Abstract
An imbalanced immune system has long been known to influence a variety of mood disorders including anxiety, obsessive-compulsive disorders and depression. In this study, we sought to model the impact of an immunocompromised state on these emotional behaviors using RAG-1−/− mice, which lack T and B cells. We also investigated the relative contribution of CD4+ or CD8+ T cells to these manifestations using RAG-1−/−/OT-II and RAG-1−/−/OT-I transgenic mice, respectively. Our results show that RAG-1−/− mice present a significant increase in digging and marble-burying activities compared with wild-type mice. Surprisingly, these anxiety-like behaviors were significantly reverted in RAG-1−/−/OT-II but not RAG-1−/−/OT-I transgenic mice. Immunodepletion experiments with anti-CD4 or anti-CD8 in C57/BL6 mice or repopulation studies in RAG-1−/− mice did not reproduce these findings. Microarray analysis of the brain of RAG-1−/− and RAG-1−/−/OT-II mice revealed a significantly different gene fingerprint, with the latter being more similar to wild-type mice than the former. Further analysis revealed nine main signaling pathways as being significantly modulated in RAG-1−/− compared with wild-type mice. Taken together, these results suggest that life-long rather than transient immunodeficient conditions influence the emotional behaviors in mice. Most interestingly, these effects seem to correlate with a specific absence of CD4+ rather than CD8+ T cells. Validation of these findings in man might provide new clues on the mechanism by which early life immune modulation might impact mood response in adults and provide a further link between immune and emotional well-being.
Similar content being viewed by others
Introduction
A correlation between mental diseases and immune dysfunction has been reported and debated in the literature since the late 1980s.1 Indeed, direct and indirect evidences in both human and animal experimental systems indicate that changes in the immune repertoire significantly influence cognitive functions2, 3 and neurodegeneration.4, 5, 6 More recent studies also suggest that a healthy immune system is a prerequisite for a balanced and functional emotional response.7, 8, 9, 10
The link between emotion and immunity has been documented in a variety of studies addressing psychosocial changes in patients treated with immunosuppressive drugs. Cyclosporine, a drug widely used in organ transplantation, has been shown to induce a range of neuropsychological problems ranging from depression to anxiety.11, 12, 13, 14, 15 Similarly, other studies described psychological side effects like anxiety, depression and obsessive-compulsive disorders in patients treated with a variety of structurally unrelated immunosuppressant including methotrexate,16 azathioprine,17 and chemotherapy.18
The recombination-activating gene RAG-1 encodes proteins necessary for immunoglobulin and T-cell receptor gene recombination. RAG-1-deficient mice have small lymphoid organs that do not contain mature B and T lymphocytes.19 Seminal work on RAG-1−/− mice by Cushman and co-workers20 reported an increased locomotor activity, reduced levels of fearfulness and no differences in spatial learning and memory. However, given the shared expression of the RAG-1 protein by lymphocytes and central nervous system tissues, the authors concluded their seminal paper stating: ‘Whether these changes are due to the loss of RAG-1 gene expression in the brain, the result of the absence of the RAG-1 gene in the immune system or some combination of both effects remains to be determined in future research’.
To address this question, McGowan et al.21 used a very elegant approach to assess the function of RAG-1 in the central nervous system and dissect it from the lack of T and B cells in the periphery.21 The authors compared the behavior of RAG-1 toRAG-2-deficient mice and found an impaired social recognition memory in the first but not the latter. Because both lines are immunodeficient and RAG-2 is not expressed in the brain, the authors claimed a specific function of RAG-1 in controlling memory formation.
In this study, we expanded on these notions and explored first if the immunodeficient state of RAG-1−/− mice had any influence on their emotional behavior and second the specific contribution of CD4+ or CD8+ T cells to these changes using RAG-1−/−/OT-II and the RAG-1−/−/OT-I transgenic lines, respectively. Second, we explored possible changes in mood-modifying circulating factors or gross brain structural differences in these mice. The results obtained suggest that CD4+ but not CD8+ T cells are capable of partially reverting anxiety-like behavior and lack of self-care characteristic of immunodeficient mice. Interestingly, these behavioral changes were mirrored in specific brain gene fingerprint. These unexpected new findings might provide a mechanistic explanation for the increased emotional distress observed in patients suffering from a wide variety of immune disorders.
Materials and methods
Mice
We used 5-week-old male mice for all the experiments. Mice were housed in groups of six per cage under specific pathogen-free conditions and with free access to food and water. Mice were housed for at least 7 days before testing. Wild-type (WT) C57BL/6 mice purchased from Charles River (Manston, UK) or RAG-1+/+ littermate were used as control. B6.SJL-Ptprca Pepcb/BoyJ-Tg(TcraTcrb)1100Mjb/J-B6.129S7-Rag1tm1Mom (RAG−/−/OT-I) and B6.SJL-Ptprca Pepcb/BoyJ-Tg(TcraTcrb)425Cbn/J-B6.129S7-Rag1tm1Mom (RAG−/−/OT-II) mice were kindly provided by Professor Hans Stauss (University College London, London, UK) and bred in our animal facility. Apart from the nest construction test, all experiments were performed during the light phase of the light–dark cycle, and no more than two tests per day were performed. All tests were conducted in a blinded manner and according to the UK Animals (Scientific Procedures) Act, 1986.
Flow cytometric analysis
Thymocytes and lymphocytes were stained in 100 μl of fluorescence-activated cell sorting buffer (phosphate-buffered saline containing 5% fetal calf serum and 0.02% of NaN2). The antibodies used were anti-CD3 phycoerythrin (clone 145-2C11), anti-CD4 fluorescein isothiocyanate (clone GK 1.5), anti-CD8 Cy5 (clone 53-6.7) (all from eBioscience, San Diego, CA, USA). Cells were labeled with the appropriate concentration of conjugated antibodies for 1 h at 4 °C as described previously.22 After labeling, cells were washed and analyzed. In all experiments, stained cells were acquired with FACScalibur flow cytometer and analyzed using the FlowJoTM software (Tree Star, Ashland, OR, USA, Oregon Corporation).
In vivo T-cell depletion
Male C57/BL6 mice (6 weeks old) received an intraperitoneal injection of anti-CD4 (250 μg; clone GK1.5; BioLegend) or anti-CD8 (250 μg; clone 53-6.7; BioLegend, San Diego, CA, USA) or immunoglobulin G control. T-cell immunodepletion was verified by staining peripheral blood mononuclear cells at different time points (Day 2, Day 5 and Day 7) after the treatment. Briefly, blood samples were collected by intracardiac puncture in syringes containing sodium citrate 3.2% (w v−1). The cells were centrifuged to pellet at 300 g and resuspended in fluorescence-activated cell sorting buffer containing 1:500 Fc blocking (anti-mouse CD16/32) and stained with anti-CD4 or anti-CD8. Red blood cells were lysed with RBC Lysis Buffer according to the manufacturer’s instruction (eBioscience).
