpaired t-test (two-tailed), p = 0.3321). All mice that underwent surgery were allowed to recover for 10 days before being subjected to the remote recall test.
Histology
Brain preparation and staining
For the excitotoxic NMDA lesion group, the animals were rapidly sacrificed with carbon dioxide after the last recall test, and their brains were rapidly removed and frozen using dry ice in methylbutane. All the brains were stored at −80 °C until cryosectioning. Coronal sections (20 μm) were cut on a cryostat and were counter-stained using hematoxylin nuclear counterstain (Vector lab) following the manufacturer’s recommendation.
To assess the expression pattern of Arc-dVenus in control (n = 3), tone (n = 2), shock (n = 2) and fear conditioned mice (n = 3), the Arc-dVenus transgenic mice were deeply anaesthetized with isoflurane 6 hrs after behavioral procedures and perfused transcardially with ice-cold PBS containing 4% paraformaldehyde. The brains were dissected, post-fixed overnight at 4 °C and cryoprotected in PBS containing 30% sucrose before freezing. All of the brains were stored at −80 °C until cryosectioning. Coronal sections (20 μm) were cut on a cryostat, mounted and imaged.
To assess the expression pattern of Fos in control, tone, shock and fear conditioned mice, the animals (n = 3/group) were rapidly sacrificed with carbon dioxide 1 hr after behavioral procedures and their brains were rapidly frozen using dry ice in methylbutane. All the brains were stored at −80 °C until the cryosectioning. Coronal sections (20 μm) were cut on a cryostat and stored the slides at −20 °C until further use.
Fluorescent in situ hybridization. Nonradioactive, digoxigenin (DIG)-labeled cRNA probes with either sense or antisense orientation were synthesized by in vitro transcription using DIG labeling mix (Roche) according to the recommendations of the manufacturer. Probes were synthesized from cDNA clones encoding Fos purchased from Dharmacon (MMM1013-202760329). For fluorescent in situ hybridization (FISH), all solutions were prepared using RNase-free reagents and diethylpyrocarbonate (DEPC)-treated double deionzide water (ddH2O).
The previously cryosectioned slides were allowed to dry for 20 min. After fixation, the sections were washed with PBS 3 times for 5 min and treated with 0.3% H2O2 (vol/vol) in PBS for 30 min, followed by 3 rounds of 5 min wash with PBS. The sections were then acetylated by 0.1% acetic anhydride (vol/vol) in triethanolamine for 10 min, followed by 2 rounds of 5 min wash with PBS and 1 round of 5 min wash with 2XSSC. Afterward, the sections were incubated in the hybridization solution (Sigma) without probe for 2 h at 70 °C and incubated in the hybridization solutions with Fos probe for 16–18 h at 70 °C. After hybridizations, the sections were washed in 5XSSC and 2XSSC for 10 min at 65 °C, followed by 30 min wash in 50% formamide (vol/vol) in 0.2X SSC at 65 °C. The sections were further washed in 0.2XSSC at room temperature and followed by one wash in TN buffer (0.1 M Tris-HCl, pH 7.5, 0.15 M NaCl) for 5 min. The sections were blocked with 1% blocking reagent (wt/vol) (Roche) in TN buffer for 1 h and incubated in the same solutions with digoxigenin antibody conjugated to horseradish peroxidase (1:1000) (PerkinElmer) for 1 h 30 min. Following the incubation with antibody, the sections were washed 3 times in TNT buffer (0.1 M Tris-HCl, pH 7.5, 0.15 M NaCl, 0.05% Tween-20 (vol/vol)) for 5 min and incubated in the amplification solution with Fluorescein plus tyramide (1:50) (PerkinElmer) for 30 min. After the amplification, the sections were washed 3 times in TNT buffer and incubated with Hoechst (1:10000) (Invitrogen) in PBS for 5 min. After 3 rounds of washing in PBS, the sections were mounted and stored at 4 °C until further analysis.
Microscopy and image analysis
All hemotoxyline stained, Arc-dVenus images and Fos FISH images were acquired with a 10X objective lens using an Olympus VS120 Whole slide scanner, which generates a proprietary vsi image at the Harvard Neurobiology Imaging Facility (NS072030).
