-
PDF
- Split View
-
Views
-
Cite
Cite
Jonathan S. F. Lee, Andrew H. Bass, Dimorphic male midshipman fish: reduced sexual selection or sexual selection for reduced characters?, Behavioral Ecology, Volume 17, Issue 4, July/August 2006, Pages 670–675, https://doi.org/10.1093/beheco/ark015
Close - Share Icon Share
Abstract
In most taxa with male dimorphisms, some males are large in body size with exaggerated secondary sexual characters (exaggerated morph), whereas other males in the same population are small and have reduced secondary sexual characters (reduced morph). What selective pressures cause male dimorphisms? Reduced morphologies may result when a) some males develop a morphology that, in the absence of sexual selection pressures for an exaggerated morphology, reduces energetic and developmental costs and/or b) some males opt for an alternative morphology that does well at an alternative behavioral tactic such as cuckoldry. The 2 mechanisms could act together, but each alone is theoretically sufficient to drive dimorphisms. Here, we tested hypothesis “b” (sexual selection for reduced characters) in the plainfin midshipman fish, Porichthys notatus. Behavioral plasticity between territoriality and cuckoldry in an exaggerated male morph (type I) allows for a direct comparison of cuckoldry by exaggerated morph males to cuckoldry by reduced morph (type II) males. Compared with type I cuckolders, type II cuckolders were able to remain near the nest for longer periods before being chased by the territorial type I male, suggesting that the reduced type II morphology allows type II males to prolong the time before attack by territorial males. Combined with other studies showing a role of sexual selection in maintaining the exaggerated morph, the data support the “sexual selection for reduced characters” hypothesis and elucidate how sexual selection can act in different ways on different males to maintain 2 male morphologies within a single species.
In many species, male competition over females leads to unequal reproductive success among males, which in turn plays a major role in shaping male behavior and morphology (Andersson 1994). Although many studies have demonstrated how sexual selection can maintain variation in behavioral reproductive tactics (Taborsky 1994; Gross 1996; Brockmann 2001; Lee 2005), fewer have addressed its role in the maintenance of variation in male morphologies.
Sexual selection can lead to exaggerated traits that help males attract females and fight other males (Zahavi 1975; Andersson 1994). Accordingly, compared with females, males of many species are often more colorful, larger in body size, and have elaborate appendages such as horns and longer tails (Andersson 1994).
In species with dimorphic males, morphological variation is bimodally distributed within a single sex (Taborsky 1994; Gross 1996). Some males are exaggerated in morphology, whereas the same structures in other males are greatly reduced (e.g., nematode: Ainsworth 1990; amphipod: Clark 1997; fig wasp: Cook et al. 1997; bee: Danforth 1991; bird: Lank et al. 1995; dung beetle: Moczek and Emlen 1999; lizard: Moore et al. 1998; mite: Radwan and Klimas 2001; earwig: Tomkins and Brown 2004; fish: Uglem et al. 2001; orangutan: Utami et al. 2002).
“Exaggerated” and “reduced” morphologies are often associated with the behavioral tactics of territoriality/courtship and cuckoldry, respectively (Gross 1996). Cuckoldry provides males the opportunity to obtain fertilizations without having to compete with other males to obtain territories or attract females (Taborsky 1994). Because cuckolders are not subject to the same sexual selective forces that lead to the development of exaggerated morphological traits in territorial/courting males, natural selection may reduce unnecessary costs and lead to the reduced morphology. This hypothesis, which we will refer to as “reduced sexual selection for the exaggerated morphology,” is compatible when the 2 morphs have equal fitness (e.g., when the benefit of earlier time to sexual maturation and reduced energy expenditure offsets the cost of having a less optimal phenotype) as well as when the 2 morphs coexist with unequal fitness under a conditional strategy (e.g., when the benefit of earlier time to sexual maturation is exceeded by the cost of having a less functional phenotype; the more functional phenotype is not adopted because the individual's low condition precludes it).