RAG1−/− repopulation studies
Purified CD4 or CD8 T cells were obtained from male C57/BL6 mice (6 weeks old) by negative selection following the manufacturer’s instructions (Dynabeads® Untouched™ Mouse CD8 Cells and Dynabeads® Untouched™ Mouse CD4 Cells; Invitrogen, Invitrogen Life Technologies Ltd, Paisley, UK). Purity was tested by fluorescence-activated cell sorter and was >98%. Cells were resuspended in phosphate-buffered saline (2 × 106/300 l) and transferred into male RAG1−/− mice (6 weeks old) by intraperitoneal injection.
Digging and marble-burying tests
Marble-burying and digging tests were carried out as described previously23 with some modifications. Briefly, mice were individually placed in a clear plastic box (14 cm × 10 cm × 11 cm) filled with approximately 5-cm-deep wood chip bedding lightly pressed to give a flat surface. The same bedding substrate was used for all the mice and flattened after each test. Fifteen glass marbles were placed on the surface in five rows of three marbles each. The latency to start digging, the number of digging bouts and the number of buried marbles (to 2/3 their depth) were recorded during the 15-min test. Two trials were performed, the second trial taking place 24 h after the first trial.
Open field activity test
The open filed test was performed as described previously with some modifications.24 The open field consisted of a white PVC arena (50 cm × 30 cm) divided into 10 cm × 10 cm squares (n=15). Mice were brought into the experimental room 15 min before testing. Each mouse was placed in one of the corner squares facing the wall, observed and recorded for 5 min. The total number of squares crossed, latency to the first rear and the total number of rears were recorded. After each test, the arena was cleaned with water to attenuate and homogenize olfactory traces. Two trials were performed, the second trial taking place 24 h after completion of the first trial.
Nest construction test
The nest construction test was performed as described previously.25 Mice were transferred into individual cages 1 h before the dark phase (1700 hours) and individually housed overnight. The results were assessed the next morning. Food, water and wood chip bedding were provided. No other environmental enrichment was added. One 2–3 g, 5 cm × 5 cm pressed cotton square (nestlet; Ancare, Ancare Bellmore, NY, USA) was placed in each cage. The weight of nesting material shredded was calculated by weighing the nestlet before and after the overnight test. The quality of the nest was evaluated on a five-point scale as detailed in Deacon.25
Plasma corticosterone and cytokine measurement
Blood was collected by intracardiac puncture performed under anesthesia. Serum was obtained from the clotted blood by centrifugation (8000 r.p.m., 5 min) and stored at −80 °C before the assay. Corticosterone concentrations were measured in diluted (1:32) plasma by EIA assay following the manufacturer’s instructions (Enzo Life Sciences, Exeter, UK). Cytokine levels in the same samples were measured (dil. 1:100) using mouse T-helper type 1 (Th1)/Th2/Th17/Th22 16plex Kit Flowcytomix and according to the manufacturer’s instructions (eBioscience).
Histology
Brains were collected either before or at the end of experiments and fixed in 4% paraformaldehyde for 72 h. Thereafter, tissues were sectioned on a sagittal or coronal plane and embedded in paraffin by our in-house histology facility. Sections (5 μm) were deparaffinized and stained with hematoxylin and eosin. In all cases, a minimum of three sections per animal was evaluated. Phase-contrast digital images were taken using the Image Image-Pro (Media Cybernetics, Rockville, MD, USA) analysis software package.
Microarray analysis
Total RNA was extracted from brains of WT (n=3), RAG−/− (n=2) and OT-II/RAG−/− (n=2) mice using RNeasy® Microarray Tissue Mini Kit (Qiagen®, West Sussex, UK). Total RNA was hybridized to Affymetrix Mouse Gene 1.0 ST array chips at UCL Genomics (London, UK) with standard Affymetrix protocols, using GeneChip Fluidics Station 450, and scanned using the Affymetrix GeneChip Scanner (Affymetrix, Santa Clara, CA, USA). Data were normalized by rma of the Bioconductor package, affy. Differentially expressed genes were identified by the Bioconductor package, limma, considering the false discovery rate (adjusted P-value <0.05). The gene and sample scoring system was made by canonical correspondence analysis. Canonical correspondence analysis is a variant of correspondence analysis, where the main data are linearly regressed onto explanatory variables (environmental variables), and subsequently the regressed data are analyzed by correspondence analysis. In this study, we regressed the whole data set onto an explanatory variable, which was defined as the difference between ‘average’ WT and ‘average’ RAG-1−/−. Detailed methodology is described elsewhere.26 Signaling pathway impact analysis was performed using the Bioconductor package, SPIA, by comparing WT and RAG-1−/−.
Real-time polymerase chain reaction
Total RNA was extracted from whole brains (n=6 for each mouse line) with RNeasy® Microarray Tissue Mini Kit (Qiagen®) according to the manufacturer’s protocol and reverse transcribed using 2 μg oligo(dT)15 primer, 10 U AMV reverse transcriptase, 40 U RNase inhibitor (all from Promega Corporation, Madison, WI, USA) and 1.25 mM each dNTP (Bioline, London, UK) for 45 min at 42 °C. Real-time polymerase chain reaction was carried out by using ABsoluteTM QPCR ROX Mix (Thermo Scientific, Epsom, UK) and fluorescent QuantiTect primers. Cycling conditions were set according to the manufacturer’s instructions. Sequence-specific fluorescent signal was detected by 7900HT Fast Real-Time PCR System (Applied Biosystems, Warrington, Cheshire, UK). mRNA data were normalized relative to glyceraldehyde 3-phosphate dehydrogenase and then used to calculate expression levels. We used the comparative Ct method to measure the gene transcription in samples. The results are expressed as relative units based on calculation of 2−ΔΔCt, which gives the relative amount of gene normalized to endogenous control (glyceraldehyde 3-phosphate dehydrogenase) and to the sample with the lowest expression set as 1.
Data analysis
Initially, we determined if the data distribution was parametric. Pairwise comparisons were made by t-test and the results expressed as mean±s.e.m. For non-parametric data, the Mann–Whitney U-test was applied, and results were expressed as medians (interquartile range).