Data analysis
Arc-dVenus and Fos+ neurons were manually counted by a blinded experimenter. TeA images were quantified from 3 sections per animal.
Statistical analyses were performed using commercial software (GraphPad Prism; GraphPad Software, Inc.) for analyzing freezing data between NMDA lesion and sham groups and quantification for Arc-dVenus and Fos+ neurons.
RNA sequencing
Manual cell sorting and RNA sequencing
Manual sorting of fluorescent cells was carried out as first described in24. Briefly, Arc-dVenus transgenic male C57BL/6 mice were sacrificed under isoflurane anesthesia 6 hrs after behavioral procedures and their brains were quickly removed and transferred into ice-cold oxygenated ACSF, containing 126 mM NaCl, 20 mM NaHCO3, 20 mM dextrose, 3 mM KCl, 1.25 mM NaH2PO4, 2 mM CaCl2, 2 mM MgCl2, 50 mM APV, 20 mM DNQX, 100 nM TTX. Acute 330 μm coronal brain slices were prepared and incubated in a protease E (1.2 mg/mL) (Sigma) containing oxygenated ACSF for 50 min. After 15 min of washes in the ACSF, TeA tissue was microdissected using a pair of fine scissors under a fluorescent dissecting microscope (Leica M165FC). The dissected TeA tissue was then triturated in ACSF using a series of three Pasteur pipettes of decreasing tip diameters, and the dissociated cells were transferred into a small petri dish. With visual control under a fluorescent dissecting microscope, Venus+ neurons were aspirated into a micropipette with a 30–50 μm tip diameter and transferred into a clean petri dish. A total of 35–60 Venus+ neurons were pooled for each sample, which were immediately lysed in 50 μl of extraction buffer (PicoPure RNA isolation kit, Arcturus, Life Technologies) and total mRNA was subsequently isolated. cDNA was synthesized using Ovation RNA-Seq System V2 kit (Nugen). We obtained approximately 6 μg of cDNA from 35–60 cells from each group. Then, the cDNA library was prepared using Ovation Ultralow DRMultiplex Systems (Nugen). Sequencing was conducted on an Illumina HiSeq2000, and is available via GEO accession GSE85128.
Analysis of Arc-dVenus RNA-sequencing data
We aligned reads in fastq format to mouse genome mm9 using RUM version 1.11 with default settings43. We analyzed only uniquely aligning reads, only genes with a total of at least 20 reads per million across all samples, and only one transcript variant per gene (the highest expressed across all samples). We called differentially expressed RefSeq transcripts based on a 5% false discovery rate (FDR) and a requisite 2-fold enrichment in Arc-dVenus+ cells. Significance is based on p.values from edgeR using an overdispersed Poisson noise model44, with corresponding FDRs from the R function p.adjust. The dendrograms and heatmaps were produced by heatmap.2 in R. The set of genes analyzed and their normalized read counts in each sample are provided in Table S1. Gene set overrepresentation analysis was performed using DAVID45 with the RefSeq genes as a background. Potassium channel enrichment was tested with a hypergeometric test that addressed whether the number of potassium channels among activity-regulated Arc-dVenus genes was unexpected based in their frequency among (1) all genes whose enrichment in Arc-dVenus+ neurons was tested and (2) genes that are activity-regulated in vitro or by regulated by visual stimulus in visual cortex.
Analysis of published sequencing data
Markers were identified from published lists of Emx1-enriched genes13, S1 pyramidal neurons46, or layer-specific markers26. Activity-regulated genes in vitro were identified as genes whose mean normalized expression increased at least 50% in previously published RNA-Seq experiments12. Visual stimulus-induced genes were taken from a published list13.
Additional Information
How to cite this article: Cho, J.-H. et al. RNA sequencing from neural ensembles activated during fear conditioning in the mouse temporal association cortex. Sci. Rep. 6, 31753; doi: 10.1038/srep31753 (2016).
Change history
16 September 2016
The PDF version of this Article previously published contained a lower resolution image for Figure 3. This has now been corrected.
References
Frankland, P. W., Bontempi, B., Talton, L. E., Kaczmarek, L. & Silva, A. J. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science 304, 881–883 (2004).
