Abstract
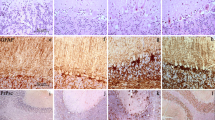
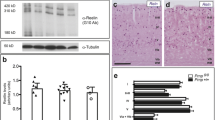
Prion protein (PrPc) is a cell membrane glycoprotein particularly abundant in the synapses. Prion diseases are characterized by the replacement of the normal PrPc by a protease-resistant, sheet-containing isoform (PrPCJD, PrPSc, PrPBSE) that is pathogenic. Creutzfeldt-Jakob disease (CJD) in humans, scrapie (Sc) in sheep and goats, and bovine spongiform encephalopathy (BSE) in cattle are typical prion diseases. Classical CJD can be presented as sporadic, infectious or familial, whereas the new variant of CJD (nvCJD) is considered a BSE-derived human disease. Spongiform degeneration, glial proliferation, involving astrocytes and microglia, neuron loss and abnormal PrP deposition are the main neuopathological findings in most human and animal prion diseases. Yet recent data point to synapses as principal targets of abnormal PrP deposition. Loss of synapses is an early abnormality in experimental scrapie. Decreased expression of crucial proteins linked to exocytosis and neurotransmission, covering synaptophysin, synaptosomal-associated protein of 25,000 mol wt (SNAP-25), synapsins, syntaxins and Rab3a occurs in the cerebral cortex and cerebellum in sporadic CJD. Moreover, impairment of glomerular synapses and attenuation of parallel fiber pre-synaptic terminals on Purkinje cell dendrites is a cardinal consequence of abnormal PrP metabolism in CJD. Accumulation of synaptic proteins in the soma and axonal torpedoes of Purkinje cells suggests additional impairment of axonal transport. Increase in nuclear DNA vulnerability leading to augmented numbers of cells bearing nuclear DNA fragments is a common feature in the brains of humans affected by prion diseases examined at post-mortem, but also in archival biopsy samples processed with the method of in situ end-labeling of nuclear DNA fragmentation. This form of cell death is reminiscent of apoptosis found in experimental scrapie in rodents. It is not clear that all forms of cell death in human and animal prion diseases are due to apoptosis. Yet new observations have shown cleaved (active) caspase-3 (17 kDa), a main executioner of apoptosis, expressed in scattered cells in the brains of mice with experimental scrapie and in the cerebellum of patients with sporadic CJD. Together, these data suggest activation of the caspase pathway of apoptosis in human and animal prion diseases.
Similar content being viewed by others
References
Prusiner SB. Cell biology and transgenic models of prion diseases. In: Collinge J, Palmer MS, editors. Prion diseases. Oxford: Oxford University Press, 1997: 130–162.
Prusiner SB. Prion diseases and the BSE crisis. Science 1997; 278: 245–251.
Prusiner SB. Biology of prions. In: Rosenberg RN, Prusiner SB, DiMauro S, Barchi RL, editors. The molecular and genetic basis of neurological disease. Oxford: Butterworth-Heinemann, 1997: 103–143.
Prusiner SB. The prion diseases of humans and animals. In: Rosenberg RN, Prusiner SB, DiMauro S, Barchi RL, editors. The molecular and genetic basis of neurological disease. Oxford: Butterworth-Heinemann, 1997: 165–186.
DeArmond SJ, Prusiner SB. Prion diseases. In: Graham DI, Lantos PL, editors. Greenfield's neuropathology. London: Arnold, 1997; 2: 235–280.
DeArmond SJ, Prusiner SB. Molecular neuropathology of prion diseases. In: Rosenberg RN, Prusiner SB, DiMauro S, Barchi RL, editors. The molecular and genetic basis of neurological disease. Oxford: Butterworth-Heinemann, 1997: 145–163.
Caughey B, Chesebro B. Prion protein and the transmissible spongiform encephalopathies. Trends Cell Biol 1997; 7: 56–62.
Stahl N, Borchelt DR, Hsiao K, Prusiner SB. Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell 1987; 51: 229–240.
Kretzschmar HA, Prusiner SB, Stowring LE, DeArmond SJ. Scrapie prion proteins are synthesized in neurons. Am J Path 1986; 122: 1–5.
