-
PDF
- Split View
-
Views
-
Cite
Cite
Javier A. Adachi, Herbert L. DuPont, Rifaximin: A Novel Nonabsorbed Rifamycin for Gastrointestinal Disorders, Clinical Infectious Diseases, Volume 42, Issue 4, 15 February 2006, Pages 541–547, https://doi.org/10.1086/499950
Close - Share Icon Share
Abstract
Rifaximin, a virtually nonabsorbed (<0.4%) rifamycin drug, has in vitro activity against aerobic and anaerobic gram-positive and gram-negative microorganisms. Because rifaximin is nonabsorbed, systemic adverse effects are unusual, and after 3 days of therapy, the fecal level of the drug reaches 8000 µg/g. Moreover, the important selection of resistant mutants by the related drug, rifampin, has not yet been observed for rifaximin. Rifaximin has been demonstrated to reduce the duration of traveler's diarrhea secondary to noninvasive bacterial pathogens and recently has been shown to reduce the occurrence of the disease when used for chemoprophylaxis. Preliminary studies have demonstrated its potential for the treatment of other gastrointestinal disorders, such as hepatic encephalopathy. Additional studies should be performed to further define the role of rifaximin in the treatment of gastrointestinal diseases in adults and children.
Rifaximin is a semisynthetic derivative of rifamycin [1, 2] that was first approved in Italy in 1987, was approved as Xifaxan (Salix Pharmaceuticals) by the US Food and Drug Administration (FDA) in 2004 for the treatment of uncomplicated traveler's diarrhea [3], and is currently approved for use in 17 countries [4]. Rifaximin's additional pyridoimidazole ring makes it virtually nonabsorbable [1, 2, 5], enabling it to achieve a high concentration in the gastrointestinal tract [6] and to be active against enteric infection or abnormal floral states [4, 7, 8].
Mechanisms of Action
Like other rifamycins, rifaximin binds the β subunit of the bacterial DNA-dependent RNA polymerase, inhibiting the initiation of chain formation in RNA synthesis [9]. Rifamycins also apparently modify bacterial pathogenicity [10], and, in subtherapeutic concentrations, they may alter attachment and tissue toxicity [11].
Pharmacokinetics
Rifaximin is not inactivated by the gastric fluids, and it is poorly absorbed, with a bioavailability of <0.4% in the blood following oral administration [12–14]. Approximately 97% of radiolabeled rifaximin is excreted in the feces as unchanged drug, with 0.32% of the dose detected in urine and without detectable levels in bile or breast milk [3, 12]. After the oral administration of 400 mg twice per day for 3 days in travelers with diarrhea, rifaximin's fecal concentration reached 8000 µg/g [6]. Topical rifaximin (5% cream) does not achieve any systemic levels [15].
Dosage adjustments for hepatic dysfunction are not necessary, because of the minimal oral absorption, even in patients with hepatic encephalopathy [3]. Likewise, dosage adjustments for renal insufficiency should not be a concern. Although pharmacokinetics and safety have not been studied in the elderly or during pregnancy, a few studies in children have been conducted in Europe.
Antimicrobial and In Vitro Activity
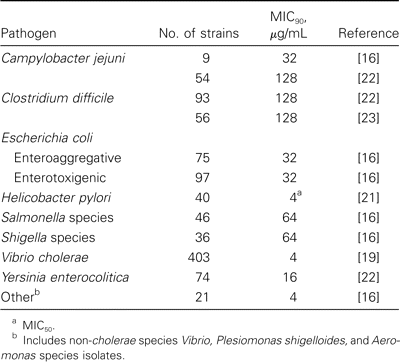
Similar to rifampin, rifaximin has broad-spectrum activity against aerobic and anaerobic gram-positive and gram-negative microorganisms [4]. Traditional MIC breakpoints for rifampin Clinical and Laboratory Standards Institute (CLSI; formerly NCCLS) criteria are based on plasma levels and systemic infection and have little relevance to treatment of an enteric infection with a nonabsorbed drug, such as rifaximin. The drug has low solubility in water and high solubility in bile, which may translate to greater antimicrobial effects in the small bowel than in the aqueous colon.
In table 1, in vitro activity of rifaximin is shown as an MIC90, with a dosage of ⩽64 µg/mL having activity against the majority of bacterial enteropathogens associated with diarrhea [3, 16–22]. The drug also has in vitro activity against Clostridium difficile, Helicobacter pylori, Yersinia enterocolitica, and Shigella species [17, 20–23], with MICs that are 80–500 times lower than the concentration of rifaximin in the feces [6]. Other gram-negative bacilli that are usually nonrelated to diarrhea (Klebsiella, Enterobacter, Proteus, Acinetobacter, and Pseudomonas species) demonstrated MIC90 at levels between 4 and 128 µg/mL [4, 17].