A second mechanism that we will refer to as “sexual selection for reduced characters” is not mutually exclusive but could independently explain reduced morphologies. Sexual selection could select for reduced characters if reduced characters provide a functional advantage over exaggerated ones (see Greene et al. 2000; Moczek and Emlen 2000). For example, a smaller and more drab morphology may make it easier to gain proximity to the female during cuckoldry via covert tactics (Gadgil 1972; Andersson 1994). This mechanism is compatible when the 2 morphs have equal fitness (e.g., when a male adopts the reduced morphology because negative frequency dependence selects for individuals that adopt the morph that is best for the rarer tactic) as well as when the 2 morphs coexist with unequal fitness (e.g., when the fitness payoff associated with the reduced morphology developed by a low-condition individual is superior to the fitness payoff associated with the exaggerated morphology developed by that low-condition individual but inferior to the fitness payoff associated with the exaggerated morphology developed by a higher condition individual).
Although the 2 mechanisms are not mutually exclusive (see Moczek and Emlen 2000), they can be separated; theoretically, each alone could sufficiently explain the evolution of reduced morphologies (see Table 1). The “reduced sexual selection for exaggerated characters” hypothesis predicts i) some energetic and/or temporal savings (e.g., earlier onset of gonadal maturation), whereas the sexual selection for reduced characters hypothesis predicts ii) a functional benefit. If both hypotheses are correct, then both “i” and “ii” should exist. However, if reduced sexual selection for exaggerated characters but not sexual selection for reduced characters is responsible, then “i” should exist and “ii” should not. If the sexual selection for reduced characters but not reduced sexual selection for exaggerated characters is responsible, then the opposite is predicted.
Two hypotheses may account for the evolutionary maintenance of reduced morphologies
| Hypothesis . | Prediction . |
|---|---|
| Reduced sexual selection for exaggerated characters | Energetic or temporal savings of reduced over exaggerated morphology |
| Sexual selection for reduced characters | Functional benefit of reduced over exaggerated morphology |
| Hypothesis . | Prediction . |
|---|---|
| Reduced sexual selection for exaggerated characters | Energetic or temporal savings of reduced over exaggerated morphology |
| Sexual selection for reduced characters | Functional benefit of reduced over exaggerated morphology |
Theoretically, each hypothesis could alone sufficiently explain reduced morphologies. However, each hypothesis is not mutually exclusive of the other and thus could also act along side the other. In this study, we test the Sexual selection for reduced characters hypothesis.
Two hypotheses may account for the evolutionary maintenance of reduced morphologies
| Hypothesis . | Prediction . |
|---|---|
| Reduced sexual selection for exaggerated characters | Energetic or temporal savings of reduced over exaggerated morphology |
| Sexual selection for reduced characters | Functional benefit of reduced over exaggerated morphology |
| Hypothesis . | Prediction . |
|---|---|
| Reduced sexual selection for exaggerated characters | Energetic or temporal savings of reduced over exaggerated morphology |
| Sexual selection for reduced characters | Functional benefit of reduced over exaggerated morphology |
Theoretically, each hypothesis could alone sufficiently explain reduced morphologies. However, each hypothesis is not mutually exclusive of the other and thus could also act along side the other. In this study, we test the Sexual selection for reduced characters hypothesis.
In this study, we test the sexual selection for reduced characters hypothesis in the midshipman fish, Porichthys notatus. This hypothesis is often assumed to be correct but is rarely directly tested (see Discussion for a definition of direct vs. indirect tests).