Moser M, Colello RJ, Pott U, Oesch B. Developmental expression of the prion protein gene in glial cells. Neuron 1995; 14: 509–517.
van Keulen LJ, Schreuder BE, Meloen RH, Poelen-van der Berg M, Mooji-Harkes G, Vromans ME, Langeveld JP. Immunohistochemical detection and localization of prion protein in brain tissue of sheep with natural scrapie. Vet Pathol 1995; 32: 299–308.
Brown DR, Besinger A, Herms JW, Kretzschmar HA. Microglia expression of the prion protein. NeuroReport 1998; 9: 1425–1429.
Brown HR, Goller NL, Rudelli RD, Merz GS, Wolfe GC, Wisniewski HM, Robakis NK. The mRNA encoding the scrapie agent protein is present in a variety of non-neuronal cells. Acta Neuropathol 1990; 80: 1–6.
Cashman NR, Loertscher R, Nalbantoglu J, Shaw I, Kascsak RJ, Bolton DC, Benheim PE. Cellular isoform of the scrapie agent protein participates in lymphocyte activation. Cell 1990; 61: 185–192.
McBride PA, Eikelenboom P, Kraal G, Fraser H, Bruce ME. PrP protein is associated with follicular dendritic cells of spleens and lymph nodes in uninfected and scrapie-infected mice. J Pathol 1992; 168: 413–418.
Brown DR, Schmidt B, Groschup MH, Kretzschmar HA. Prion protein expression in muscle cells and toxicity of a prion protein fragment. Eur J Cell Biol 1998; 75: 29–37.
Caughey B, Race RE, Chesebro B. Detection of prion protein mRNA in normal and scrapie-infected tissues and cell lines. J Gen Virol 1988; 69: 711–716.
Wopfner F, Weindenhofer G, Schneider R, von Brunn A, Gilch S, Schwarz TF, Werner T, Schatzl HM. Analysis of 27 mammalian and 9 avian PrPs reveals high conservation of flexible regions of the prion protein. J Mol Biol 1999; 289: 1163–1178.
Borchelt DR, Koliatsos VE, Guarnieri M, Pardo CA, Sisodia SS, Price DL. Rapid anterograde transport of the cellular prion glycoprotein in the peripheral and central nervous system. J Biol Chem 1994; 269: 14711–14714.
Fournier JG, Escaig-Haye F, Billette de Villemeur T, Robain O. Ultrastructural localization of cellular prion protein (PrPC) in synaptic boutons of normal hamster hippocampus. CR Acad Sci III 1995; 318: 339–344.
Salès N, Rodolfo K, Hassig R, Fancheaux B, DiGianbardino L, Moya KL. Cellular prion protein localization in rodent and primate brain. Eur J Neurosci 1998; 10: 2464–2471.
Haeberlé AM, Ribaut-Barassin C, Bombarde G, Mariani J, Hussmann G, Grassi J, Bailly Y. Synaptic prion immunoreactivity in the rodent cerebellum. Microsc Res Tech 2000; 50: 66–76.
Fournier JG, Escaig-Haye F, Grigoriev V. Ultrastructural localization of prion proteins: physiological and pathological implications. Microsc Res Tech 2000; 50: 77–88.
Brown DR. Prion and prejudice: normal protein and the synapse. Trends Neurosci 2001; 24: 85–90.
Herms JW, Tings T, Gall S, Madlung A, Giese A, Siebert H, Schürmann P, Windl O, Brose N, Kretzschmar HA. Evidence of presynaptic location and function of the prion protein. J Neurosci 1999;15: 8866–8875.
Westaway D, Telling G, Priola S. Prions. Proc Natl Acad Sci USA 1998; 95: 11030–11031.
Ferrer I, Blanco R, Carmona M, Puig B, Ribera R, Rey MJ, Ribalta T. Prion protein expression in senile plaques in Alzheimer's disease. Acta Neuropathol 2001; 101: 49–56.
