Abstract
Exposure to solar UV radiation is the origin of most skin cancers, including deadly melanomas. Melanomas are quite different from keratinocyte-derived tumours and exhibit a different mutation spectrum in the activated oncogenes, possibly arising from a different class of DNA damage. In addition, some data suggest a role for UVA radiation in melanomagenesis. To get further insight into the molecular mechanisms underlying induction of melanoma, we quantified a series of UV-induced DNA damage in primary cultures of normal human melanocytes. The results were compared with those obtained in keratinocytes from the same donors. In the UVB range, the frequency and the distribution of pyrimidine dimers was the same in melanocytes and keratinocytes. UVA was also found to produce thymine cyclobutane dimer as the major DNA lesion with an equal efficiency in both cell types. In contrast, following UVA-irradiation a large difference was found for the yield of 8-oxo-7,8-dihydroguanine; the level of this product was 2.2-fold higher in melanocytes than in keratinocytes. The comet assay showed that the induction of strand breaks was equally efficient in both cell types but that the yield of Fpg-sensitive sites was larger in melanocytes. Our data show that, upon UVA irradiation, oxidative lesions contribute to a larger extent to DNA damage in melanocytes than in keratinocytes. We also observed that the basal level of oxidative lesions was higher in the melanocytes, in agreement with a higher oxidative stress that may be due to the production of melanin. The bulk of these results, combined with qPCR and cell survival data, may explain some of the differences in mutation spectrum and target genes between melanomas and carcinomas arising from keratinocytes.
Similar content being viewed by others
References
G. Walker, Cutaneous melanoma: how does ultraviolet light contribute to melanocyte transformation?, Future Oncol., 2008, 4, 841–856.
N. Maddodi, V. Setaluri, Role of UV in cutaneous melanoma, Photochem. Photobiol., 2008, 84, 528–536.
D. C. Whiteman, C. A. Whiteman, A. C. Green, Childhood sun exposure as a risk factor for melanoma: a systematic review of epidemiologic studies, Cancer, Causes Control, 2001, 12, 69–82.
S. Jiveskog, B. Ragnarsson-Olding, A. Platz, U. Ringborg, N-ras mutations are common in melanomas from sun-exposed skin of humans but rare in mucosal membrane or unexposed skin, J. Invest. Dermatol., 1998, 111, 757–761.
S. Pavey, P. Johansson, L. Packer, J. Taylor, M. Stark, P. M. Pollock, G. J. Walker, G. M. Boyle, U. Harper, S. J. Cozzi, K. Hansen, L. Yudt, C. Schmidt, P. Hersey, K. A. Ellem, M. G. O’Rourke, P. G. Parsons, P. Meltzer, M. Ringner, N. K. Hayward, Microarray expression profiling in melanoma reveals a BRAF mutation signature, Oncogene, 2004, 23, 4060–4067.
P. M. Pollock, U. L. Harper, K. S. Hansen, L. M. Yudt, M. Stark, C. M. Robbins, T. Y. Moses, G. Hostetter, U. Wagner, J. Kakareka, G. Salem, T. Pohida, P. Heenan, P. Duray, O. Kallioniemi, N. K. Hayward, J. M. Trent, P. S. Meltzer, High frequency of BRAF mutations in nevi, Nat. Genet., 2003, 33, 19–20.
J. A. Curtin, J. Fridlyand, T. Kageshita, H. N. Patel, K. J. Busam, H. Kutzner, K. H. Cho, S. Aiba, E. B. Brocker, P. E. LeBoit, D. Pinkel, B. C. Bastian, Distinct sets of genetic alterations in melanoma, N. Engl. J. Med., 2005, 353, 2135–2147.
R. B. Setlow, Spectral regions contributing to melanoma: a personal view, J. Invest. Dermatol. Symp. Proc., 1999, 4, 46–49.
D. L. Mitchell, A. A. Fernandez, R. S. Nairn, R. Garcia, L. Paniker, D. Trono, H. D. Thames, I. Gimenez-Conti, Ultraviolet A does not induce melanomas in a Xiphophorus hybrid fish model, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 9329–9334.