Midshipman fish
Juvenile male midshipman fish adopt alternative growth trajectories leading to 1 of 2 reproductive morphs known as type I and type II that have, respectively, the exaggerated and reduced morphologies (review: Bass 1996). Some type I males are territorial, defending nests under rocks (Arora 1948; Crane 1981). Type I males can be large, reaching 350 g after several years of growth (DeMartini 1988; Bass et al. 1996) (type I males are DeMartini's pair-spawner males). Type I males acoustically court females with advertisement “hums” and emit aggressive vocalizations to other males (Ibara et al. 1983; Brantley and Bass 1994; McKibben and Bass 1998). Hums draw females to the nests of territorial males, where they lay eggs on the nest substrate and depart soon after spawning is completed. The external somatic traits that best distinguish type I and type II males are body size, head shape, and ventral coloration (Bass 1996; Bass and McKibben 2003). Compared with type I males, type II males are smaller with narrower heads and ventral coloration more similar to that of females than type I males (Bass 1996). The wider heads and mouths of type I males are used in combat with other males (mouth locking) and in the holding and positioning of females during spawning (Brantley and Bass 1994). Type II males cuckold and have never been observed to hold territories (Brantley and Bass 1994).
The reduced morphology of type II males potentially could be explained by the reduced sexual selection for the exaggerated morphology hypothesis, that is, a reduced investment in exaggerated structures that function in territoriality and courtship (see above). Simultaneously or alternatively, the reduced morphology of type II males could be explained by the sexual selection for reduced characters hypothesis.
A recent study on type I males showed that they exhibit behavioral plasticity from territoriality/courtship to cuckoldry (Lee and Bass 2004). Of those type I males that cuckolded, larger ones were able to gain closer proximity to the spawning pair. Given that proximity to the nest is likely to facilitate fertilization, especially in nesting fishes that externally fertilize (Taborsky 1994 and references therein), how could sexual selection for reduced characters have a part in the evolutionary maintenance of the much reduced morphology of type II males if the ability of type I cuckolders to get close to the nest increases with body size? Does sexual selection for reduced characters play a role in the evolution of the reduced morphology, or could reduced sexual selection for the exaggerated morphology alone explain the reduced morphology of type II males? These questions guided the design of the current study that tests the sexual selection for reduced characters hypothesis in type II male midshipman fish.
In our previous study, the positive correlation between type I cuckolder body size and access to the nest likely occurred because larger bodies aided in aggressive interactions with the resident male, after the resident male had attacked them (Lee and Bass 2004). In the present study, we focus on the time interval before the resident male attacks and test the hypothesis that the reduced type II morphology aids sneaky cuckoldry—that is, that the reduced morphology of type II males allows them to prolong the time that they are near the nest before being attacked by the resident type I male.
METHODS
Study animals
Type I and type II males were collected from the Hood Canal, Washington, at Seal Rock Beach in May and June. We brought them to the Big Beef Creek Field Station (University of Washington), attached color-coded beads to their dorsal tissue, and placed them in fiberglass aquaria (1.8 ×1.8 × 0.5 m). Animals were confirmed as type I and II males at the conclusion of the experiment after anesthetization in MS222 and dissection; several traits distinguish the 2 morphs, prominent among which are relative gonad size and the size and coloration of the sonic muscle (see Bass 1996).
Experimental design
A shelter, which would eventually be adopted by a large type I male as a nest, was positioned at each of the 4 aquarium corners. A shelter consisted of a ceramic tile (40.5 × 40.5 cm) resting on a rim of bricks. In nature, nests vary in the numbers and sizes of entrances. Because nest entrance characteristics may affect dynamics of alternative reproductive tactics (see Svensson and Kvarnemo 2003), we varied the brick arrangement to produce entrances of varying numbers and sizes. All 3 nest treatments, treatments “a” through “c,” included a single main entrance that was produced by leaving a 5-cm break in the rim of bricks. Treatments “b” and “c” had 3 additional openings that were either 2.5 cm (nest treatment b) or 6 cm (nest treatment c) in width. These 3 nest treatments together approximate a large portion of the range of variation seen in the field (22 nests surveyed along a transect, number of entrances per nest = 1.9 [range 1–4], entrance width = 7.6 cm [range 1.5–16 cm], entrance height = 3.5 cm [range 1–6.5 cm], methods for measuring entrances follow those in Lee and Barlow 2001). All nests within a single aquarium were of the same treatment.