Speilhaupter C, Schätzl HM. PrPC directly interacts with proteins involved in signaling pathways. J Biol Chem 2001; 276: 44604–44612
Büeler H, Fisher M, Lang Y, Blurthmann H, Lipp HP, DeArmond SJ, Prusiner SB, Aguet M, Weissmann C. Normal development and behavior of mice lacking the neuronal surface PrP protein. Nature 1992; 365: 577–582.
Manson JC, Clarke AR, Hooper ML, Aitchison L, McConnell I, Hope J. 129/Ola mice carrying a null mutation in PrP that abolishes mRNA production are developmentally normal. Mol Neurobiol 1994; 8: 121–127.
Collinge J, Whittington MA, Sidle KCL, Smith J, Palmer MS, Clarke AR, Jeffreys JGR. Prion protein is necessary for normal synaptic function. Nature 1994; 370: 295–297.
Tobler I, Gaus SE, Deboer T, Achermann P, Fisher M, Rülicke T, Moser M, Oesh B, McBride PA, Manson JC. Altered circadian activity rhythms and sleep in mice devoid of prion protein. Nature 1986; 380: 639–642.
Herms JW, Kretzschmar HA, Tilz S, Keller BU. Patch-clamp analysis of synaptic transmission to cerebellar Purkinje cells of prion protein knockout mice. Eur J Neurosci 1995; 12: 2508–2512.
Lledó PM, Tremblay P, DeArmond SJ, Prusiner SB, Nicoll RA. Mice deficient for prion protein exhibit normal neuronal excitability and synaptic transmission in the hippocampus. Proc Natl Acad Sci USA 1996; 93: 2403–2407.
Colling SB, Collinge J, Jefferys JG. Hippocampal slices from prion protein null mice: disruption of Ca2-activated K+ currents. Neurosci Lett 1996; 209: 49–52.
Sakaguchi S, Katamine S, Nishida N, Moriuchi R, Shigematzu K, Sugimoto T, Nakatni T, Kataoka Y, Houtani H, Shirabe S, Okada H, Hasegawa S, Myamoto T, Noda T. Loss of cerebellar Purkinje cells in aged mice homozygous for a disrupted PrP gene. Nature 1996; 380: 528–531.
Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science 1982; 216: 136–144.
Prusiner SB. Scrapie prions. Annu Rev Microbiol 1989; 43: 345–374.
Prusiner SB. Molecular biology of prion diseases. Science 1991; 252: 1515–1522.
Pan KM, Baldwin M, Nguyen J, Gasset M, Serban A, Groth D, Mehlhom I, Huang Z, Fletterick RJ, Cohen FE, Prusiner SB. Conversion of alpha-helices into the beta-sheets features in the formation of the scrapie prion proteins. Proc Natl Acad Sci USA 1993; 90: 10962–10966.
Aguzzi A, Weissmann C. Prion research: the next frontiers. Nature 1997; 389: 795–798.
Soto C, Saborio GP. Prions: disease propagation and disease therapy by conformational transmission. Trends Mol Med 2001; 7: 109–114.
Hadlow WJ. Neuropathology and the scrapie-kuru connection. Brain Pathol 1995; 5: 27–32.
Wells GAH, Wilesmith JW. The neuropathology and epidemiology of bovine spongiform encephalopathy. Brain Pathol 1995; 5: 91–103.
Collinge J, Palmer MS, editors. Prion diseases. Oxford: Oxford University Press, 1997.
McLean CA, Ironside JW, Alpers MP, Brown PW, Cervenakova L, Anderson RM, Masters CL. Comparative neuropathology of kuru with the new variant of Creutzfeldt-Jakob disease. Evidence for strain and agent predominating over genotype of host. Brain Pathol 1998; 8: 429–438.
Masters CL, Gajdusek DC. The spectrum of Creutzfeldt-Jakob disease and the virus-induced subacute spongiform encephalopathies. In: Thomas Smith W, Cavanagh JB, editors. Advances in neuropathology. Edinburgh: Churchill Livingstone, 1982; 2: 139–143.
