Abstract
The stingless bees are among the most abundant and ecologically important social invertebrates in tropical communities. The Neotropical stingless bee Melipona quadrifasciata has two subspecies: M. quadrifasciata quadrifasciata and M. quadrifasciata anthidioides. The main difference between subspecies are the yellow metassomal stripes, which are continuous in M. q. quadrifasciata and discontinuous in M. q. anthidioides. Recently, two populations were described with continuous stripes and inhabiting clearly disjunct areas in relation to M. q. quadrifasciata. We sequenced 852 bp of the mtDNA COI gene from 145 colonies from 56 localities, and for the first time performed a detailed phylogeographic study of a neotropical stingless bee. Phylogenetic analyses revealed the existence of two clades exhibiting a south to north distribution: southern populations comprise the subspecies M. q. quadrifasciata, and northern populations are composed of M. q. anthidioides and two disjunct populations with continuous stripes. The divergence time of these two phylogroups was estimated between 0.233 and 0.840 million years ago in the Pleistocene, a period of climatic changes and geomorphological alterations in the Neotropical region. No evidence of genetic structure in relation to the tergal stripes was found, indicating that the morphological trait regarding the pattern of stripes on tergites is not an accurate diagnostic for the subspecies of M. quadrifasciata.
Zusammenfassung
Stachellose Bienen gehören zu den häufigsten und ökologisch bedeutsamsten wirbellosen Tieren in tropischen Lebensgemeinschaften. Die neotropische stachellose Biene Melipona quadrifasciata hat zwei Unterarten: M. quadrifasciata quadrifasciata und M. quadrifasciata anthidioides. Das hauptsächliche Unterscheidungsmerkmal dieser beiden Unterarten sind gelbe metasomale Streifen, die bei M. q. quadrifasciata durchgehend, und bei M. q. anthidioides unterbrochen sind. Die Art ist entlang der Ostküste Brasiliens verbreitet, wobei M. q. quadrifasciata im Süden und M. q. anthidioides im Norden des Verbreitungsgebiets vorkommt.Vor kurzem wurden zwei Populationen mit zusammenhängenden Streifen beschrieben, die nördlich des von M. q. quadrifasciata besiedelten Gebietes in disjunkten Arealen vorkommen.
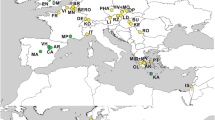
In dieser Studie untersuchen wir die populationsgenetische Struktur und die historische Demographie von M. quadrifasciata mit Hilfe von Sequenzen der mitochondrialen DNA aus umfangreichem Probenmaterial (Abb. 1 und Tab. S1, online material). Dabei untersuchen wir zwei Hauptfragen: (i) korreliert die morphologische Variation (Verteilung des Streifenmusters auf dem Abdomen) mit der Variation der mitochondrialen DNA? Mit anderen Worten, stammen die Populationen mit zusammenhängenden Streifen aus derselben mütterlichen Linie ab? (ii) Welche demographischen Ereignisse können die beobachteten phylogeographischen Muster erklären?
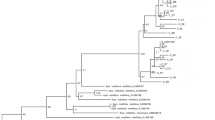
Zur Beantwortung dieser Fragen sequenzierten wir 852 bp der Untereinheit1 des Cytochromoxidase (CoI) Gens aus der mitochondrialen DNA von 145 Völken (1 Arbeiterin pro Volk), die entlang des Verbreitungsgebiets der Art gesammelt worden waren (Abb. 1). Die Ergebnisse von phylogeographischen Tests und Koaleszenzberechnungen machten deutlich, dass es zwei unterschiedliche Gruppen gibt: eine südliche Klade, die aus M. q. quadrifasciata besteht, und eine nördliche, die M. q. anthidioides sowie die beiden disjunkten Populationen mit durchgehenden Streifen beinhaltet. Interessanterweise sind also Formen, die durchgehende Streifen gemeinsam haben, nicht unbedingt monophyletisch; umgekehrt haben Individuen mit verschiedener Morphologie denselben mtDNA Haplotypen gemeinsam (H14 in Abb. 3). Wir schlagen vor, die durchgehenden tergalen Streifen in den diskjunkten Populationen als einen anzestralen Polymorphismus zu betrachten, da nach den Ergebnissen anderer Studien das Muster aus zusammenhängenden Streifen innerhalb der Untergattung Melipona (Melipona) ein plesiomorphes Merkmal zu sein scheint. Der Zeitpunkt der Divergenz der beiden Gruppen wurde auf die Zeit des Pleistozäns, zwischen 0,233 und 0,840 Millionen Jahren vor der Gegenwart bestimmt (online material, Abb. S2 und Tab. S1), die durch Klimaänderungen und geomorphologische Umbildungen in der neotropischen Region charakterisiert war. Ähnliche phylogeographische Muster wurden bei endemischen Wirbeltieren des Atlantischen Regenwaldes nachgewiesen, zum Beispiel bei Schlangen, Vögeln und Amphibien.