In a previous study (Lee and Bass 2004), type I males of the 3 highest body mass ranks (means: 170, 204, and 237 g) held territories and did not cuckold, whereas some males of the other 5 body mass ranks did cuckold (means: 71, 85, 108, 126, and 151 g). We wanted to compare cuckoldry by type I and type II males, so in each aquarium, we placed 4 large (>200 g) type I males that we expected to hold territories, 2 type I males that we expected to cuckold (50–134 g), and 2 type II males (15–51 g).
Eight sets of 2 type I putative cuckolders and 2 type II males were rotated among treatments but never reused within the same nest treatment. Similarly, 20 putative type I territorial males were reused among, but never within, the same treatment.
To initiate a spawning, we added a female to each tank at midnight (see Brantley and Bass [1994] for extended documentation of spawning). Beginning at 5:30 AM, the following morning, we used a small submersible camcorder (Micro Video Products, MVC-2000-WP-LED) to view the inside of each nest and determine if the female was spawning. In most cases, the female had begun spawning, but in some instances, the female was still outside the nests. In the latter case, we waited until the female entered a nest and began to spawn before beginning observations. If the female did not begin spawning by 7:30 AM, we removed her and collected no data.
Once we determined that a female had begun to spawn, we set up a camcorder to point down into the aquarium, recording cuckolder movement into and around the nest. We recorded until the spawning ended or for the duration of 8 h, whichever occurred first. We ended the spawning after 4 h on days when there was no cloud cover to ensure that the water temperature would not rise above 22 °C, which is the highest observed in natural nest sites (recording required removing the top covering of each aquarium, which left it susceptible to heating from the sun; temperature is from personal observations). In total, we recorded 21 spawnings at an average of 5.5 h per spawning.
We wanted to compare type I cuckoldry with type II cuckoldry. Previous observations suggested that cuckoldry by both type I and type II males could occur from 3 positions: 1) at the nest periphery, where the cuckolding male would sit and occasionally release sperm from outside the nest; 2) at the entrance of the nest with the tail inserted into the nest, where the cuckolding male would sit and occasionally release sperm directly into the nest; and 3) inside the nest, where a male would release sperm from within the nest (for type II cuckolders, see Brantley and Bass 1994; for type I cuckolders, see Lee and Bass 2004). Sperm release in midshipman fish can be identified by rapid anal fin quivering (Brantley and Bass 1994; also see Neat and Locatello 2002 for a blenny). Positions 2 and 3 allow the release of sperm directly into the nest. Therefore, for each treatment, we calculated the time spent with the tail-in and with the body inside the nest as a percentage of the duration of the observed portion of the spawning. We also recorded the number of attempts made by each fish to insert the tail into the nest. A tail-in behavior ended when 1) the cuckolder entered the nest entirely, 2) the cuckolder left the nest voluntarily (cuckolder leaves the nest slowly, with no apparent chase by the resident), or 3) the cuckolder left the nest after being chased by the resident male (cuckolder rapidly darts away from nest, often coinciding with or preceding the visually apparent chase of the resident male, and sometimes after a bite or bite-and-hold by the resident male, also see Brantley and Bass 1994).
Statistical methods
To incorporate both random and fixed effects, we used a mixed model (restricted maximum likelihood [REML(t1)] estimation method, SAS Institute, Inc., Cary, North Carolina) to determine whether there were any differences in the abilities of type I and type II males to gain proximity to the nest during cuckoldry and whether that relationship changed with nest treatment. Male type (I or II) and nest treatment (a, b, or c) were set as fixed factors. Because groups that each contained 2 type I males and 2 type II males were rotated through each nest treatment, the group was set as a random effect.
Also with a mixed model, we examined the effects of male type and nest treatment on the number of attempts at tail-in and the time at tail-in before a chase by the resident male (this considers tail-in events that ended “involuntarily,” i.e., in a chase).