Rifaximin has a lower MIC against gram-positive bacteria, with an MIC90 at dosages ranging from ⩽0.01 to 0.5 µg/mL, than it does against methicillin-resistant Staphylococcus aureus (MIC90, 8–16 µg/mL) and enterococcus (MIC90, 4–16 µg/mL) [4, 17]. The drug is also active against anaerobes, with an MIC90 at dosages between 0.25 and 128 µg/mL, and it is active against Gardnerella vaginalis, Mobilincus species, Cryptosporidium parvum, and Blastocystis hominis [20, 24].
Effect In Gastrointestinal Flora and The Development of Resistance
Surprisingly, rifaximin has little effect on the normal gastrointestinal flora. After 3 cycles of receiving 1800 mg per day for 10 days, followed by 25 days free of medication, there was an initial decrease in the gastrointestinal flora (enterococcus, Escherichia coli, Lactobacillus species, Bifidobacterium species, Bacteroides species, and Clostridium perfringens), followed by normalization after 1 month, without Candida species overgrowth [25]. Our group has also shown that rifaximin has minimal effects on E. coli and enterococcus flora after 3–14 days of therapy [26, 27]. One finding of the study was a 6-log increase in the median growth of intestinal coliform counts on culture media containing rifaximin 64 µg/mL, after 7 and 14 days of rifaximin administration in 3 of 6 groups tested [27]. The MIC50 and MIC90 of coliforms from the rifaximin groups combined showed a value that was 1 dilution higher than the isolates obtained from the placebo group. It will be important to monitor for increasing resistance as the drug is used more broadly, especially for chemoprophylaxis. Concerns about the broad use of an antimicrobial drug in the prevention of traveler's diarrhea include the development of drug-resistant enteric flora, because of its impact on superinfection (e.g., C. difficile diarrhea), and the emergence of resistant bacteria that spreads extra-intestinally (e.g., vancomycin-resistant enterococcus).
Theoretically, the high number of bacteria in the gastrointestinal tract could increase the probability of spontaneous chromosomal point mutations with resistance to rifaximin. It is well known that rifampin is a major stimulant of resistance, but the development of resistance to rifaximin appears to occur with a lower frequency, possibly because of causes related to pharmacokinetics or differences in the mechanism of action [26, 28]. Currently, there is no evidence of cross-resistance between rifampin and rifaximin, but this possibility should be studied further.
One public health concern about the broad use of rifaximin is the possibility of the induction of resistant strains of Mycobacterium tuberculosis that lessen the therapeutic value of rifampin. Studies using guinea pigs and 1 clinical study have demonstrated that there is no induction of resistance between rifampin and rifaximin. Moreover, M. tuberculosis resistance to rifampin apparently is not plasmid associated, and the risk of horizontal transmission of resistance appears to be low [29–34].
Clinical Studies
Traveler's Diarrhea
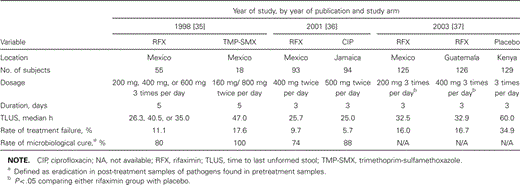
Therapy. Rifaximin was approved in May 2004 by the FDA for the treatment of traveler's diarrhea caused only by noninvasive strains of E. coli in patients ⩾12 years of age. The approved dosage is 200 mg 3 times per day for 3 days, and the drug should not be used in cases complicated by fever or blood in the stool or in patients with diarrhea due to invasive pathogens. This approval was made on the basis of findings of 3 randomized, double-blinded clinical trials in travelers with diarrhea acquired in Guatemala, Mexico, Jamaica, and Kenya [35–37], in which reduction in the duration of postenrollment diarrhea was observed in travelers given rifaximin, compared with travelers given placebo, or in which an equivalent response to rifaximin was observed throughout the duration, compared with the response to ciprofloxacin. The study design of all 3 studies was basically identical. All subjects were at least 16–18 years of age and had traveler's diarrhea, which was defined as ⩾3 unformed stools during the 24 h prior to enrollment accompanied by ⩾1 symptom of enteric infection (abdominal pain, excess gas, nausea or vomiting, fecal urgency, tenesmus, or fever [temperature, ⩾37.8 C]) and with a duration of ⩽72 h. The primary endpoint for all studies was the time to the last unformed stool, which was defined as the time between taking the first dose of medication until the passage of the last unformed stool after which wellness was documented. Secondary endpoints were the improvement of diarrhea (⩾50% reduction in the number of unformed stools over a 24-h period of time), the number of unformed stools for each 24-h period, treatment failure, and eradication of pretreatment bacterial pathogens in post-treatment stool samples (or microbiological cure of infection with enterotoxigenic E. coli and Salmonella, Shigella, Campylobacter, Aeromonas, Plesiomonas, and Vibrio species, among others).