Beck E, Daniel PM. Neuropathology of transmissible spongiform encephalopathies. In: Prusiner SB, McKinley MP, editors. Prions: novel infectious pathogens causing scrapie and Creutzfeldt-Jakob disease. Orlando: Academic Press, 1987: 331–385.
DeArmond SJ, Prusiner SB. Etiology and pathogenesis of prion diseases. Am J Pathol 1995; 146: 785–811.
Ironside JW. Review: Creutzfeldt-Jakob disease. Brain Pathol 1996; 6: 379–388.
Will RG, Ironside JW, Zeidler M, Cousens SN, Estebeiro K, Alperovitch A, Pocchiari M, Hofman A, Smith PG. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet 1996; 347: 921–925.
Collinge J, Palmer MS, Sidle KCL, Gowland I, Medori R, Ironside J, Lantos. Transmission of fatal familial insomnia to laboratory animals. Lancet 1995; 346: 569–570.
Telling GC. Prion protein genes and prion diseases: studies in transgenic mice. Neuropathol Appl Neurobiol 2000; 26: 209–220.
Muramoto T, Kitamoto J, Tateishi J, Goto I. The sequential development of abnormal prion protein accumulation in mice with Creutzfeldt-Jakob disease. Am J Pathol 1992; 140: 1411–1420.
Muramoto T, Kitamoto J, Tateishi J, Goto I. Accumulation of abnormal prion protein in mice with Creutzfeldt-Jakob disease via intra-peritoneal route: a sequential study. Am J Pathol 1993; 143: 1470–1479.
Büeler H, Aguzzi A, Sailer A, Greiner RA, Autenreid P, Aguet M, Weissmann C. Mice devoid of PrP are resistant to scrapie. Cell 1993; 73: 1339–1347.
Prusiner SB, Groth D, Serban A, Koehler R, Foster D, Torchia M, Burton D, Yang SL, DeArmond SJ. Ablation of the prion protein (PrP) gene in mice prevents scrapie and facilitates production of anti-PrP antibodies. Proc Natl Acad Sci USA 1993; 90: 10608–10612.
Sailer A, Büeler H, Fischer M, Aguzzi A, Weissmann C. No propagation of prions in mice devoid of PrP. Cell 1994; 77: 967–968.
Brown DR, Herms J, Kretzschmar HA. Mouse cortical cells lacking cellular PrP survive in culture with a neurotoxic PrP fragment. NeuroReport 1994; 5: 2057–2060.
Fischer M, Rülicke T, Raeber A, Sailer A, Moser M, Oesch B, Brandner S, Aguzzi A, Weissmann C. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J 1996; 15: 1255–1264.
Hsiao KK, Scott M, Foster D, Groth DF, DeArmond SJ, Prusiner SB. Spontaneous neurodegeneration in transgenic mice with mutant prion protein. Science 1990; 250: 1587–1590.
DeArmond SJ, Kristensson K, Bowler RP. PrPSC causes nerve cell death and stimulates astrocyte proliferation: a paradox. Progr Brain Res 1992; 94: 437–446.
Bruce ME, McBride PA, Farquhar CF. Precise targeting of the pathology of the syaloglycoprotein PrP and vacuolar degeneration in mouse scrapie. Neurosci Lett 1989; 102: 1–6.
Kitamoto T, Shin RW, Don-una K, Tonokane N, Miyazono N, Muramoto T, Tateishi J. Abnormal isoform of prion protein accumulates in the synaptic structures of the central nervous system in patients with Creutzfeldt-Jakob disease. Am J Pathol 1992; 140: 1285–1294.
Parchi P, Castellani R, Capellari S, Ghetti B, Young K, Chen SG, Farlow M, Dickson DW, Sima AAF, Trojanowski JQ, Petersen RB, Gambetti P. Molecular basis of phenotyping variability in sporadic Creutzfeldt-Jakob disease. Ann Neurol 1996; 39: 767–778.
Ferrer I, Ribera R, Blanco R, Marti E. Expression of proteins linked to exocytosis and neurotransmission in patients with Creutzfeldt-Jakob disease. Neurobiol Dis 1999; 6: 92–100.