E. C. De Fabo, F. P. Noonan, T. Fears, G. Merlino, Ultraviolet B but not ultraviolet A radiation initiates melanoma, Cancer Res., 2004, 64, 6372–6376.
S. G. Coelho, V. J. Hearing, UVA tanning is involved in the increased incidence of skin cancers in fair-skinned young women, Pigm. Cell Melanoma Res., 2010, 23, 57–63.
D. E. Brash, J. A. Rudolph, J. A. Simon, A. Lin, G. J. McKenna, H. P. Baden, A. J. Halperin, J. Ponten, A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma, Proc. Natl. Acad. Sci. U. S. A., 1991, 88, 10124–10128.
A. Ziegler, D. J. Leffel, S. Kunala, H. W. Sharma, P. E. Shapiro, A. E. Bale, D. E. Brash, Mutation hotspots due to sunlight in the p53 gene of nonmelanoma skin cancers, Proc. Natl. Acad. Sci. U. S. A., 1993, 90, 4216–4220.
J. Jans, W. Schul, Y. G. Sert, Y. Rijksen, H. Rebel, A. P. Eker, S. Nakajima, H. van Steeg, F. R. de Gruijl, A. Yasui, J. H. Hoeijmakers, G. T. van der Horst, Powerful skin cancer protection by a CPD-photolyase transgene, Curr. Biol., 2005, 15, 105–115.
Y. H. You, D. H. Lee, J. H. Yoon, S. Nakajima, A. Yasui, G. P. Pfeifer, Cyclobutane pyrimidine dimers are responsible for the vast majority of mutations induced by UVB irradiation in mammalian cells, J. Biol. Chem., 2001, 276, 44688–44694.
E. D. Pleasance, R. K. Cheetham, P. J. Stephens, D. J. McBride, S. J. Humphray, C. D. Greenman, I. Varela, M. L. Lin, G. R. Ordonez, G. R. Bignell, K. Ye, J. Alipaz, M. J. Bauer, D. Beare, A. Butler, R. J. Carter, L. N. Chen, A. J. Cox, S. Edkins, P. I. Kokko-Gonzales, N. A. Gormley, R. J. Grocock, C. D. Haudenschild, M. M. Hims, T. James, M. M. Jia, Z. Kingsbury, C. Leroy, J. Marshall, A. Menzies, L. J. Mudie, Z. M. Ning, T. Royce, O. B. Schulz-Trieglaff, A. Spiridou, L. A. Stebbings, L. Szajkowski, J. Teague, D. Williamson, L. Chin, M. T. Ross, P. J. Campbell, D. R. Bentley, P. A. Futreal, M. R. Stratton, A comprehensive catalogue of somatic mutations from a human cancer genome, Nature, 2010, 463, 191–197.
T. Hocker, H. S. Tsao, Ultraviolet radiation and melanoma: A systematic review and analysis of reported sequence variants, Hum. Mutat., 2007, 28, 578–588.
Y. Wang, J. J. DiGiovanna, J. B. Stern, T. J. Hornyak, M. Raffeld, S. G. Khan, K. S. Oh, M. C. Hollander, P. A. Dennis, K. H. Kraemer, Evidence of ultraviolet type mutations in xeroderma pigmentosum melanomas, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 6279–6284.
H. Davies, G. R. Bignell, C. Cox, P. Stephens, S. Edkins, S. Clegg, J. Teague, H. Woffendin, M. J. Garnett, W. Bottomley, N. Davis, N. Dicks, R. Ewing, Y. Floyd, K. Gray, S. Hall, R. Hawes, J. Hughes, V. Kosmidou, A. Menzies, C. Mould, A. Parker, C. Stevens, S. Watt, S. Hooper, R. Wilson, H. Jayatilake, B. A. Gusterson, C. Cooper, J. Shipley, D. Hargrave, K. Pritchard-Jones, N. Maitland, G. Chenevix-Trench, G. J. Riggins, D. D. Bigner, G. Palmieri, A. Cossu, A. Flanagan, A. Nicholson, J. W. C. Ho, S. Y. Leung, S. T. Yuen, B. L. Weber, H. F. Siegler, T. L. Darrow, H. Paterson, R. Marais, C. J. Marshall, R. Wooster, M. R. Stratton, P. A. Futreal, Mutations of the BRAF gene in human cancer, Nature, 2002, 417, 949–954.