Table 2 summarizes the published clinical trials of rifaximin therapy of traveler's diarrhea. In the first study, rifaximin given at a dosage of 200, 400, or 600 mg 3 times per day for 5 days (n = 55) achieved lower values for the time to the last unformed stool, compared with trimethoprim-sulfamethoxazole (TMP-SMX) given at a dosage of 160/800 mg twice per day for 5 days (n = 18), although the reduction in illness was nonstatistically significant [35]. In the second study, rifaximin given at a dosage of 400 mg twice per day for 3 days (n = 93) was as effective as ciprofloxacin given at a dosage of 500 mg twice per day for 3 days (n = 94) [36]. In the last multicenter, placebo-controlled trial, rifaximin given at a dosage of 200 mg (n = 125) or 400 mg (n = 126) 3 times per day for 3 days significantly shortened the time to the last unformed stool, compared with placebo (n = 129) [37]. The number of unformed stools per 24-h period and the number of subjects considered to be healthy after receiving therapy were similar between subjects receiving rifaximin or ciprofloxacin. The rate of microbiologic cure associated with rifaximin was lower than the rates associated with TMP-SMX and ciprofloxacin, but greater than the rate associated with placebo. We feel that the important effect of an antibacterial drug in the treatment of traveler's diarrhea is the reduction of illness, rather than the eradication of a pathogen from a nonsterile gut, especially in an environment where travelers do not represent the important reservoir of enteric pathogens [38].
Controlled clinical trials of rifaximin in the treatment of traveler's diarrhea.
Although the approved dosage for the treatment of traveler's diarrhea is 200 mg twice per day for 3 days, a dosage of 400 mg 3 times per day is apparently equally effective and may be better tolerated by patients [37]. It is our opinion that either dose is equally effective and can be recommended.
Chemoprophylaxis. More recently, in 1 randomized, double-blind, placebo-controlled study [27], our group demonstrated that rifaximin is effective for the prevention of traveler's diarrhea. Within 72 h after their arrival in Mexico, 210 travelers were randomized to receive either placebo or 1 of the following 3 dosages of rifaximin for 2 weeks: 200 mg once per day, 200 mg twice per day, or 200 mg 3 times per day. The percentage of subjects who received rifaximin who did not have traveler's diarrhea during the follow-up period of 2 weeks (i.e., the percentage of subjects receiving rifaximin who were free of disease) was 75%–85%, a significantly better rate than the rate among subjects who received placebo alone (42%) [27]. Rifaximin fulfills the characteristics of an ideal drug for the prevention of traveler's diarrhea. It is active in vitro, it is safe (because it is nonabsorbed), and it has a low potential for inducing resistance [39]. Given the importance of the morbidity associated with traveler's diarrhea and the potential for development of chronic irritable bowel syndrome [40], prompt antimicrobial therapy or prevention of diarrhea during high-risk travel is advisable. Rifaximin prophylaxis is likely to become more widespread because of its safety and efficacy. Studies are being planned for Thailand to evaluate the drug's ability to prevent invasive forms of traveler's diarrhea caused by Campylobacter, Shigella, and Salmonella species. A volunteer study demonstrated that rifaximin was effective in preventing experimental shigellosis [41] and provided evidence that the drug would be active against the development of invasive forms of enteric infection.
Other Possible Indications for Rifaximin Therapy (Non-FDA Approved)
Infectious diarrhea. Several uncontrolled clinical trials have suggested that rifaximin is an effective alternative for the treatment of infectious diarrhea in nontravelers [42–48]. In an open-label study, 20 hospitalized adults with acute enterocolitis treated with rifaximin experienced clinical improvement, with an 80% pathogen-eradication rate [42]. In a double-blind study, 121 elderly patients with diarrhea received rifaximin or placebo, with better reduction in the number of unformed stools and in the duration of symptoms in the rifaximin group [43].