Similar content being viewed by others
References
Avise J.C. (2008) Phylogeography: retrospect and prospect, J. Biogeogr. 36, 3–15.
Bandelt H.J., Forster P., Röhl A. (1999) Medianjoining networks for inferring intraspecific phylogenies, Mol. Biol. Evol. 16, 37–48.
Batalha-Filho H., Melo G.A.R., Waldschmidt A.M., Campos L.A.O., Fernandes-Salomão T.M. (2009) Geographic distribution and spatial differentiation in the color pattern of abdominal stripes of the Neotropical stingless bee Melipona quadrifasciata (Hymenoptera, Apidae), Zoologia 26, 213–219.
Burnham K.P., Anderson D.R. (2002) Model selection and multimodel inference: a pratical informationtheoretic approach, 2nd ed., Springer, New York.
Cabanne G.S., d’Horta F.M., Sari E.H.R., Santos F.R., Miyaki C.Y. (2008) Nuclear and mitochondrial phylogeography of the Atlantic forest endemic Xiphorhynchus fuscus (Aves: Dendrocolaptidae): Biogeography and systematic implications, Mol. Phylogenet. Evol. 49, 760–773.
Cabanne G.S., Santos F.R., Miyaki C.Y. (2007) Phylogeography of Xiphorhynchus fuscus (Passeriformes, Dendrocolaptidae): vicariance and recent demographic expansion in southern Atlantic forest, Biol. J. Linn. Soc. 91, 73–84.
Caccone A., Sbordoni V. (2001) Molecular biogeography, evolutionary rates, and morphological adaptations to cave life: a case study using Bathysciine beetles and sequence data from the mitochondria CO1 gene, Evolution 55, 122–130.
Camargo J.M.F., Pedro S.M.R. (2007) Meliponini, in: Moure J.S., Urban D., Melo G.A.R. (Eds.), Catalogue of Bees (Hymenoptera, Apoidea) in the Neotropical Region, Sociedade Brasileira de Entomologia, Curitiba, pp. 272–578.
Carnaval A.C., Moritz C. (2008) Historical climate modelling predicts patterns of current biodiversity in the Brazilian Atlantic forest, J. Biogeogr. 35, 1187–1201.
Carnaval A.C., Hickerson M.J., Haddad C.F.B., Rodrigues M.T., Moritz C. (2009) Stability predicts genetic diversity in the Brazilian Atlantic forest hotspot, Science 323, 785–789.
Cruz D.O., Jorge D.M.M., Pereira J.O.P., Torres D.C., Soares C.E.A., Freitas B.M., Grangeiro T.B. (2006) Intraspecific variation in the first internal transcribed spacer (ITS1) of the nuclear ribosomal DNA in Melipona subnitida (Hymenoptera, Apidae), an endemic stingless bee from northeastern Brazil, Apidologie 37, 376–386.
Dick C.W., Roubik D.W., Gruber K.F., Bermingham E. (2004) Long-distance gene flow and cross-Andean dispersal of lowland rainforest bees (Apidae: Euglossini) revealed by comparative mitochondrial DNA phylogeography, Mol. Ecol. 13, 3775–3785.
Ducke A. (1916) Enumeração dos Hymenopteros colligidos pela Comissão e Revisão das espécies de abelhas do Brasil, Comm. Lin. Teleg. Estr. M. Gr. Amaz. 35, 3–171.
Dupanloup I., Schneider S., Excoffier L. (2002) A simulated annealing approach to define the genetic structure of populations, Mol. Ecol. 11, 2571–2581.
Edwards S.V., Beerli P. (2000) Perspective: gene divergence, population divergence, and the variance in coalescence time in phylogeographic studies, Evolution 54, 1839–1854.