Results
Immune repertoire of RAG-1−/− , RAG-1−/− /OT-I and RAG-1−/− /OT-II
Crossing OT-I and OT-II TCR transgenic mice onto RAG-1−/− background generates mice with a single population of mature CD8+ or CD4+ T cells. Figure 1 shows a typical immature and mature T-cell profiles of RAG-1−/−, RAG-1−/−/OT-I and RAG-1−/−/OT-II compared with WT C57BL/6 control mice. Control mice show a typical profile with a 1:2 ratio of CD8+ and CD4+ single-positive T cells in the thymus as well as in the periphery (Figure 1, first top and bottom panels, respectively). As expected, the majority of RAG-1−/− thymocytes are CD4 and CD8 double-negative cells and have no mature CD4 or CD8 single-positive T cells in the periphery (Figure 1, second top and bottom panels, respectively).19 The presence of OT-I and OT-II TCR transgene overcomes the block at the stage of double-negative 3 (DN3) of RAG-1−/− thymocytes and allows the generation of a peripheral T-cell immune repertoire constituted by 72% of CD8+ in RAG-1–/–/OT-I and 65% of CD4+ T cells RAG-1–/–/OT-II mice (Figure 1, third top and bottom panels).27
Immune repertoire of RAG-1−/−, RAG-1−/−/OT-I and RAG-1−/−/OT-II mice. Thymocytes and lymphocytes from male wild-type (WT), RAG-1−/−, RAG-1−/−/OT-I and RAG-1−/−/OT-II mice were analyzed for CD4 and CD8 expression. The dot plots show the T-cell profiles of RAG-1−/−, RAG-1−/−/OT-I and RAG-1−/−/OT-II mice in the thymus (upper panel) and in the periphery (lower panel) compared with WT C57BL/6 control mice. The percentage of the cells in each quadrant is given.
Increased digging and marble-burying behavior of RAG-1−/− mice
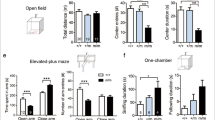
We first investigated differences in anxiety- and obsessive-compulsive-like behavior in WT and RAG-1−/− mice using the digging and marble-burying tests. As shown in Figure 2a, RAG-1−/− mice presented a significant increase (two- to threefold) in the number of digging bouts and buried marble compared with WT mice. The latency to dig was higher in control mice compared with RAG-1−/− mice, although the difference was not significant.
Increased digging and marble-burying behavior of RAG-1−/− mice (upper panel). The bar graphs in a shows the total number of digging bouts, buried marbles and the latency to dig (expressed in seconds) measured during the 15min marble-burying test. The bar graphs in b show the total number of rears, the latency to rear (expressed in seconds) and the total number of squares crossed assessed during the 5-min open field test. Values are expressed as mean±s.e.m. of six mice and are representative of n=3–4 separate experiments. **P<0.01 and ***P<0.001 indicate significant values compared with wild-type (WT) C57BL/6 control mice (Mann–Whitney U-test).
To further corroborate the anxiety-like behavior and simultaneously rule out possible intrinsic impairment in locomotor activity, we used the open field activity test. Here, we considered a number of parameters including exploration (number of rears and latency) and locomotor activity (number of squares crossed). Although RAG-1−/− mice showed a slight reduction compared with the mice in all the parameters observed, these differences were not statistically significant (Figure 2b).
CD4+ but not CD8+ T cells revert the increased digging and marble-burying behavior of RAG-1−/− mice
We next tested the hypothesis that the presence of CD4+ or CD8+ T cells might influence the heightened digging and marble-burying behavior of the RAG-1−/− mice. RAG-1–/–/OT-I showed no difference in either number of bouts, buried marbles or latency compared with RAG-1−/− mice (Figure 3a). In contrast, RAG-1–/–/OT-II behaved differently from RAG-1−/− mice, showing a significant reduction in the number of bouts (about 25%) and buried marbles (about 20%), and almost doubled latency time (P<0.05; Figure 3b).
CD4+ but not CD8+ T cells revert the increased digging and marble-burying behavior of RAG-1−/− mice. The bar graphs show the total number of digging bouts, buried marbles and the latency to dig (expressed in seconds) in RAG-1−/−/OT-I (a) or RAG-1−/−/OT-II (b) compared with RAG-1−/− during the 15-min marble-burying test. Values are expressed as mean±s.e.m. of six mice and are representative of n=3–4 separate experiments. **P<0.01 indicates significant values compared with RAG-1−/− mice (Mann–Whitney U-test).
With the open field test, we observed no difference in the behavior of RAG-1–/–/OT-I and RAG-1–/– (Figure 4a). Similarly, RAG-1–/–/OT-II showed no significant difference in either the number of rears or of squares crossed as compared with RAG-1−/−, except for an increase in the latency, which this time reached a statistical significance (Figure 4b). When we compared the number of center entries (considered an anxiety-like behavior parameter) in the three lines, we observed a significant reduction in RAG-1−/− mice compared with WT mice. However, this difference was not significantly reverted in either RAG-1−/−/OT-I or RAG-1−/−/OT-II (Figure 5).
No differences in the open field activity between RAG-1−/−/OT-I, RAG-1−/−/OT-II and RAG-1−/−. The bar graphs show the total number of rears, the latency to rear (expressed in seconds) and squares crossed in RAG-1−/−/OT-I (a) or RAG-1−/−/OT-II (b) compared with RAG-1−/− during the 5-min open field test. Values are expressed as mean±s.e.m. of six mice and are representative of n=3–4 separate experiments. *P<0.05 indicates significant values compared with RAG-1−/− mice (Mann–Whitney U-test).
CD4+ but not CD8+ T cells might revert the decreased number of center entries showed by RAG-1−/− mice. The bar graphs show the comparison of the entries into the center between RAG-1−/− and wild-type mice (left panel), RAG-1−/− and RAG−/−/OT-I (middle panel) and RAG−/− and RAG−/−/OT-II (right panel) during the 5-min open field test. Values are expressed as mean±s.e.m. of six mice and are representative of n=3–4 separate experiments. **P<0.01 indicates significant values compared with wild-type C57BL/6 control mice (Mann–Whitney U-test).
Transient depletion of CD4+ or CD8+ T cells does not affect the emotional behavior of C57/BL6 mice
We next wondered if we could reproduce the results obtained with RAG-1–/–/OT-I and RAG-1–/–/OT-II using anti-CD4- or anti-CD8-depleting antibodies in C57/BL6 mice. As shown in Figure 6, neither anti-CD4 nor anti-CD8 antibodies significantly modified the digging, or marble-burying activities or the latency to digging (top, middle and bottom panels, respectively) compared with control IgG-treated mice at the indicated times.
Depletion of CD4 or CD8 T cells does not induce anxiety-like behavior in C57/BL6 mice. C57/BL6 mice received an intraperitoneal injection of anti-CD4 (250 μg), anti-CD8- (250 μg) depleting antibodies or IgG control, and then tested in the digging and marble-burying test. The bar graphs show the total number of digging bouts, buried marbles and the latency to dig (expressed in seconds) in mice treated as indicated and assessed before the treatment (day 0) or after 2, 5 and 7 days (day 2, day 5 and day 7, respectively). Values are expressed as mean±s.e.m. of 6–8 mice.
To further confirm these results, we sought to investigate if the repopulation of RAG-1−/− with T cells would rescue the increased anxiety observed in these mice. Consistent with previous results, reconstitution of RAG-1−/− mice with purified CD4 or CD8 T cells did not affect the increase in the number of digging bouts compared with vehicle phosphate-buffered saline-injected mice (Figure 7).