Although there are limited data regarding the effectiveness of rifaximin in children with bacterial diarrhea, and although rifaximin is not approved for use in children, a pediatric suspension form is available in some countries. The first study involving children with infectious diarrhea was conducted in 1984 and showed that rifaximin was significantly superior to placebo [44]. In a small study of 46 children receiving antimicrobial prophylaxis for genito-urinary disorders with acute recurrent diarrhea, 93% of rifaximin-treated children experienced an improved condition, compared with 44% of children receiving oral rehydration alone [45]. Finally, a study that compared rifaximin with rehydration in the treatment of 146 children with acute diarrhea showed reduction of the duration of diarrhea in the rifaximin group [46].
On the basis of its in vitro activity [17, 20, 22], rifaximin is being evaluated for possible efficacy in the treatment of C. difficile–associated diarrhea. One small randomized study demonstrated similar efficacy in reducing the duration of diarrhea when either rifaximin or oral vancomycin was given to patients with C. difficile–associated diarrhea [47]. A large, multicenter, clinical trial of rifaximin versus oral vancomycin is currently being planned in the United States.
Hepatic encephalopathy. Several studies have demonstrated that rifaximin may be an alternative to current treatment options for hepatic encephalopathy. In a dose-ranging, randomized, double-blind study of 54 patients with cirrhosis that compared dosages of rifaximin at 200 mg, 400 mg, and 800 mg 3 times per day for 7 days, the 2 highest dosages demonstrated improvement of symptoms of hepatic encephalopathy and reduction of blood ammonia levels [49].
Three double-blind studies compared rifaximin at a dosage of 400 mg 3 times per day with lactulose for the treatment of hepatic encephalopathy [49–51]. In those studies, improved cognitive function and reduction of ammonia levels favored rifaximin treatment, compared with treatment with lactulose. Rifaximin was shown to be comparable to neomycin in the treatment of hepatic encephalopathy [52–54].
A randomized, double-blind, multicenter study comparing rifaximin with lactilol (dosage, 20 g 3 times per day) demonstrated similar efficacy for both drugs, with a more significant reduction of ammonia levels associated with rifaximin [55]. However, neither rifaximin nor lactilol were beneficial in the prevention of hepatic encephalopathy following transjugular intrahepatic portosystemic shunt [56]. Rifaximin has been given “orphan” status by the FDA for use in the treatment of hepatic encephalopathy; the drug's status is important for its use as treatment for hepatic encephalopathy and for having its cost reimbursed by insurance plans.
Small bowel bacterial overgrowth, intestinal gas, and gas-associated symptoms. Rifaximin appears to be effective in reducing small bowel flora, with subsequent reduction of the production of intestinal gas (e.g., H2 and methane), resulting in the improvement of abdominal distension, bloating, flatulence, and excessive gas production. In a double-blind study, 34 patients with irritable bowel syndrome were randomized to receive rifaximin or activated charcoal. The rifaximin group experienced reduction in the production of H2, together with a decreased severity of symptoms and abdominal girth. There were no changes in bloating, abdominal pain, or production of CH4 in either group [57].
Rifaximin may help to resolve the malabsorption secondary to small-bowel bacterial overgrowth, because of its bile-soluble and nonabsorbable properties. In a small, randomized, double-blind study [58], 10 patients received rifaximin at a dosage of 400 mg 3 times per day, and 11 patients received chlortetracycline at a dosage of 333 mg 3 times per day for 7 days. Normalization of breath-hydrogen test results occurred in 70% of rifaximin-treated versus 27% of tetracycline-treated patients (P < .01).
Inflammatory bowel disease. A small, randomized, double-blind, placebo-controlled study of 26 patients with ulcerative colitis refractory to steroids demonstrated a nonsignificant improvement of condition in 64% of subjects in the rifaximin group, compared with 42% of subjects who received placebo [13]. In another recent, open-label study, 10 patients with active ulcerative colitis were treated with rifaximin plus mesalamine for 4 weeks, resulting in 70% clinical remission, sparing the use of steroids [59]. In a subset of patients with pouchitis (inflammation of the ileal reservoir after pouch surgery for ulcerative colitis) refractory to other treatments, 89% of the 18 patients treated with rifaximin plus ciprofloxacin for 15 days showed clinical improvement (including 33% of patients in remission) [60]. Also, for patients with active Crohn disease, an open-label study of rifaximin administered for 16 weeks demonstrated clinical remission in 62% of patients [61].
From the data available, it appears that rifaximin may be of value in a subset of patients with irritable bowel disease. Additional controlled clinical trials are needed to confirm the preliminary data and to identify the patients for whom rifaximin treatment should be recommended.