Ewing B., Green P. (1998) Base-calling of automated sequencer traces using phred. II. Error probabilities, Genome Res. 8, 186–194.
Ewing B., Hillier L., Wendl M.C., Green P. (1998) Base-calling of automated sequencer traces using phred. I. Accuracy assessment, Genome Res. 8, 175–185.
Excoffier L., Smouse P.E., Quattro J.M. (1992) Analyses of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data, Genetics 131, 479–491.
Farrell B.D. (2001) Evolutionary assembly of the milkweed fauna: cytochrome oxidase 1 and the age of Tetraopes beetles, Mol. Phylogenet. Evol. 18, 469–478.
Fernandes-Salomão T.M., Rocha R.B., Campos L.A.O., Araújo E.F. (2005) The first internal transcribed spacer (ITS1) of Melipona species (Hymenoptera, Apidae, Meliponini): characterization and phylogenetic analysis, Insectes Soc. 52, 11–18.
Fu Y.X. (1997) Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection, Genetics 147, 915–925.
Gordon D., Abajian C., Green P. (1998) Consed: A graphical tool for sequence finishing, Genome Res. 8, 195–202.
Grazziotin F.G., Monzel M., Echeverrigaray S., Bonatto S.L. (2006) Phylogeography of the Bothrops jararaca complex (Serpentes: Viperidae): past fragmentation and island colonization in the Brazilian Atlantic Forest, Mol. Ecol. 15, 3969–3982.
Higgins D., Thompson J., Gibson T., Thompson J.D., Higgins D.G., Gibson T.J. (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice, Nucleic Acids Res. 22, 4673–4680.
Huelsenbech J.P., Ronquist F. (2001) MrBAYES: Bayesian inference of phylogenetic tree, Bioinformatics 17, 754–755.
Kerr W.E. (1951) Estudos sobre a genética de populações de Himenópteros em geral e dos Apíneos sociais em particular, Tese para livre docência, Ann. Esc. Sup. Agric. L. Queiroz 8, 219–354.
Kraus F.B., Weinhold S., Moritz R.F.A. (2008) Genetic structure of drone congregations of the stingless bee Scaptotrigona mexicana, Insectes Soc. 55, 22–27.
Melo M.S., Fernandes L.A., Coimbra A.M., Ramos R.G.N. (1989) O Graben (Terciário?) de Sete Barras, Vale do Ribeira do Iguape, SP, Rev. Brasil. Geoc. 19, 260–262.
Moreto G., Arias M.C. (2005) Detection of mitochondrial DNA restriction site differences between the subspecies of Melipona quadrifasciata Lepeletier (Hymenoptera: Apidae, Meliponini), Neo. Entomol. 34, 381–385.
Moure J.S. (1975) Notas sobre as espécies de Melipona descritas por Lepetelier em 1836 (Hymenoptera, Apidae), Rev. Brasil. Biol. 3, 15–17.
Moure J.S., Kerr W.E. (1950) Sugestões para a modificação da sistemática do gênero Melipona (Hymenoptera, Apoidea), Dusenia 1, 105–129, + 2 estampas.
Nielsen R., Wakeley J. (2001) Distinguishing migration from isolation: a Markov chain Monte Carlo approach, Genetics 158, 885–896.
Nogueira-Neto P. (1954) Notas bionômicas sobre meliponíneos: III — Sobre a enxameagem, Arq. Mus. Nac. 42, 419–451.
Nylander J.A.A., Ronquist F., Huelsenbeck J.P., Nieves-Aldrey J.L. (2004) Bayesian phylogenetic analysis of combined data, Syst. Biol. 53, 47–67.
Page R.D.M. (2001) TreeView (Win32) 1.6.6, Acquired in: http://taxonomy.zoology.gla.ac.uk/rod/rod.html
Pereira J.B.S., Almeida J.R. (1996) Biogeografia e Geomorfologia, in: Guerra A.J.T., Cunha S.B. (Eds.), Geomorfologia e Meio Ambiente. Bertrand Brasil, Rio de Janeiro, pp. 195–247.
Posada D., Krandall K.A. (1998) MODELTEST: testing the model of DNA substitution, Bioinformatics 14, 817–818.