Repopulation of RAG1−/− mice with CD4 or CD8 T cells does not affect their anxiety-like behavior. RAG1−/− mice received an intraperitoneal injection of purified CD4 (2 × 106) or CD8 (2 × 106) T cells and then tested in the digging and marble-burying test. The bar graphs show the total number of digging bouts, buried marbles and the latency to dig (expressed in seconds) in mice assessed before the cell transfer (day 0) or after 4 and 7 days (day 4 and day 7, respectively). Values are expressed as mean±s.e.m. of 6–8 mice.
CD4+ T cells revert the impaired nest construction of RAG-1−/− mice
To explore the impact of T cells on other emotional behavior, we tested the three transgenic lines for their nesting activity, a standard test for measuring activities of daily living. Figure 8a shows representative pictures of the results obtained, whereas in Figure 8b, we report the quantitative results. Measurement of nestlet shredding showed a decreased ability of RAG-1−/− mice to perform this task compared with WT mice (Figure 8b). Similar to previous analysis, this impaired behavior was significantly reverted in RAG-1−/−/OT-II but not RAG-1−/−/OT-I mice (Figure 8b). Comparable results were obtained scoring nest quality (Supplementary Figure 1).
CD4+ but not CD8+ T cells revert the impaired nest construction of RAG-1−/− mice. (a) Representative pictures of the nestlet shredding activity of wild-type (WT), RAG-1−/−, RAG-1−/−/OT-I and RAG-1−/−/OT-II during an overnight test. (b) Quantitative analysis of nestlet shredding activity expressed as grams of nestlet shredded after an overnight test. Values are expressed as mean±s.e.m. of six mice and are representative of n=3–4 separate experiments. §P<0.05 and **P<0.01 indicate significant values compared with WT C57BL/6 control and RAG−/− mice, respectively (Mann–Whitney U-test).
No differences in systemic or gross brain structure between RAG-1−/− , RAG-1–/– /OT-I and RAG-1–/– /OT-II mice
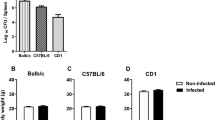
We next investigated whether the behavioral changes observed were due to changes in known behavioral modulating factors such as corticosterone. As shown in Figure 9a, there were no significant differences in the levels of circulating corticosterone between WT and RAG-1−/− mice or between RAG-1−/− and RAG-1−/−/OT-I and RAG-1−/−/OT-II mice.
No differences in corticosterone or inflammatory cytokine serum levels between RAG-1−/−, RAG-1–/–/OT-I and RAG-1–/–/OT-II mice. Levels of corticosterone (a) or interleukin (IL)-17, IL-18 and interferon (IFN)-γ (b) in the serum of wild-type (WT), RAG-1−/−, RAG-1−/−/OT-I and RAG-1−/−/OT-II. Values are expressed as ng ml−1 or as pg ml−1 and are cumulative of n=2–3 experiments.
Cytokines can induce behavioral changes (also known as sickness behavior28), a consequence of their modulatory effects on brain function. When we scanned the same samples for classical Th1, Th2, Th17 and ThGM-CSF cytokines, only interleukin (IL)-17, IL-18 and interferon-γ could be detected. However, none of these mediators was differentially modulated in RAG-1−/− and RAG-1−/−/OT-I and RAG-1−/−/OT-II mice compared with WT mice (Figure 9b), excluding the possibility that cytokines released by T cells or a latent state of infection as being responsible for the behavioral changes observed.
Similarly, analysis of general brain morphology and architecture of WT, RAG-1−/−, RAG-1−/−/OT-I and RAG-1−/−/OT-II mice showed comparable hematoxylin–eosin (Figures 10a–d, respectively) or luxol fast blue staining (data not shown) ruling out any contribution of infiltrated immune cells or general neuronal defect in the altered emotional behavior of tested mice.
No differences in gross brain structure between RAG-1−/−, RAG-1–/–/OT-I and RAG-1–/–/OT-II mice. The pictures show the coronal hematoxylin and eosin -stained sections of brain from wild-type (a), RAG-1−/− (b), RAG-1−/−/OT-I (c) and RAG-1−/−/OT-II (d). The higher magnification represents the hippocampal area. The figures are representative of n=3–4 mice.
Brain gene fingerprint of RAG-1−/− and RAG-1−/− /OT-II
To unveil cellular and molecular mechanisms potentially responsible for the observed changes in emotional behavior, we took an unbiased approach and compared whole brain gene fingerprint of WT, RAG-1−/−, RAG-1−/−/OT-II mice. The flowchart in Figure 11a summarizes the results of this analysis. From the 34 760 probes present in the chip, 6635 were significantly modulated (P<0.05). This corresponded to 782 differentially expressed genes with a fold change (FC) value <−1 or >1, and 111 of them were annotated genes (genes with Entrez ID.)
Heatmap and Canonical Correspondence Analysis on Microarray data of brain from wild-type (WT), RAG-1−/− and RAG-1–/–/OT-II mice. (a) Genes were filtered by a moderated t-statistics and fold change (FC). The heatmap analysis used annotated genes only (genes with EntrezID). (b) Hierarchical clustering and heatmap analysis of the filtered genes. RAG-1−/− samples showed a distinct cluster. (c) Similarity analysis for the features of RAG-1−/− in comparison with WT. Note that RAG-1−/−/OT-II mice had low scores and were equivalent to WT mice.
Hierarchical clustering and heatmap analysis of these selected 111 genes and brain samples (Figure 11b and Table 1) showed that RAG-1−/− mice showed a distinct pattern of gene expression compared with RAG-1−/−/OT-II and WT mice. This result also suggested that RAG-1−/−/OT-II was similar to WT at the transcriptomic level. To further determine whether and how much RAG-1−/−/OT-II was more similar to WT than RAG-1−/−, we analyzed the similarities between the samples based on the gene expression pattern, focusing on the difference between WT and RAG-1−/−, which is our major interest in this study (see Materials and methods). The similarity analysis using these 111 genes showed that RAG-1−/−/OT-II was far more similar to WT than RAG-1−/− (Figure 11c). Importantly, the result of this similarity analysis was very similar using all the differentially expressed genes (data not shown), indicating that the result is robust and not dependent on the selection of genes by FC and annotation. These results were compatible with those of the behavioral analysis.
Pathway analysis provided further clues on the main differences between WT and RAG-1−/−. Using a third-generation pathway analysis approach,29 nine pathways were identified as been significantly modulated in RAG-1−/− compared with WT: two being activated (Parkinson’s disease and RNA transport) and seven inhibited (Huntington’s diseases, Alzheimer’s disease, extracellular matrix–receptor interaction, olfactory transduction, focal adhesion, calcium signaling and small-cell lung cancer; summarized in Table 2 and reported singularly in Supplementary Figures 2–10).