J. Cadet, T. Douki, J. L. Ravanat, P. Di Mascio, Sensitized formation of oxidatively generated damage to cellular DNA by UVA radiation, Photochem. Photobiol. Sci., 2009, 8, 903–911.
S. Courdavault, C. Baudouin, M. Charveron, A. Favier, J. Cadet, T. Douki, Larger yield of cyclobutane dimers than 8 oxo-7,8-dihydroguanine in the DNA of UVA-irradiated human skin cells, Mutat. Res., Fundam. Mol. Mech. Mutagen., 2004, 556, 135–142.
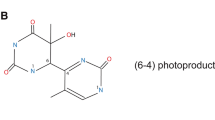
T. Douki, A. Reynaud-Angelin, J. Cadet, E. Sage, Bipyrimidine photoproducts rather than oxidative lesions are the main type of DNA damage involved in the genotoxic effect of solar UVA radiation, Biochemistry, 2003, 42, 9221–9226.
C. Kielbassa, L. Roza, B. Epe, Wavelength dependence of oxidative DNA damage induced by UV and visible light, Carcinogenesis, 1997, 18, 811–816.
S. Mouret, C. Baudouin, M. Charveron, A. Favier, J. Cadet, T. Douki, Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 13765–13770.
D. Perdiz, P. Grof, M. Mezzina, O. Nikaido, E. Moustacchi, E. Sage, Distribution and repair of bipyrimidine photoproducts in solar UV-irradiated mammalian cells. Possible role of Dewar photoproducts in solar mutagenesis, J. Biol. Chem., 2000, 275, 26732–26742.
S. Frelon, T. Douki, J.-L. Ravanat, C. Tornabene, J. Cadet, High performance liquid chromatography-tandem mass spectrometry measurement of radiation-induced base damage to isolated and cellular DNA, Chem. Res. Toxicol., 2000, 13, 1002–1010.
T. Douki, J. Cadet, Individual determination of the yield of the main-UV induced dimeric pyrimidine photoproducts in DNA suggests a high mutagenicity of CC photolesions, Biochemistry, 2001, 40, 2495–2501.
T. Douki, M. Court, S. Sauvaigo, F. Odin, J. Cadet, Formation of the main UV-induced thymine dimeric lesions within isolated and cellular DNA as measured by high performance liquid chromatography-tandem mass spectrometry, J. Biol. Chem., 2000, 275, 11678–11685.
S. Mouret, S. Sauvaigo, A. Peinnequin, A. Favier, J. C. Beani, M. T. Leccia, E6*oncoprotein expression of human papillomavirus type-16 determines different ultraviolet sensitivity related to glutathione and glutathione peroxidase antioxidant defence, Exp. Dermatol., 2005, 14, 401–410.
M. W. Pfaffl, G. W. Horgan, L. Dempfle, Relative expression software tool (REST (c)) for group-wise comparison and statistical analysis of relative expression results in real-time PCR, Nucleic Acids Res., 2002, 30, e36.
S. M. Deleeuw, S. Janssen, J. Simons, P. H. M. Lohman, B. J. Vermeer, A. A. Schothorst, The UV action spectra for the clone-forming ability of cultured human melanocytes and keratinocytes, Photochem. Photobiol., 1994, 59, 430–436.
P. Larsson, E. Andersson, U. Johansson, K. Ollinger, I. Rosdahl, Ultraviolet A and B affect human melanocytes and keratinocytes differently. A study of oxidative alterations and apoptosis, Exp. Dermatol., 2005, 14, 117–123.
L. P. Lund, G. S. Timmins, Melanoma, long wavelength ultraviolet and sunscreens: Controversies and potential resolutions, Pharmacol. Ther., 2007, 114, 198–207.
M. A. Cotter, J. Thomas, P. Cassidy, K. Robinette, N. Jenkins, S. R. Florell, S. Leachman, W. E. Samlowski, D. Grossman, N-acetylcysteine protects melanocytes against oxidative stress/damage and delays onset of ultraviolet induced melanoma in mice, Clin. Cancer Res., 2007, 13, 5952–5958.