To avoid pseudoreplication in these mixed models, for each spawning in each tank, we averaged the type I cuckolder values into one data point; the same was done for the type II cuckolders. In all experiments, individuals were repeated among treatments but were never used more than once within a treatment (randomized block design). If a male did not cuckold at all in a nest treatment, he was excluded from analyses (thus, an incomplete randomized block design). Where appropriate, we performed post hoc tests and corrected for multiple tests with the Tukey test.
RESULTS
Time near the nest
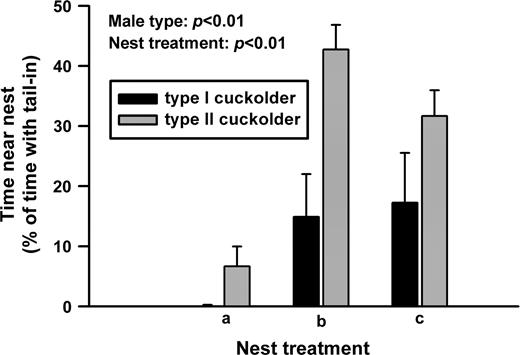
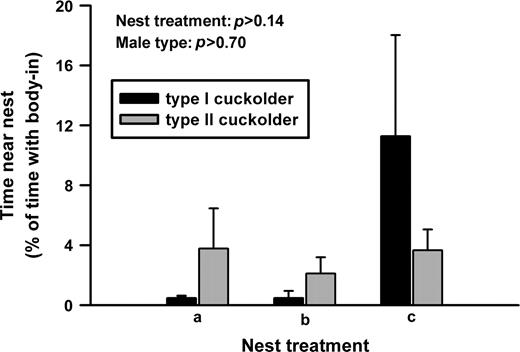
As in previous experiments (Lee and Bass 2004), the largest type I males took the shelters and acoustically courted females (also see Brantley and Bass 1994). The other type I males and the type II males cuckolded spawnings. Type II cuckolders spent a significantly greater amount of time in the tail-in position than type I cuckolders (Figure 1; df = 8, F = 11.68, P < 0.01). Nest treatment had a significant effect (Figure 1; df = 10, F = 8.82, P < 0.01), as did the interaction between male type and nest treatment (Figure 1; df = 10, F = 5.85, P < 0.03). A post hoc test comparing nest treatments showed that both type I cuckolders and type II cuckolders generally did better at gaining access to the spawning pair when there existed more than one nest entrance (nest treatments b and c; differences in tail-in time between nest treatments: a and b, P < 0.01; a and c, P < 0.02; b and c, P > 0.80). For time spent with the entire body inside the nest by cuckolders, we found no significant effects of male type (Figure 2; df = 8, F = 0.13, P > 0.7), nest treatment (Figure 2; df = 10, F = 2.36, P > 0.14), or the interaction between male type and nest treatment (Figure 2; df = 10, F = 2.07, P > 0.17).
Type II cuckolders spent more time with their tails inserted into the nest during cuckoldry than did type I cuckolders.
Type I and type II males did not differ in the time spent with their bodies entirely inside the nest during cuckoldry.
Entrance size
For nest treatment b, where cuckolders could choose between the main entrance and smaller entrances, type II cuckolders did not spend a greater proportion of their tail-in time in the 2.5-cm crevices versus the 5-cm main entrance than did type I cuckolders (median percentage of tail-in time spent in crevices as opposed to in the main entrance—type II cuckolders [n = 5]: 98.9% [range: 68.7–100%]; type I cuckolders [n = 7]: 99.9% [range: 0–100%]; P > 0.9, Mann–Whitney U test statistic = 17.5).