Discussion
The idea that a balanced mental state is a directly associated with general well-being can be traced back to the time of Decimus Iunius Iuvenalis. He was the first to state that a healthy mind is found in a healthy body (‘mens sana in corpore sano’). However, evidences gathered in our modern time suggests also the reverse, that is, that a corpore sano, and in particular a healthy immune system, might contribute to our mental well-being. In this study, we sought to provide direct experimental evidence of this hypothesis investigating first the emotional behavior of mice genetically void of T and B cells (the RAG-1−/− mice) and secondly assessing the specific contribution of CD4+ or CD8+ T cells.
Our results show a significant increase in anxiety-like behavior in RAG-1−/− mice as evaluated by the number of center entries in the open field test as well as the increased digging and marble-burying activities. Most interestingly, we also observed that CD4+ but not CD8+ T cells are able to revert significantly the exaggerated emotional response of RAG-1−/− mice. These results were not due to T-cell activation upon exposure of the mice to the behavioral paradigms (Supplementary Figure 11). The notion that CD4+ T cells has a preferential role as ‘mood stabilizer’ compared with CD8+ T cells has long been suggested. Early studies on adult patients suffering from anxiety and obsessive-compulsive disorders have demonstrated immunological alterations including a significant increase of CD8+ and decrease of CD4+ lymphocytes compared with the healthy control group.30 The same abnormality has been observed in patients with autism, a disorder characterized also by obsessive-compulsive symptoms and anxiety disorders.31 A recent study has also demonstrated in a quantitative manner an inverse relationship between CD4 count and hospital-associated anxiety and depression.32 Finally, in one of his recent review AH Miller33 provided a comprehensive account of the multiple links between CD4+ T cells and depression, highlighting the importance of trafficking of T cells to the brain following stress as a way to reduce stress-induced anxiety-like behavior.33 However, a direct functional association between T cells and altered emotional behavior is still missing.
One of the major drawbacks in the life of patients suffering from anxiety-like behavior and/or obsessive-compulsive disorders is their inability to perform normal daily activities. Insightful epidemiological studies suggest that this ‘inability to cope’ might be due to an emotional rather than a cognitive impairment.34 Our results are in line with this hypothesis and show a significant reduction in self-caring of RAG-1−/− mice compared with WT mice, as evaluated by the nesting test. Although these results suggest a link between self-neglect, anxiety-like behavior and immune suppression, more studies are needed to corroborate this hypothesis. These could include burrowing and hoarding, two other methods to test and quantify experimentally daily living activities.35
Nestlet shredding together with marble burying is also a reliable experimental model for obsessive-compulsive disorders and anxiety.36 We could not observe a significant difference in nestlet shredding after 60 min (data not shown), whereas we found a dramatic reduction in nest construction after an overnight test. We are tempted to explain these findings with the fact that, contrary to innate cells, adaptive immune cells are more involved in chronic disease and hence they usually exert their functions over a long period of time. In a similar way, one might speculate that immunosuppressed patients might present more difficulties in coping with long-term illness while being able to face problems as they came in.
Textbook immunology reports CD4+ T cells as ‘helper’ cells because of their ability to modulate other cell functions, and these ‘helping’ properties may go beyond antigen-presenting cell-mediated immunity. Groundbreaking studies by Kipnis and Schwartz37, 38, 39 has put CD4+ T cells at the center stage of neuroimmunology.2 Repopulation of scid mice with T cells from WT donors has been shown to improve significantly the impaired cognitive functions of these mice.40 Most interestingly, circulating and patrolling CD4+ T cells have been reported to convey constantly protective signaling to the brain, thus contributing to what is now known as ‘protective autoimmunity’.41, 42, 43, 44 In light of this concept, antigen-specific T cells, like myelin basic protein T-cell clones,45 circulate through the brain and sustain key neuronal processes and function such as neurogenesis, cognition and memory.39 Considering that the OT-II TCR transgene recognizes a non-endogenous antigenic peptide like OVA323–339, it is tempting to speculate that, at difference from cognition and memory, emotional behavior might require a less-stringent condition, that is, the simple presence of circulating CD4+ T cells. Indeed, their circulation through the brain or meningeal spaces, as suggested by Derecki et al.,46 might be enough to restore the emotional impairment that flares in immunocompromised conditions.46
Previous studies assessing the role of T cells on brain functions have used immunodepleting antibodies or cellular replacement in immunodeficient host like the scid mice.40 When we adopted similar approaches in our settings, we could not find any significant differences. Neither the depletion of T cells in C57/BL6 mice using anti-CD4 or anti-CD8 antibodies nor the repopulation of RAG-1−/− with purified CD4 or CD8 T cells had any effect on emotional behavior. The reason behind this apparent discrepancy might lay in the nature of the immunodeficient state that has been studied. In the RAG-1−/− mice, we have investigated how the absence of immune cells from prenatal development to adulthood influences neuronal networks and emotional behavior, whereas the immunodepletion or repopulation experiments refer to transient conditions (up to 2 weeks in our tests). This is quite an important aspect when one considers that neuropsychiatric disorders have often been linked to problems occurring at the developmental stage.47, 48, 49 Inflammation and infection at pre- and perinatal stages have been proved to be as powerful as maternal stress and trauma in causing long-term consequences on neuronal development and mental health.50, 51, 52 These clinical evidences hold true in experimental settings. Both peri- and prenatal administration of immunomodulatory agents such as TLR3 ligands and viral mimic polyinosine-polycytidylic, or TLR4 ligand and bacterial surrogate lipopolysaccharide, induced the development of schizophrenia- and autism-related behavioral changes including decreased exploratory activity and social interaction as adults.53, 54, 55, 56, 57, 58
Previous studies have shown that, like in pregnancy,59 CD4+ T cells with a skewed Th2 phenotype contribute to a controlled and trophic microenvironment in the brain upregulating neurotrophic factors such as glial-derived neurotrophic factor, brain-derived neurotrophic factor and insulin growth factor 1, or suppressing inflammatory mediators like tumor necrosis factor-α and IL-6.46, 60 Conversely, direct and indirect evidence have linked Th1 and Th17 cells to emotional disorders. T cells from individuals with generalized anxiety disorders show an enhanced capability to differentiate in Th17 cells.61, 62 Experimental evidences also suggest that Th17 cells preferentially accumulate in the brain of mice subjected to chronic restrain stress, whereas mice deficient in the RORγT (transcription factor necessary for Th17 differentiation) exhibited resistance to learned helplessness.63 T-bet (transcription factor necessary for Th1 differentiation) knockout mice show significantly reduced depressive-like behaviors provoked by repeated restraint stress.64 This is in line with clinical studies showing, for instance, the contribution of Th1 cytokines to the pathogenesis of neuropsychiatric manifestations of systemic lupus erythematosus.65 In light of these findings, we decided to investigate whether the absence or presence of circulating CD4+ T cells would impact neuronal gene development. Our microarray analysis provided us with a number of interesting findings.