S. Q. Wang, R. Setlow, M. Berwick, D. Polsky, A. A. Marghoob, A. W. Kopf, R. S. Bart, Ultraviolet A and melanoma: A review, J. Am. Acad. Dermatol., 2001, 44, 837–846.
X. Z. Song, N. Mosby, J. Yang, A. Xu, Z. Abdel-Malek, A. L. Kadekaro, alpha-MSH activates immediate defense responses to UV-induced oxidative stress in human melanocytes, Pigm. Cell Melanoma Res., 2009, 22, 809–818.
H. T. Wang, B. Choi, M. S. Tang, Melanocytes are deficient in repair of oxidative DNA damage and UV-induced photoproducts, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 12180–12185.
S. Courdavault, C. Baudouin, M. Charveron, B. Canghilem, A. Favier, J. Cadet, T. Douki, Repair of the three main types of bipyrimidine DNA photoproducts in human keratinocytes exposed to UVB and UVA radiations, DNA Repair, 2005, 4, 836–844.
S. M. Deleeuw, W. I. M. Simons, B. J. Vermeer, A. A. Schothorst, Comparison of melanocytes and keratinocytes in ultraviolet-induced DNA-damage per minimum erythema dose sunlight - Applicability of ultraviolet action spectra for risk estimates, J. Invest. Dermatol., 1995, 105, 259–263.
A. A. Schothorst, L. M. Evers, K. C. Noz, R. Filon, A. A. Vanzeeland, Pyrimidine dimer induction and repair in cultured human skin keratinocytes or melanocytes after irradiation with monochromatic ultraviolet-radiation, J. Invest. Dermatol., 1991, 96, 916–920.
A. R. Young, C. S. Potten, O. Nikaido, P. G. Parsons, J. Boenders, J. M. Ramsden, C. A. Chadmick, Human melanocytes and keratinocytes exposed to UVB or UVA in vivo show comparable levels of thymine dimers, J. Invest. Dermatol., 1998, 111, 936–940.
M. Brenner, V. J. Hearing, The protective role of melanin against UV damage in human skin, Photochem. Photobiol., 2008, 84, 539–549.
S. M. De Leeuw, N. P. M. Smit, M. Van Veldhoven, E. M. Pennings, S. Pavel, J. Simons, A. A. Schothorst, Melanin content of cultured human melanocytes and UV-induced cytotoxicity, J. Photochem. Photobiol., B, 2001, 61, 106–113.
C. Kowalczuk, M. Priestner, C. Baller, A. Pearson, N. Cridland, R. Saunders, K. Wakamatsu, S. Ito, Effect of increased intracellular melanin concentration on survival of human melanoma cells exposed to different wavelengths of UV radiation, Int. J. Radiat. Biol., 2001, 77, 883–889.
H. Z. Hill, G. J. Hill, K. Cieszka, P. M. Plonka, D. L. Mitchell, M. F. Meyenhofer, P. T. Xin, R. E. Boissy, Comparative action spectrum for ultraviolet light killing of mouse melanocytes from different genetic coat color backgrounds, Photochem. Photobiol., 1997, 65, 983–989.
H. Y. Park, M. Kosmadaki, M. Yaar, B. A. Gilchrest, Cellular mechanisms regulating human melanogenesis, Cell. Mol. Life Sci., 2009, 66, 1493–1506.
S. Mouret, M.-T. Leccia, J.-L. Bourrain, T. Douki, J.-C. Beani Individual, photosensitivity of human skin and UVA-induced pyrimidine dimers in DNA, J. Invest. Dermatol., 2011, 131, 1539–1546.
T. Tadokoro, N. Kobayashi, B. Z. Zmudzka, S. Ito, K. Wakamatsu, Y. Yamaguchi, K. S. Korossy, S. A. Miller, J. Z. Beer, V. J. Hearing, UV-induced DNA damage and melanin content in human skin differing in racial/ethnic origin, FASEB J., 2003, 17, 1177–1179.
Y. Yamaguchi, K. Takahashi, B. Z. Zmudzka, A. Kornhauser, S. A. Miller, T. Tadokoro, W. Berens, J. Z. Beer, V. J. Hearing, Human skin responses to UV radiation: pigment in the upper epidermis protects against DNA damage in the lower epidermis and facilitates apoptosis, FASEB J., 2006, 20, 1486–1488.