Ferrer I, Puig B, Blanco R, Marti E. Prion protein deposition and abnormal synaptic protein expression in the cerebellum in Creutzfeldt-Jakob disease. Neuroscience 2000; 97: 715–726.
Jefferys JGR, Empson RM, Wittington MA, Prusiner SB. Scrapie infection of transgenic mice leads to network and intrinsic dysfunction of cortical and hippocampal neurons. Neurobiol Dis 1994; 1: 25–30.
Johnston AR, Black C, Fraser J, McLeod N. Scrapie infection alters the membrane and synaptic properties of mouse hippocampal CA1 pyramidal neurons. J Physiol Lond 1997; 500: 1–15.
Tiller-Borcich JK, Urich H. Abnormal arborisation of Purkinje cell dendrites in Creutzfeldt-Jakob disease: a manifestation of neuronal plasticity? J Neurol Neurosurg Psychiatr 1986; 49: 581–584.
Ferrer I, Kulisewski J, Vázquez J, González A, Pineda M. Purkinje cells in degenerative diseases of the cerebellum and its connections: a Golgi study. Clin Neuropathol 1988; 7: 237–242.
Berciano J, Berciano MT, Polo JM, Figols J, Ciudad J, Lafarga M. Creutzfeldt-Jakob disease with severe involvement of cerebral white matter and cerebellum. Virchows Arch A, Pathol Anat 1990; 417: 533–538.
Parchi P, Giese A, Capwellari S, Brown P, Schulz-Schaeffer W, Windl O, Zerr I, Budka H, Kopp N, Piccardo P, Poser S, Rojiani A, Streichemberger N, Julien J, Vital C, Ghetti B, Grambetti P, Kretzschmar H. Classification of sporadic Creutzfeldt-Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann Neurol 1999; 46: 224–233.
Kitamoto T, Don-ura K, Muramoto T, Niyazono M, Tateishi J. The primary structure of the prion protein influences the distribution of abnormal prion protein. Am J Pathol 1992; 141: 271–272.
Clinton J, Forsyth C, Royston MC, Roberts GW. Synaptic degeneration is the primary neuropathological feature in prion diseases: a preliminary study. NeuroReport 1993; 4: 65–68.
Jeffrey M, Fraser JR, Halliday BWG, Fowler N, Goodsir CM, Brown DA. Early unsuspected neuron and axon terminal loss in scrapie-infected mice revealed by morphometry and immunohistochemistry. Neuropathol Appl Neurobiol 1995; 21: 41–49.
Jeffrey M, Halliday WG, Bell J, Johnston AR, MacLeod NK, Ingham C, Sayers AR, Brown DA, Fraser JR. Synapse loss associated with abnormal PrP precedes neuronal degeneration in the scrapie-infected murine hippocampus. Neuropathol Appl Neurobiol 2000; 26: 41–54.
Forloni G, Angeretti N, Chiesa R, Monzani E, Salmona M, Bugiani O, Tagliavini F. Neurotoxicity of a prion protein fragment. Nature 1993; 362: 543–546.
Brown DR, Herms J, Schmidt B, Kretzschmar HA. Different requirements for the neurotoxicity of fragments PrP and β-amyloid. Eur J Neurosci 1997; 9: 162–169.
Brown DR, Pitschke M, Riesner D, Kretzschmar HA. Cellular effects of a neurotoxic prion protein peptide are related to its β-sheet configuration. Neurosci Res Commun 1998; 23: 119–128.
Müller WEG, Ushijima H, Schröder HC, Forrest JM, Schatton WF, Rytik PG, Heffner-Lauc M. Cytoprotective effect of NMDA receptor antagonists on prion protein (PrionSc)-induced toxicity in rat cortical cell cultures. Eur J Pharmacol 1993; 246: 261–267.
Schätzl HM, Laszlo L, Holtzman DM, Tatzelt J, DeArmond SJ, Weiner RI, Mobley WC, Prusiner SB. A hypothalamic neuronal cell line persistently infected with scrapie prions exhibits apoptosis. J Virol 1997; 71: 8821–8831.