A large number of micro-RNA(s) was found among the genes that were mostly downregulated. These gene expression modulators have been recently highlighted for their role in mental heath and are becoming increasingly popular in this field.66 Literature search for known targets of the micro-RNA we have identified provided us with few indications (Table 3). Further studies are needed to investigate the functions of these micro-RNA and their role in emotional behavior. Several quantitative polymerase chain reaction validated genes (data not shown) in our screening are known to control a variety of neuropsychological conditions. Synuclein-γ has been associated with Parkinson’s disease67 and reported to be implicated in both cognitive and emotional functions.68 Von Willebrand factor has been shown to be significantly increased in schizophrenia,69, 70, 71 whereas changes in polycystic kidney disease 1 and tetratricopeptide repeat and ankyrin repeat containing 1) expression have been associated with bipolar disorders.72, 73, 74 S100a10, a recently suggested potential biomarker for suicide risk in mental disorders,75 was upregulated in RAG-1−/− and downregulated in RAG-1−/−/OT-II to WT level. Other interesting differentially expressed genes included ephrin type-B receptor 1 (Ephb1), whose genetic deletion in mice causes neuronal loss in the substantia nigra and spontaneous locomotor hyperactivity,76 myeloid/lymphoid or mixed-lineage leukemia 2 (Mll2), whose activity is required for memory formation,77 and Churchill domain containing protein 1 (Churc1), a neuronal development gene implicated with the occurrence of autism.78
Some of the implications of these changes in gene expression have been investigated with SPIA pathway analysis software. This showed inhibition of signaling pathways that control neurodegenerative disorders, such as Alzheimer’s and Huntington’s disease, in WT compared with RAG-1−/− mice further supporting the emerging view of these diseases as endowed with an autoimmune-related diseases component.79, 80, 81 Further differences were observed in a wide range of neuronal, sensory and basic cellular pathways that will be explored in future studies to detail the complex crosstalk between the neuronal and immune systems.
In conclusion, the results of this study shed new light on the complex crosstalk between the immune system and our emotional well-being, although future investigations are needed to corroborate our hypothesis. In fact, it would be interesting to explore the possible contribution of B cells to the emotional behavior of the RAG-1−/− mice as well as to confirm these results in mice expressing TCR transgene with different strength of signaling. Equally important, one might speculate the existence of CD4+ T-cell-specific factors that control emotional behaviors and their exploitation for the treatment of wide variety of mental disorders.
Beyond these experimental questions, the most important challenge for the future is to understand how T cells influence behavior and vice versa. The answer might lay in shared signaling pathway like RAG or the immune synapse: a signaling complex that has been named after the neuromuscular synapse and that allows the exchanges of information between antigen-presenting cells and T cells.82, 83, 84 Along these lines, recent studies have shown the existence of a subset of memory T cell in mice that produces acetylcholine in response to noradrenaline providing another way by which the immune system communicate with the nervous system.85, 86 Taken together, these findings might help the design of new therapies for mental health by restoring an impaired or absent immune system as observed in several autoimmune diseases.
References
Irwin M, Patterson T, Smith TL, Caldwell C, Brown SA, Gillin JC et al. Reduction of immune function in life stress and depression. Biol Psychiatry 1990; 27: 22–30.
Ziv Y, Schwartz M . Immune-based regulation of adult neurogenesis: implications for learning and memory. Brain Behav Immun 2008; 22: 167–176.
Yirmiya R, Goshen I . Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun 2011; 25: 181–213.
Aktas O, Kieseier B, Hartung HP . Neuroprotection, regeneration and immunomodulation: broadening the therapeutic repertoire in multiple sclerosis. Trends Neurosci 2010; 33: 140–152.
German DC, Eagar T, Sonsalla PK . Parkinson’s disease: a role for the immune system. Curr Mol Pharmacol 2011 (e-pub ahead of print).
Lucin KM, Wyss-Coray T . Immune activation in brain aging and neurodegeneration: too much or too little? Neuron 2009; 64: 110–122.
Littrell J . The mind–body connection: not just a theory anymore. Soc Work Health Care 2008; 46: 17–37.
Costa-Pinto FA, Palermo-Neto J . Neuroimmune interactions in stress. Neuroimmunomodulation 2010; 17: 196–199.
Rook GA, Lowry CA, Raison CL . Lymphocytes in neuroprotection, cognition and emotion: is intolerance really the answer? Brain Behav Immun 2011; 25: 591–601.
Barak Y . The immune system and happiness. Autoimmun Rev 2006; 5: 523–527.
Sato Y, Takayanagi Y, Onaka T, Kobayashi E . Impact of cyclosporine upon emotional and social behavior in mice. Transplantation 2007; 83: 1365–1370.
von Horsten S, Exton MS, Schult M, Nagel E, Stalp M, Schweitzer G et al. Behaviorally conditioned effects of cyclosporine A on the immune system of rats: specific alterations of blood leukocyte numbers and decrease of granulocyte function. J Neuroimmunol 1998; 85: 193–201.
von Horsten S, Exton MS, Voge J, Schult M, Nagel E, Schmidt RE et al. Cyclosporine A affects open field behavior in DA rats. Pharmacol Biochem Behav 1998; 60: 71–76.
Bechstein WO . Neurotoxicity of calcineurin inhibitors: impact and clinical management. Transpl Int 2000; 13: 313–326.
Wijdicks EF . Neurotoxicity of immunosuppressive drugs. Liver Transpl 2001; 7: 937–942.
van der Meer A, Wulffraat NM, Prakken BJ, Gijsbers B, Rademaker CM, Sinnema G . Psychological side effects of MTX treatment in juvenile idiopathic arthritis: a pilot study. Clin Exp Rheumatol 2007; 25: 480–485.
van der Hoeven J, Duyx J, de Langen JJ, van Royen A . Probable psychiatric side effects of azathioprine. Psychosom Med 2005; 67: 508.
Pandey M, Sarita GP, Devi N, Thomas BC, Hussain BM, Krishnan R . Distress, anxiety, and depression in cancer patients undergoing chemotherapy. World J Surg Oncol 2006; 4: 68.
Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE . RAG-1-deficient mice have no mature B and T lymphocytes. Cell 1992; 68: 869–877.
Cushman J, Lo J, Huang Z, Wasserfall C, Petitto JM . Neurobehavioral changes resulting from recombinase activation gene 1 deletion. Clin Diagn Lab Immunol 2003; 10: 13–18.
McGowan PO, Hope TA, Meck WH, Kelsoe G, Williams CL . Impaired social recognition memory in recombination activating gene 1-deficient mice. Brain Res 2011; 1383: 187–195.