Quezada-Euán J.J.G., Paxton R.J., Palmer K.A., Itza W.D.J.M., Tay W.T., Oldroyd B.P. (2007) Morphological and molecular characters reveal differentiation in a Neotropical social bee, Melipona beecheii (Apidae: Meliponini), Apidologie 38, 247–258.
Ramos-Onsins S.E., Rozas J. (2002) Statistical properties of new neutrality tests against population growth, Mol. Biol. Evol. 19, 2092–2100.
Rozas J., Sánchez-DeIBarrio J.C., Messeguer X., Rozas R. (2003) DnaSP, DNA polymorphism analyses by the coalescent and other methods, Bioinformatics 19, 2496–2497.
Saadi A., Machette M.N., Haller K.M., Dart R.L., Bradley L., Souza A.M.P.D. (2002) Map and Database of Quaternary Faults and Lineaments in Brazil, International Lithosphere ProgramTask Group II-2 Co-Chairman (Western Hemisphere), Denver, Colorado.
Schwarz H. (1932) The genus Melipona IV. The type genus of the Meliponidae or stingless bees, Bull. Am. Mus. Nat. Hist. 63, 231–460.
Silveira F.A., Martines R.B. (2009) A new species of Mydrosoma Smith with a key to Brazilian species of the genus and a discussion on the classification of the Dissoglottini (Hymenoptera: Colletidae), Zootaxa 2105, 32–42.
Silveira F.A., Melo G.A.R., Almeida E.A.B. (2002) Abelhas Brasileiras: Sistemática e Identificação, Fundação Araucária, Belo Horizonte.
Silvestre D., Dowton M., Arias M.C. (2008) The mitochondrial genome of the stingless bee Melipona bicolor (Hymenoptera, Apidae, Meliponini): Sequence, gene organization and a unique tRNA translocation event conserved across the tribe Meliponini, Genet. Mol. Biol. 31, 451–460.
Soucy S.L., Danforth B.N. (2002) Phylogeography of the socially polymorphic sweat bee Halictus rubicundus (Hymenoptera: Halictidae), Evolution 56, 330–341.
Souza R.O., Moretto G., Arias M.C., Del Lama M.A. (2008) Differentiation of Melipona quadrifasciata L. (Hymenoptera, Apidae, Meliponini) subspecies using cytochrome b PCR-RFLP patterns, Genet. Mol. Biol. 30, 445–450.
Swofford D.L. (1998) PAUP*-A computer program for phylogenetic Inference using Maximum Parsimony and other methods), version 4, Sinauer Associates, Sunderland.
Tajima F. (1983) Evolutionary relationship of DNA sequences in finite populations, Genetics 105, 437–460.
Tamura K., Dudley J., Nei M., Kumar S. (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0, Mol. Biol. Evol. 24, 1596–1599.
Tanaka H., Roubik D.W., Kato M., Gunsalam G. (2001) Phylogenetic position of Apis nuluensis of northern Borneo and phylogeography of A. cerana as inferred from mitochondrial DNA sequences, Insectes Soc. 48, 44–51.
Tuomisto H. (2007) Interpreting the biogeography of South America, J. Biogeogr. 34, 1294–1295.
Waldschmidt A.M., Barros E.G., Campos L.A.O. (2000) A molecular marker distinguishes the subspecies Melipona quadrifasciata quadrifasciata and Melipona quadrifasciata anthidioides (Hymenoptera: Apidae, Meliponini), Genet. Mol. Biol. 23, 609–611.
Waldschmidt A.M., Marco-Júnior P., Barros E.G., Campos L.A.O. (2002) Genetic analysis of Melipona quadrifasciata Lep. (Hymanoptera: Apidae, Meliponini) with RAPD markers, Braz. J. Biol. 62, 923–928.
Zink R.M., Barrowclough G.F. (2008) Mitochondrial DNA under siege in avian phylogeography, Mol. Ecol. 17, 2107–2121.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript editor: Marina Meixner
Rights and permissions
About this article
Cite this article
Batalha-Filho, H., Waldschmidt, A.M., Campos, L.A.O. et al. Phylogeography and historical demography of the neotropical stingless bee Melipona quadrifasciata (Hymenoptera, Apidae: incongruence between morphology and mitochondrial DNA. Apidologie 41, 534–547 (2010). https://doi.org/10.1051/apido/2010001
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1051/apido/2010001