Frankland, P. W. & Bontempi, B. The organization of recent and remote memories. Nat. Rev. Neurosci. 6, 119–130 (2005).
Josselyn, S. A., Köhler, S. & Frankland, P. W. Finding the engram. Nat. Rev. Neurosci. 16, 521–534 (2015).
Sacco, T. & Sacchetti, B. Role of secondary sensory cortices in emotional memory storage and retrieval in rats. Science 329, 649–656 (2010).
Kwon, J.-T., Jhang, J., Kim, H.-S., Lee, S. & Han, J.-H. Brain region-specific activity patterns after recent or remote memory retrieval of auditory conditioned fear. Learn. Mem. 19, 487–494 (2012).
Tischmeyer, W. & Grimm, R. Activation of immediate early genes and memory formation. Cell. Mol. Life Sci. 55, 564–574 (1999).
Lin, Y. et al. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature 455, 1198–1204 (2008).
Johansen, J. P., Cain, C. K., Ostroff, L. E. & LeDoux, J. E. Molecular mechanisms of fear learning and memory. Cell 147, 509–524 (2011).
Ressler, K. J., Paschall, G., Zhou, X.-L. & Davis, M. Regulation of synaptic plasticity genes during consolidation of fear conditioning. J. Neurosci. 22, 7892–7902 (2002).
Spiegel, I. et al. Npas4 regulates excitatory-inhibitory balance within neural circuits through cell-type-specific gene programs. Cell 157, 1216–1229 (2014).
Peixoto, L. et al. How data analysis affects power, reproducibility and biological insight of RNA-seq studies in complex datasets. Nucleic Acids Res. 43, 7664–7674 (2015).
Kim, T.-K. et al. Widespread transcription at neuronal activity-regulated enhancers. Nature 465, 182–187 (2010).
Mardinly, A. R. et al. Sensory experience regulates cortical inhibition by inducing IGF1 in VIP neurons. Nature 531, 371–375 (2016).
Ainsley, J. A., Drane, L., Jacobs, J., Kittelberger, K. A. & Reijmers, L. G. Functionally diverse dendritic mRNAs rapidly associate with ribosomes following a novel experience. Nat. Commun. 5, 4510 (2014).
Tayler, K. K., Tanaka, K. Z., Reijmers, L. G. & Wiltgen, B. J. Reactivation of neural ensembles during the retrieval of recent and remote memory. Curr. Biol. 23, 99–106 (2013).
Mudge, J. M. & Harrow, J. Creating reference gene annotation for the mouse C57BL6/J genome assembly. Mamm. Genome 26, 366–378 (2015).
McDonald, A. J. Cortical pathways to the mammalian amygdala. Prog. Neurobiol. 55, 257–332 (1998).
Shi, C. J. & Cassell, M. D. Cortical, thalamic, and amygdaloid projections of rat temporal cortex. J. Comp. Neurol. 382, 153–175 (1997).
Romanski, L. M. & LeDoux, J. E. Equipotentiality of thalamo-amygdala and thalamo-cortico-amygdala circuits in auditory fear conditioning. J. Neurosci. 12, 4501–4509 (1992).
Boatman, J. A. & Kim, J. J. A thalamo-cortico-amygdala pathway mediates auditory fear conditioning in the intact brain. Eur. J. Neurosci. 24, 894–900 (2006).
Eguchi, M. & Yamaguchi, S. In vivo and in vitro visualization of gene expression dynamics over extensive areas of the brain. Neuroimage 44, 1274–1283 (2009).
Montag-Sallaz, M. & Montag, D. Learning-induced arg 3.1/arc mRNA expression in the mouse brain. Learn. Mem. 10, 99–107 (2003).
Rudinskiy, N. et al. Orchestrated experience-driven Arc responses are disrupted in a mouse model of Alzheimer’s disease. Nat. Neurosci. 15, 1422–1429 (2012).
Hempel, C. M., Sugino, K. & Nelson, S. B. A manual method for the purification of fluorescently labeled neurons from the mammalian brain. Nat. Protoc. 2, 2924–2929 (2007).
Okaty, B. W., Sugino, K. & Nelson, S. B. A quantitative comparison of cell-type-specific microarray gene expression profiling methods in the mouse brain. PLos One 6, e16493 (2011).