S. E. Freeman, H. Hacham, R. W. Gange, D. J. Maytum, J. C. Sutherland, B. M. Sutherland, Wavelength dependence of pyrimidine dimer formation in DNA of human skin irradiated in situ with ultraviolet light, Proc. Natl. Acad. Sci. U. S. A., 1989, 86, 5605–5609.
F. E. Quaite, B. M. Sutherland, J. C. Sutherland, Action spectrum for DNA damage in alfalfa lowers predicted impact of ozone depletion, Nature, 1992, 358, 576–578.
S. Mouret, C. Philippe, J. Gracia-Chantegrel, A. Banyasz, S. Karpati, D. Markovitsi, T. Douki, UVA-induced cyclobutane pyrimidine dimers in DNA: a direct photochemical mechanism?, Org. Biomol. Chem., 2010, 8, 1706–1711.
Y. Jiang, M. Rabbi, M. Kim, C. H. Ke, W. Lee, R. L. Clark, P. A. Mieczkowski, P. E. Marszalek, UVA generates pyrimidine dimers in DNA directly, Biophys. J., 2009, 96, 1151–1158.
Z. Kuluncsics, D. Perdiz, E. Brulay, B. Muel, E. Sage, Wavelength dependence of ultraviolet-induced DNA damage distribution: involvement of direct or indirect mechanisms and possible artefacts, J. Photochem. Photobiol., B, 1999, 49, 71–80.
J. C. Sutherland, K. P. Griffin, Absorption spectrum of DNA for wavelengths greater than 300 nm, Radiat. Res., 1981, 86, 399–409.
F. L. Meyskens, P. Farmer, J. P. Fruehauf, Redox regulation in human melanocytes and melanoma, Pigm. Cell Res., 2001, 14, 148–154.
J.-L. Ravanat, C. Saint-Pierre, P. Di Mascio, G. R. Martinez, M. H. Medeiros, J. Cadet, Damage to isolated DNA mediated by singlet oxygen, Helv. Chim. Acta, 2001, 84, 3702–3709.
S. Gidanian, M. Mentelle, F. L. Meyskens, P. J. Farmer, Melanosomal damage in normal human melanocytes induced by UVB and metal uptake - A basis for the pro-oxidant state of melanoma, Photochem. Photobiol., 2008, 84, 556–564.
L. Marrot, J. P. Belaidi, C. Jones, P. Perez, J. R. Meunier, Molecular responses to stress induced in normal human Caucasian melanocytes in culture by exposure to simulated solar UV, Photochem. Photobiol., 2005, 81, 367–375.
J. J. Yohn, D. A. Norris, D. G. Yrastorza, I. J. Buno, J. A. Leff, S. S. Hake, J. E. Repine, Disparate antioxidant enzyme activities in cultured human cutaneous fibroblasts, keratinocytes, and melanocytes, J. Invest. Dermatol., 1991, 97, 405–409.
Q. Y. Wei, J. E. Lee, J. E. Gershenwald, M. I. Ross, P. F. Mansfield, S. S. Strom, L. E. Wang, Z. Z. Guo, Y. W. Qiao, C. I. Amos, M. R. Spitz, M. Duvic, Repair of UV light-induced DNA damage and risk of cutaneous malignant melanoma, J. Natl. Cancer Inst., 2003, 95, 308–315.
Author information
Authors and Affiliations
Corresponding author
Additional information
Contribution to the themed issue on the biology of UVA.
Electronic supplementary information (ESI) available: Fig. S1: emission spectra of the UV lamps and Table S1: list and expression of studied DNA repair genes.. See DOI: 10.1039/c1pp05185g
§ This work was supported by a grant from the French “Agence National pour la Recherche” (ANR-07-PCVI-0004-01).
Rights and permissions
About this article
Cite this article
Mouret, S., Forestier, A. & Douki, T. The specificity of UVA-induced DNA damage in human melanocytes. Photochem Photobiol Sci 11, 155–162 (2012). https://doi.org/10.1039/c1pp05185g
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c1pp05185g