Paschalidis N, Huggins A, Rowbotham NJ, Furmanski AL, Crompton T, Flower RJ et al. Role of endogenous annexin-A1 in the regulation of thymocyte positive and negative selection. Cell Cycle 2010; 9: 784–793.
Deacon RM . Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nat Protoc 2006; 1: 122–124.
Deacon RM . Housing, husbandry and handling of rodents for behavioral experiments. Nat Protoc 2006; 1: 936–946.
Deacon RM . Assessing nest building in mice. Nat Protoc 2006; 1: 1117–1119.
Ono M, Tanaka RJ, Kano M, Sugiman T . Visualising the cross-level relationships between pathological and physiological processes and gene expression: analyses of haematological diseases. PLoS One 2013; 8: e53544.
Barnden MJ, Allison J, Heath WR, Carbone FR . Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol 1998; 76: 34–40.
Sharpley CF, Agnew LL . Cytokines and depression: findings, issues, and treatment implications. Rev Neurosci 2011; 22: 295–302.
Tarca AL, Draghici S, Khatri P, Hassan SS, Mittal P, Kim JS et al. A novel signaling pathway impact analysis. Bioinformatics 2009; 25: 75–82.
Marazziti D, Presta S, Pfanner C, Gemignani A, Rossi A, Sbrana S et al. Immunological alterations in adult obsessive-compulsive disorder. Biol Psychiatry 1999; 46: 810–814.
Yonk LJ, Warren RP, Burger RA, Cole P, Odell JD, Warren WL et al. CD4+ helper T cell depression in autism. Immunol Lett 1990; 25: 341–345.
Zena-Castillo D, Mezones-Holguin E, Valdiviezo-Garcia G, La-Chira-Alban A, Rodriguez-Morales AJ, Dickson-Gonzalez S . Impact of hospital-associated anxiety and depression on the CD4 counts of naive HIV/AIDS patients from locations in Northern Peru. Int J Infect Dis 2009; 13: e75–e76.
Miller AH . Depression and immunity: a role for T cells? Brain Behav Immun 2010; 24: 1–8.
Huppert JD, Simpson HB, Nissenson KJ, Liebowitz MR, Foa EB . Quality of life and functional impairment in obsessive-compulsive disorder: a comparison of patients with and without comorbidity, patients in remission, and healthy controls. Depress Anxiety 2009; 26: 39–45.
Deacon R . Assessing burrowing, nest construction, and hoarding in mice. J Vis Exp 2012; 59: e2607.
Li X, Morrow D, Witkin JM . Decreases in nestlet shredding of mice by serotonin uptake inhibitors: comparison with marble burying. Life Sci 2006; 78: 1933–1939.
Schwartz M . The emergence of a new science of the mind: immunology benefits the mind. Mol Psychiatry 2010; 15: 337–338.
Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M . T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Natl Acad Sci USA 2004; 101: 8180–8185.
Kipnis J, Gadani S, Derecki NC . Pro-cognitive properties of T cells. Nat Rev Immunol 2012; 12: 663–669.
Brynskikh A, Warren T, Zhu J, Kipnis J . Adaptive immunity affects learning behavior in mice. Brain Behav Immun 2008; 22: 861–869.
Kipnis J, Mizrahi T, Hauben E, Shaked I, Shevach E, Schwartz M . Neuroprotective autoimmunity: naturally occurring CD4+CD25+ regulatory T cells suppress the ability to withstand injury to the central nervous system. Proc Natl Acad Sci USA 2002; 99: 15620–15625.
Schwartz M, Kipnis J . Protective autoimmunity and neuroprotection in inflammatory and noninflammatory neurodegenerative diseases. J Neurol Sci 2005; 233: 163–166.
Schwartz M, Ziv Y . Immunity to self and self-maintenance: a unified theory of brain pathologies. Trends Immunol 2008; 29: 211–219.
Schwartz M, Shechter R . Protective autoimmunity functions by intracranial immunosurveillance to support the mind: The missing link between health and disease. Mol Psychiatry 2010; 15: 342–354.
Barouch R, Schwartz M . Autoreactive T cells induce neurotrophin production by immune and neural cells in injured rat optic nerve: implications for protective autoimmunity. FASEB J 2002; 16: 1304–1306.
Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR et al. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med 2010; 207: 1067–1080.
Aas M, Navari S, Gibbs A, Mondelli V, Fisher HL, Morgan C et al. Is there a link between childhood trauma, cognition, and amygdala and hippocampus volume in first-episode psychosis? Schizophr Res 2012; 137: 73–79.
Aas M, Dazzan P, Fisher HL, Morgan C, Morgan K, Reichenberg A et al. Childhood trauma and cognitive function in first-episode affective and non-affective psychosis. Schizophr Res 2011; 129: 12–19.
Pawlby S, Hay D, Sharp D, Waters CS, Pariante CM . Antenatal depression and offspring psychopathology: the influence of childhood maltreatment. Br J Psychiatry 2011; 199: 106–112.
Monk C, Fitelson EM, Werner E . Mood disorders and their pharmacological treatment during pregnancy: is the future child affected? Pediatr Res 2011; 69 (Part 2): 3R–10R.
Harvey L, Boksa P . Prenatal and postnatal animal models of immune activation: relevance to a range of neurodevelopmental disorders. Dev Neurobiol 2012; 72: 1335–1348.
Schlotz W, Phillips DI . Fetal origins of mental health: evidence and mechanisms. Brain Behav Immun 2009; 23: 905–916.
Ellis S, Mouihate A, Pittman QJ . Neonatal programming of the rat neuroimmune response: stimulus specific changes elicited by bacterial and viral mimetics. J Physiol 2006; 571 (Pt 3): 695–701.
Hsiao EY, McBride SW, Chow J, Mazmanian SK, Patterson PH . Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc Natl Acad Sci USA 2012; 109: 12776–12781.
Hsiao EY, Patterson PH . Placental regulation of maternal–fetal interactions and brain development. Dev Neurobiol 2012; 72: 1317–1326.
Hodyl NA, Walker FR, Krivanek KM, Clifton VL, Hodgson DM . Prenatal endotoxin exposure alters behavioural pain responses to lipopolysaccharide in adult offspring. Physiol Behav 2010; 100: 143–147.
Hodyl NA, Krivanek KM, Lawrence E, Clifton VL, Hodgson DM . Prenatal exposure to a pro-inflammatory stimulus causes delays in the development of the innate immune response to LPS in the offspring. J Neuroimmunol 2007; 190: 61–71.
Mouihate A, Galic MA, Ellis SL, Spencer SJ, Tsutsui S, Pittman QJ . Early life activation of toll-like receptor 4 reprograms neural anti-inflammatory pathways. J Neurosci 2010; 30: 7975–7983.
Saito S, Nakashima A, Shima T, Ito M . Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol 2010; 63: 601–610.