Tasic, B. et al. Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat. Neurosci. 19, 335–346 (2016).
Peter, M. et al. Induction of immediate early genes in the mouse auditory cortex after auditory cued fear conditioning to complex sounds. Genes Brain Behav. 11, 314–324 (2012).
Letzkus, J. J. et al. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature 480, 331–335 (2011).
Lacar, B. et al. Nuclear RNA-seq of single neurons reveals molecular signatures of activation. Nat. Commun. 7, 11022 (2016).
Hengen, K. B., Torrado Pacheco, A., McGregor, J. N., Van Hooser, S. D. & Turrigiano, G. G. Neuronal Firing Rate Homeostasis Is Inhibited by Sleep and Promoted by Wake. Cell, 10.1016/j.cell.2016.01.046 (2016).
Keck, T. et al. Synaptic scaling and homeostatic plasticity in the mouse visual cortex in vivo . Neuron 80, 327–334 (2013).
Shepherd, J. D. et al. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron 52, 475–484 (2006).
Maffei, A. & Turrigiano, G. G. Multiple modes of network homeostasis in visual cortical layer 2/3. J. Neurosci. 28, 4377–4384 (2008).
Desai, N. S., Rutherford, L. C. & Turrigiano, G. G. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat. Neurosci. 2, 515–520 (1999).
Yassin, L. et al. An embedded subnetwork of highly active neurons in the neocortex. Neuron 68, 1043–1050 (2010).
Barth, A. L., Gerkin, R. C. & Dean, K. L. Alteration of neuronal firing properties after in vivo experience in a FosGFP transgenic mouse. J. Neurosci. 24, 6466–6475 (2004).
Sanders, J., Cowansage, K., Baumgärtel, K. & Mayford, M. Elimination of dendritic spines with long-term memory is specific to active circuits. J. Neurosci. 32, 12570–12578 (2012).
Zhang, W. & Linden, D. J. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat. Rev. Neurosci. 4, 885–900 (2003).
Kandel, E. R. The molecular biology of memory storage: a dialogue between genes and synapses. Science 294, 1030–1038 (2001).
Alberini, C. M. & Kandel, E. R. The regulation of transcription in memory consolidation. Cold Spring Harb. Perspect. Biol. 7, a021741 (2015).
Gruene, T. et al. Activity-dependent structural plasticity after aversive experiences in amygdala and auditory cortex pyramidal neurons. Neuroscience 328, 157–164 (2016).
Paxinos, G. & Franklin, K. B. J. The Mouse Brain in Stereotaxic Coordinates. (Gulf Professional Publishing, 2004).
Grant, G. R. et al. Comparative analysis of RNA-Seq alignment algorithms and the RNA-Seq unified mapper (RUM). Bioinformatics 27, 2518–2528 (2011).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Huang, D. W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009).
Zeisel, A. et al. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 347, 1138–1142 (2015).
Acknowledgements
We thank Suzuko Yorozu for her technical contributions to RNA-Seq. We thank Ben Okaty for assistance with RNA-Seq and for comments on the manuscript. JHC is supported by a postdoctoral fellowship from the Canadian Institute of Health Research. This work was supported by R21 MH104785 and R01 MH101528.
Author information
Authors and Affiliations
Contributions
J.-H.C. and J.M.G. designed the project; J.-H.C. and B.S.H. performed excitotoxic NMDA lesions. J.-H.C. performed and analyzed histological and behavioral experiments. J.-H.C. and J.M.G. performed RNA-seq and J.M.G. performed bioinformatics analysis. J.-H.C. and J.M.G. wrote the manuscript with help from B.S.H.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Cho, JH., Huang, B. & Gray, J. RNA sequencing from neural ensembles activated during fear conditioning in the mouse temporal association cortex. Sci Rep 6, 31753 (2016). https://doi.org/10.1038/srep31753
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep31753
This article is cited by
-
Persistent transcriptional programmes are associated with remote memory
Nature (2020)
-
The lateral neocortex is critical for contextual fear memory reconsolidation
Scientific Reports (2019)
-
Engram-specific transcriptome profiling of contextual memory consolidation
Nature Communications (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.