Number of attempts and success per attempt
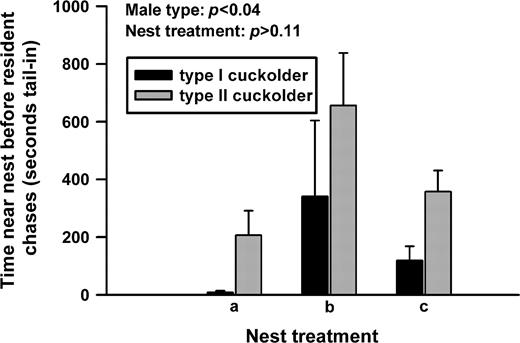
The number of attempts at tail-in was not affected by male type or nest treatment (df = 7, F = 0.08, P > 0.78 and df = 10, F = 0.63, P > 0.55, respectively), but the interaction between the 2 was significant (df = 7, F = 5.56, P < 0.04). Male type had a significant effect on the time tail-in before receiving a chase from the resident male (Figure 3); type I cuckolders received a chase after a shorter time lag following the insertion of the tail into the nest than did type II cuckolders (df = 7, F = 7.15, P < 0.04; the same statistical differences resulted if we analyzed all tail-in events regardless of whether or not they ended in chases by the resident). Neither nest treatment (Figure 3; df = 10, F = 2.69, P > 0.11) nor the interaction between male type and nest treatment had any significant effect on time at tail-in before chase (Figure 3; df = 6, F = 1.82, P > 0.24).
Type II cuckolders were able to remain tail-in longer than were type I cuckolders before receiving a chase from the resident, territorial type I male.
DISCUSSION
Methods of testing hypotheses about dimorphisms
Two approaches, namely analyzing variation either within a morph or between morphs, can be taken when studying the adaptive benefit of exaggerated and reduced morphologies. In many species, male dimorphisms are thought to be coupled to behavioral tactic: exaggerated morphology males are behaviorally rigid, holding territories, and not cuckolding if excluded from territories, whereas reduced-morphology males are behaviorally rigid, cuckolding but not holding territories even if territories are available (see Introduction). As a consequence, investigators are limited to studying variation within each morph (intramorph method) and extrapolating findings to differences between morphs. Although these studies have greatly illuminated our understanding of dimorphisms, the method is indirect in that it relies on the extrapolation of a trend within one morph to learn about differences between 2 morphs (see Moczek and Emlen [2000] for a discussion).
Behavioral plasticity within the type I morph in midshipman fish allows for a direct test—a comparison between cuckoldry by exaggerated morphology (type I male) cuckolders and cuckoldry by reduced morphology (type II male) cuckolders. Here, we compared the time each was able to spend near the nest, and most importantly, the time each was able to spend near the nest before being chased by the territorial type I male.
Cuckolders differed in their success at gaining access to the nest depending on the sizes of the nest openings, showing that the nest configuration has important consequences on the degree to which the resident is cuckolded. However, the type I cuckolders never did better than the type II cuckolders at either tail-in or body-in time, regardless of the nest treatment. Thus, type II cuckolders spent significantly more time at the tail-in position than did the type I cuckolders.
This difference between type I and type II cuckolders could arise through 2 mechanisms. First, the inequality in time tail-in may reflect differences in the number of attempts at being near the nest (i.e., motivation) rather than differences in ability. Second, type II males may have been more successful on a per-attempt basis; the inequality in the time tail-in may reflect differences in the ability to remain near the nest once an attempt has been made.
To test the second mechanism, which is the mechanism that is relevant to our main hypothesis, we compared type II cuckolders with type I cuckolders on a per-attempt basis. On average, the type II cuckolders were able to spend more time tail-in before receiving a chase from the resident. This difference was most extreme in nest treatments a and c, where the type II males were able to be tail-in 4–6 times longer before being chased than were the type I males. These results support the second mechanism—type II males were able to remain near the nest for longer periods before being chased.