Fairbairn DW, Carnahan KG, Thwaits RN, Grisby RV, Holyoak GR, O'Neill KL. Detection of apoptosis-induced DNA cleavage in scrapie-infected sheep brain. FEMS Microbiol Lett 1994; 115: 341–346.
Giese A, Groschup M, Hess B, Kretzschmar H. Neuronal cell death in scrapie-infected mice is due to apoptosis. Brain Pathol 1995; 5: 213–221.
Lucassen PJ, Williams A, Chung WC, Fraser H. Detection of apoptosis in murine scrapie. Neurosci Lett 1995; 198: 185–188.
Williams A, Lucassen PJ, Ritchie D, Bruce M. PrP deposition, microglial activation and neuronal apoptosis in murine scrapie. Exp Neurol 1997; 144: 433–438.
Giese A, Brown DR, Groschup MH, Feldmann C, Haist I, Kretzschmar HA. Role of microglia in neuronal cell death in prion disease. Brain Pathol 1998; 8: 449–457.
Dorandeu A, Wingerstmann L, Chrétien F, Delisle MB, Vital C, Parchi P, Montagna P, Lugarcsi E, Ironside JW, Budka H, Gambetti P, Gray F. Neuronal apoptosis in fatal familial insomnia. Brain Pathol 1998; 8: 531–537.
Gray F, Chrétien F, Adle-Biassette H, Dorandeu A, Ereau T, Delisle MB, Kopp N, Ironside JW, Vital C. Neuronal apoptosis in Creutzfeldt-Jakob disease. J Neuropathol Exp Neurol 1999; 58: 321–328.
Ferrer I. Nuclear DNA fragmentation in Creutzfeldt-Jakob disease: does a mere positive in situ nuclear end-labeling indicate apoptosis? Acta Neuropathol 1998; 97: 5–12.
Park SK, Choi SI, Jin JK, Choi EK, Kim JI, Carp RI, Kim YS. Differential expression of Bax and Bcl-2 in the brains of hamsters infected with 263K scrapie agent. NeuroReport 2000; 11: 1677–1682.
Puig B, Ferrer I. Cell death in the cerebellum in Creutzfeldt-Jakob disease. Acta Neuropathol 2001; 102: 207–215.
Siso S, Puig B, Varea R, Vidal E, Acin C, Prinz M, Montrasio F, Badiola J, Aguzzi A, Pumarola M, Ferrer I. Abnormal synaptic protein expression and cell death in murine scrapie. Acta Neuropathol 2002; 103: 615–626.
Giese A, Kretzschmar HA. Prion-induced neuronal damage. The mechanisms of neuronal destruction in the subacute spongiform encephalopathies. Curr Topics Microbiol Immunol 2001; 253: 203–217.
Lampert PW, Gajdusek DC, Gibbs Jr CJ. Pathology of dendrites in subacute spongiform virus encephalopathies. Adv Neurol 1975; 12: 465–469.
Gonatas NK, Terry RD, Weiss M. Electron microscopic study in two cases of Jakob-Creutzfeldt disease. J Neuropathol Exp Neurol 1965; 24: 575–598.
Bignami A, Forno LS. Status spongiosus in Jakob-Creutzfeldt disease. Electron microscopic study of a cortical biopsy. Brain 1970; 93: 89–93.
Choi SI, Ku WK, Choi EK, Kim J, Lea H, Carp RI, Wisniewski HM, Kim YS. Mitochondrial dysfunction induced by oxidative stress in the brains of hamsters infected with the 263K scrapie agent. Acta Neuropathol 1998; 96: 279–286.
Boellard JW, Schlote W, Tateishi J. Neuronal autophagy in experimental Creutzfeldt-Jakob's disease. Acta Neuropathol 1989; 78: 410–418.
Jeffrey M, Scott JR, Williams A, Fraser H. Ultrastructural features of spongiform encephalopathy transmitted to mice from three species of bovidae. Acta Neuropathol 1992; 84: 559–569.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferrer, I. Synaptic pathology and cell death in the cerebellum in Creutzfeldt-Jakob disease. Cerebellum 1, 213–222 (2002). https://doi.org/10.1080/14734220260418448
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1080/14734220260418448