Gadani SP, Cronk JC, Norris GT, Kipnis J . IL-4 in the brain: a cytokine to remember. J Immunol 2012; 189: 4213–4219.
Ferreira TB, Kasahara TM, Barros PO, Vieira MM, Bittencourt VC, Hygino J et al. Dopamine up-regulates Th17 phenotype from individuals with generalized anxiety disorder. J Neuroimmunol 2011; 238: 58–66.
Vieira MM, Ferreira TB, Pacheco PA, Barros PO, Almeida CR, Araujo-Lima CF et al. Enhanced Th17 phenotype in individuals with generalized anxiety disorder. J Neuroimmunol 2010; 229: 212–218.
Beurel E, Harrington LE, Jope RS . Inflammatory T helper 17 cells promote depression-like behavior in mice. Biol Psychiatry 2012; 73: 622–630.
Kim SJ, Lee H, Joung HY, Lee G, Lee HJ, Shin MK et al. T-bet deficient mice exhibit resistance to stress-induced development of depression-like behaviors. J Neuroimmunol 2011; 240–241: 45–51.
Postal M, Pelicari KO, Sinicato NA, Marini R, Costallat LT, Appenzeller S . Th1/Th2 cytokine profile in childhood-onset systemic lupus erythematosus. Cytokine 2013; 61: 785–791.
O’Connor RM, Dinan TG, Cryan JF . Little things on which happiness depends: microRNAs as novel therapeutic targets for the treatment of anxiety and depression. Mol Psychiatry 2012; 17: 359–376.
Nishioka K, Wider C, Vilarino-Guell C, Soto-Ortolaza AI, Lincoln SJ, Kachergus JM et al. Association of alpha-, beta-, and gamma-Synuclein with diffuse lewy body disease. Arch Neurol 2010; 67: 970–975.
Kokhan VS, Bolkunov AV, Ustiugov AA, Van’kin GI, Shelkovnikova TA, Redkozubova OM et al. Targeted inactivation of gamma-synuclein gene affects anxiety and exploratory behaviour of mice. Zh Vyssh Nerv Deiat Im I P Pavlova 2011; 61: 85–93.
Brietzke E, Teixeira AL . Similar immune profile in bipolar disorder and schizophrenia: selective increase in soluble tumor necrosis factor receptor I and von Willebrand factor. Bipolar Disord 2010; 12: 453–454.
Ho-Yen DO, Kay DS, Henderson R, Todd AS, Naylor GJ . Factor VIII-related antigen and procoagulant activity in manic-depressive patients. J Affect Disord 1983; 5: 209–213.
Hope S, Melle I, Aukrust P, Steen NE, Birkenaes AB, Lorentzen S et al. Similar immune profile in bipolar disorder and schizophrenia: selective increase in soluble tumor necrosis factor receptor I and von Willebrand factor. Bipolar Disord 2009; 11: 726–734.
Kimmel RJ, Kovacs I, Vrabel C, Wood B, Schalling M, Kelsoe JR . Cosegregation of bipolar disorder and autosomal-dominant medullary cystic kidney disease in a large family. Am J Psychiatry 2005; 162: 1972–1974.
Wylie K, de Silva D, Jerram T, Mindham RH . Simultaneous kidney disease and manic-depressive psychosis. Br J Psychiatry 1993; 162: 275–276.
Chen DT, Jiang X, Akula N, Shugart YY, Wendland JR, Steele CJ et al. Genome-wide association study meta-analysis of European and Asian-ancestry samples identifies three novel loci associated with bipolar disorder. Mol Psychiatry 2013; 18: 195–205.
Zhang L, Su TP, Choi K, Maree W, Li CT, Chung MY et al. P11 (S100A10) as a potential biomarker of psychiatric patients at risk of suicide. J Psychiatr Res 2011; 45: 435–441.
Richards AB, Scheel TA, Wang K, Henkemeyer M, Kromer LF . EphB1 null mice exhibit neuronal loss in substantia nigra pars reticulata and spontaneous locomotor hyperactivity. Eur J Neurosci 2007; 25: 2619–2628.
Kerimoglu C, Agis-Balboa RC, Kranz A, Stilling R, Bahari-Javan S, Benito-Garagorri E et al. Histone-methyltransferase MLL2 (KMT2B) is required for memory formation in mice. J Neurosci 2013; 33: 3452–3464.
Griswold AJ, Ma D, Sacharow SJ, Robinson JL, Jaworski JM, Wright HH et al. A de novo 1.5 Mb microdeletion on chromosome 14q23.2–23.3 in a patient with autism and spherocytosis. Autism Res 2011; 4: 221–227.
Villoslada P, Moreno B, Melero I, Pablos JL, Martino G, Uccelli A et al. Immunotherapy for neurological diseases. Clin Immunol 2008; 128: 294–305.
Wisniewski T, Konietzko U . Amyloid-beta immunisation for Alzheimer’s disease. Lancet Neurol 2008; 7: 805–811.
Cardoso F . Huntington disease and other choreas. Neurol Clin 2009; 27: 719–736, vi.
Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A . Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 1998; 395: 82–86.
Reichardt P, Dornbach B, Gunzer M . APC, T cells, and the immune synapse. Curr Top Microbiol Immunol 2010; 340: 229–249.
Valitutti S, Dupre L . Plasticity of immunological synapses. Curr Top Microbiol Immunol 2010; 340: 209–228.
Rosas-Ballina M, Olofsson PS, Ochani M, Valdes-Ferrer SI, Levine YA, Reardon C et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 2011; 334: 98–101.
Woodman I . Neuroimmunology: nervous ChAT. Nat Rev Immunol 2011; 11: 720.
Acknowledgements
We thank Dr Mangesh Thorat (Cancer Research UK Center for Epidemiology, Mathematics and Statistics) for helping us with brain section picture acquisition and Dr Dianne Cooper for carefully reading the manuscript. MO is a Human Frontier Science Program Long-term Fellow.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Translational Psychiatry website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by/3.0/
About this article
Cite this article
Rattazzi, L., Piras, G., Ono, M. et al. CD4+ but not CD8+ T cells revert the impaired emotional behavior of immunocompromised RAG-1-deficient mice. Transl Psychiatry 3, e280 (2013). https://doi.org/10.1038/tp.2013.54
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2013.54
Keywords
This article is cited by
-
Neonatal immune challenge poses a sex-specific risk for epigenetic microglial reprogramming and behavioral impairment
Nature Communications (2023)
-
T cells: an emerging cast of roles in bipolar disorder
Translational Psychiatry (2023)
-
Zika virus vertical transmission in interferon receptor1-antagonized Rag1−/− mice results in postnatal brain abnormalities and clinical disease
Acta Neuropathologica Communications (2022)
-
Basic principles of neuroimmunology
Seminars in Immunopathology (2022)
-
Neuromodulation by the immune system: a focus on cytokines
Nature Reviews Immunology (2021)