Sexual selection and male dimorphisms
In the Introduction, we presented a paradox: How can sexual selection lead to the reduced morphology if, among the type I males in our previous study (Lee and Bass 2004), bigger type I cuckolders did better than smaller type I cuckolders at gaining access to the nest? Data from this and previous studies provide support for the following resolution. The exaggerated morphology displayed by large type I males reflects sexual selection for territoriality, courtship, and when the type I male is engaged in cuckoldry, the ability to remain near the nest after the first aggressive maneuver by the resident male. Simultaneously in other males in the same population, the reduced morphology of type II males reflects sexual selection for the ability to prolong the time near the nest before being attacked by the resident. Greene et al. (2000) found that among male lazuli buntings, yearlings with dull plumages were more tolerated in territories close to other males than were yearlings with brighter plumages. Although the mechanism through which proximity to territories is achieved is different from that in midshipman fish, both findings show how reduced morphologies can have beneficial fitness consequences.
Evidence that the exaggerated morphology of large type I males is driven at least in part by sexual selection comes from previous studies. Compared with smaller type I males, larger type I males are able to obtain bigger nests (DeMartini 1988) and to provide lengthier care for offspring (Lee 1996). Compared with type II males, type I males also have larger sonic muscles and associated neural circuitry, which are used in mate attraction and intrasexual competition (Brantley et al. 1993; Bass et al. 1996). Several pieces of evidence support the hypothesis that sexual selection may further work to maintain the exaggerated morphology even when type I males practice a reproductive tactic, cuckoldry, that is alternative to that for which they are usually assumed to be specialized (territoriality and courtship). These include the observations that: 1) in our previous study, bigger type I cuckolders were able to attain greater proximity to the spawning pair than were smaller type I cuckolders (Lee and Bass 2004), 2) large type I cuckolders engage in aggressive interactions with the resident territorial type I male after the initial attack by the resident male (Lee and Bass 2004), 3) fighting ability increases with body mass in both midshipman fish (JSF Lee, unpublished data) and other fishes (Barlow 1983; Rowland 1989; Oliveira and Almada 1996; Kroon et al. 2000; Barlow and Lee 2005), and 4) aggressive interactions in midshipman fish are accompanied by agonistic vocalizations (Brantley and Bass 1994; Lee and Bass 2004) that are dependent on large sonic muscles and associated neural circuitry (see above).
Why do type I males cuckold? For type I males, the highest fitness payoff is likely realized by large, territorial type I males that hold territories on high-quality nest sites (see DeMartini 1988; Lee 1996). This conclusion derives from the observation that the largest type I males will always be territorial if a large nest site is available (Lee and Bass 2004). Data from this and previous studies (cited above) support the hypothesis that disruptive selection favors both the reduced type II morphology and the exaggerated morphology of large type I males. However, because type I males need several years to reach full size, young type I males possess a morphology that is exaggerated but not yet fully grown (see review in Bass 1996, also see Rak 1995). Small- and medium-sized type I males practice territoriality on low-quality nest sites and cuckoldry as “best of a bad situation” tactics that are part of a partially conditional strategy, until they are larger and better able to successfully practice the high-payoff tactic of territoriality on high-quality nest sites (see Lee and Bass 2004). When younger and smaller type I males are territorial, they are apparently excluded from high-quality nest sites by larger type I males (see Lee and Bass 2004). When they cuckold, young type I males are likely unable to partake in aggressive cuckoldry against large residents because they are too small to effectively challenge the large resident males (see Lee and Bass 2004). The tactic of aggressive cuckoldry is thus limited to type I cuckolders that are similar in size to the resident. Given the sizes of the type I cuckolders used in this experiment, relative to the sizes of the resident males used in this experiment, we neither expected nor observed aggression from the type I cuckolders toward the resident type I males. Instead, the cuckoldry observed in type I males in this study appeared identical to that of type II males. Results from this study suggest that, even though young type I males are forced to attempt sneaky cuckoldry because of their inability to successfully challenge large resident males, young type I males are inferior to type II cuckolders at sneaky cuckoldry because their morphology does not function well at avoiding detection by resident males.