Diverticular disease. Some studies have suggested that rifaximin (in combination with fiber or mesalazine) could be beneficial in the treatment and prevention of nonsevere, uncomplicated diverticular disease, effecting a better and faster relief of symptoms and a lower incidence of diverticulitis, recurrence, and rectal bleeding [62, 63].
Adverse Effects and Safety Profile
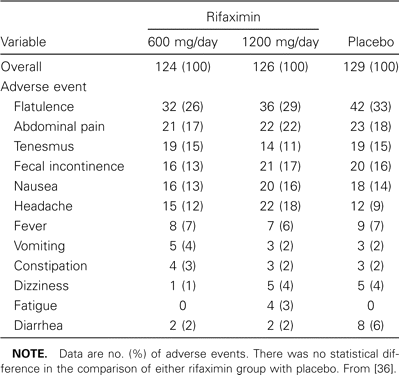
The 3 clinical trials of rifaximin in patients with traveler's diarrhea mentioned above (cumulative number of subject, 500) demonstrated that this drug was well tolerated, with minor adverse experiences occurring at rates similar to that observed among placebo recipients (table 3) [35–37]. Although headache was more common in the rifaximin group in 1 study, the integrated adverse-event data from all studies that compared placebo with rifaximin showed a nonsignificant difference. Rifaximin is unlikely to have drug interactions with other medications metabolized by the cytochrome P-450 system [3, 37, 64, 65]. Postmarketing surveillance conducted from 1996 to 2002 in Europe and other countries revealed an excellent safety profile for rifaximin (Salix Pharmaceuticals, unpublished data).
Adverse events in a placebo-controlled trial of rifaximin in the treatment of traveler's diarrhea.
Limitations of Rifaximin In Enteric Infections
Because of the drug's nonabsorption, rifaximin should not be used to treat systemic infections or mucosally invasive enteric infections. Rifaximin is not recommended for the treatment of dysenteric diarrhea secondary to Shigella, Salmonella, or Campylobacter species infection or for the treatment of complicated diarrhea with fever, systemic toxicity, or bloody diarrhea [3, 37]. Travelers relying on rifaximin for self-administered therapy should be informed that the drug is not effective for treatment of bloody diarrhea or diarrhea with fever.
Depending on the dosage and the comparative drug, rifaximin may be slightly more expensive than some available treatments. The current approximate cost is $3.80 per 200 mg tablet, for a total of $34.20 for the recommended course of treatment of traveler's diarrhea. Chemoprophylaxis of traveler's diarrhea costs ∼$7.60 per day at risk, if 2 tablets are taken each day. The recommended dosage of rifaximin to treat traveler's diarrhea is 3 times per day, compared with the recommended dosage of quinolones or azalides, which can be given once per day.
The only contraindication of rifaximin use is history of hypersensitivity to a rifamycin drug [3]. Because of its lack of absorption, rifaximin should be safe for use in the pediatric population and in pregnant and breastfeeding women. Rifaximin is currently not approved for these populations.
Dosing and Product Availability
Rifaximin is available in the United States only as a 200-mg tablet [3]. In a few other countries, a pediatric suspension is available (dose size, 100 mg and/or 5 mL) [44, 45].
Conclusions and Future Considerations
Rifaximin is effective in the treatment of nondysenteric and afebrile bacterial diarrhea found in ∼90% of travelers with illness. Recently, rifaximin was successfully used for prophylaxis of traveler's diarrhea.
Additional trials should evaluate the therapeutic value of this medication in the treatment other intestinal disorders for which preliminary data suggest its potential value. These disorders include small-bowel bacterial overgrowth, inflammatory bowel disease, diverticular disease, and hepatic encephalopathy. Also, additional studies should be conducted to help define the mechanisms of action, the role in alteration of bacterial virulence, and the safety and effectiveness of this antibiotic in special populations (e.g., pregnant women and children).
Additional areas in which rifaximin could be evaluated for efficacy and safety include the following: treatment and prevention of C. difficile infection, prevention of spontaneous bacterial peritonitis in patients with liver disease, and prophylaxis of immunosuppressed patients (e.g., neutropenic patients). An unlikely use but a potential need is the treatment and prevention of widespread foodborne outbreaks related to bioterrorism.
Acknowledgments
Dr. Zhi-Dong Jiang, Dr. Karen J. Vigil, and Mrs. Margaret W. DuPont provided valuable assistance to this review.
Financial support. Salix Pharmaceuticals, Alfa Wassermann (Italy), and the National Institutes of Health (DK 56338).
Potential conflicts of interest. H.L.D. is serving as a consultant to and has received research grants from Salix Pharmaceuticals and Alfa Wassermann SPA. J.A.A.: no conflicts.





Comments