Although not the focus of this study, reduced sexual selection for the exaggerated morphology likely plays a role in the maintenance of the type II morphology. Developing a small body size and small sonic muscles likely requires less energy than the development of a large body and large sonic muscles. This energy savings appears to be accompanied by a temporal savings—type II males mature earlier than type I males (Bass et al. 1996, also see review in Bass 1996). The earlier time of sexual maturation is a characteristic trait of the reduced morphs of many species (e.g., see Gross and Charnov 1980; Ryan et al. 1992; Bass et al. 1996; Kurdziel and Knowles 2002).
In this study, which focused on the external morphology of type II males, we have provided evidence that supports the hypothesis that if selection for reduced energetic and temporal investment (in response to reduced sexual selection for the exaggerated morphology) helps maintain the reduced morphology in type II males, it is not the sole selective force. The hypothesis that the reduced morphology is at least partially driven by sexual selection to increase time before attack by the resident male is supported by data showing that type II cuckolders were able to remain penetrated into the nest for longer periods of time before being chased than were type I cuckolders.
In combination with previous studies on type I males (see above), we have provided support for the hypothesis that while sexual selection for territoriality, courtship, and aggressive cuckoldry after attack by the resident male select for the exaggerated morphology in type I males, simultaneously in other males of the same population, sexual selection for a morphology that delays the attack by the resident male during cuckoldry selects for the reduced morphology in type II males.
We thank Julia Frey and Emily Lang for extensive help with field collections and the collection of data from video. For discussion, we are grateful to Elizabeth Adkins-Regan, Julia Frey, Emily Lang, Kern Reeve, Paul Sherman, and the participants of the Cornell Behavior Lunch Bunch. Mark Hauber, Kern Reeve, Paul Sherman, and 3 anonymous reviewers provided critical comments on the manuscript. Eliot Brenowitz and Gordy George provided logistical support at the Big Beef Creek Field Station (University of Washington). This project was funded by a research grant from the National Science Foundation (NSF) (IBN9987341, 0516748) to A.H.B. and grants to J.S.F.L. from the American Museum of Natural History (Lerner-Grey Fund), the American Society of Ichthyologists and Herpetologists (Raney Fund), and the Animal Behavior Society (Student Research Grant) and by an NSF Graduate Research Fellowship to J.S.F.L.
References
Ainsworth R.
Arora HL.
Barlow GW.
Barlow GW, Lee JSF.
Bass AH, Horvath BJ, Brothers EB.
Bass AH, McKibben JR.
Brantley RK, Bass AH.
Brantley RK, Tseng J, Bass AH.
Clark RA.
Cook JM, Compton SG, Herre EA, West SA.
Crane JM.
Danforth BN.
DeMartini EE.
Greene E, Lyon BE, Muehter VR, Ratcliffe L, Oliver SJ, Boag PT.
Gross MR.
Gross MR, Charnov EL.
Ibara RM, Penny LT, Ebeling AW, van Dykhuizen G, Caillet G.
Kroon FJ, de Graaf M, Liley NR.
Kurdziel JP, Knowles LL.
Lank DB, Smith CM, Hanotte O, Burke T, Cooke F.
Lee AO.
Lee JSF.
Lee JSF, Barlow GW.
Lee JSF, Bass AH.
McKibben JR, Bass AH.
Moczek AP, Emlen DJ.
Moczek AP, Emlen DJ.
Moore MC, Hews DK, Knapp R.
Neat FC, Locatello L.
Oliveira RF, Almada VC.
Radwan J, Klimas M.
Rak RS.
Rowland WJ.
Ryan MJ, Pease CM, Morris MR.
Svensson O, Kvarnemo C.
Taborsky M.
Tomkins JL, Brown GS.
Uglem I, Galloway TF, Rosenqvist G, Folstad I.
Utami SS, Goossens B, Bruford MW, de Ruiter JR, van Hooff J.