-
PDF
- Split View
-
Views
-
Cite
Cite
Angela Bahr, Stefan Sommer, Beat Mattle, Anthony B. Wilson, Mutual mate choice in the potbellied seahorse (Hippocampus abdominalis), Behavioral Ecology, Volume 23, Issue 4, July-August 2012, Pages 869–878, https://doi.org/10.1093/beheco/ars045
Close - Share Icon Share
Abstract
Models of sexual selection often assume dichotomous sex roles, with one sex competing for access to mates, while the other sex is choosy. However, it is well known that mating decisions are realized by integrating information across multiple traits, the relative importance of which may be sex specific. While a large body of work has investigated the influence of sexual signals on mating behavior, such traits have typically been studied in isolation, oversimplifying the multimodal communication associated with natural mating behavior. We investigated the impact of 2 key traits (Major Histocompatibility Class II beta-chain [MHIIb] olfactory cues and body size) on mate choice decisions in the potbellied seahorse (Hippocampus abdominalis), a species considered to have female competition and male choice. We used a hierarchical experimental design (1. olfactory cues only, 2. olfactory and visual cues, and 3. free interaction) to investigate behavioral preferences and mating success of female and male seahorses under increasing levels of multimodal stimulation. Our data show that female seahorses prefer and mate with MHIIb-dissimilar males, while male seahorses mate randomly with respect to this trait. Conversely, males prefer and mate with large females, while females show no size-based mating preference. The multimodal integration of sex-specific mate preferences in mating behavior of the potbellied seahorse suggests the existence of mutual mate choice in this species. The results presented here suggest that more comprehensive studies of mating behavior, considering both female and male preferences for multiple traits, may lead to a more nuanced understanding of how sexual selection operates in natural populations.
INTRODUCTION
Mate choice decisions involve the integration of information provided via a wide range of behavioral, morphological, olfactory, and vocal signals (Andersson 1994; Candolin 2003). The importance of such cues during mate choice depends not only on environmental conditions, but may also differ between the sexes (Andersson 1994; Houde 2001). Models of sexual selection have classically assumed that mate choice decisions are dictated by the choosy sex, neglecting the influence of mate preferences of the competitive sex on realized mating behavior (e.g., Darwin 1871; Houle and Kondrashov 2002; but see Johnstone et al. 1996; Bergstrom and Real 2000). This bias has been reinforced by the majority of behavioral studies, which even today tend to focus exclusively on the mating preferences of the presumptively choosy sex (typically the female) (e.g., Candolin 2003; but see Ahnesjö 2010). A growing appreciation for the potential importance of mutual mate choice is reflected in an increasing number of empirical studies investigating male and female mating preferences, which suggest that both sexes may often discriminate when making mate choice decisions (Jones and Hunter 1993; Sandvik et al. 2000; Rypstra et al. 2003, reviewed in Hooper and Miller 2008; Edward and Chapman 2011).
Bony fishes are well suited for the study of sexual selection, due to their exceptional diversity, not only in terms of species numbers but also in reproductive patterns, parental care modes, and mating patterns (reviewed in Amundsen 2003). When mating preferences of both male and female teleosts have been investigated under similar experimental conditions, sex-specific differences in trait preferences have often been detected. The most widely studied visual cues, for example, are body size and color patterns. The majority of studies on fish species incorporating both female and male preference experiments have found a preference of large mates by males, but not by females (e.g., Nerophis ophidion [Berglund et al. 1986], Hippocampus abdominalis [Mattle and Wilson 2009], Gymnogobius isaza [Morimoto et al. 2010]), while others have observed the opposite pattern (e.g., Micropterus dolomieu [Hanson and Cooke 2009], Pomatoschistus minutus [Kvarnemo and Forsgren 2000]), and/or preferences of large mates by both sexes (e.g., Syngnathus typhle [Berglund et al. 1986; Sandvik et al. 2000] and Poecilia reticulata [Reynolds and Gross 1992; Herdman et al. 2004]). Size-based preferences are thought to reflect the fecundity benefits of mating with larger individuals and/or the competitive advantages of large body size. Preferences for more colorful or ornamented mates have also been detected in both males (e.g., Nerophis ophidion [Berglund et al. 1986], Gobiusculus flavescens [Amundsen and Forsgren 2001], Syngnathus typhle [Berglund and Rosenqvist 2001]), and females (e.g., Gasterosteus aculeatus [Bakker 1993]).
The genes of the major histocompatibility complex (MHC/Major Histocompatibility [MH] in Actinopterygii) play an important role in determining individual odor (reviewed in Penn 2002). In addition to the importance of MHC genes as an integral part of the vertebrate adaptive immune system, MHC-mediated odor cues have been shown to be important in mate choice, individual recognition, and inbreeding avoidance (Penn and Potts 1999; Penn 2002; Boehm and Zufall 2006; Milinski 2006). According to the “divergent allele advantage” model, individuals with diverse MHC alleles are able to recognize a broader spectrum of pathogen-derived antigens than individuals carrying similar alleles (Wakeland et al. 1990; Sommer 2005). MHC-based mate choice may offer several advantages to the choosy individual (reviewed in Penn and Potts 1999): First, parents may be able to actively enhance the immunocompetence of their offspring. Second, a moving target is provided during host–parasite coevolution. Finally, inbreeding can be avoided by rejecting MHC-identical mates. As all three of these benefits can be realized by mating with MHC-dissimilar individuals, behavioral studies of MHC-based mating preferences have typically focused on disassortative mating in females and/or males. Olfactory and mating preferences for MHC-dissimilar mates have been detected in a wide range of vertebrate species (e.g., Homo sapiens [Wedekind et al. 1995; Wedekind and Furi 1997], Mus spp. [reviewed in Penn and Potts 1999], Salmo salar [Landry et al. 2001; Consuegra and de Leaniz 2008], Oncorhynchus tshawytscha [Neff et al. 2008], Rhodeus ocellatus [Agbali et al. 2010], but see: Paterson and Pemberton 1997; Huchard et al. 2010). At the same time, there is increasing evidence that an intermediate MHC-diversity may sometimes be preferred during mate choice, due to an interaction between MHC allele number and T-cell repertoire size (G. aculeatus [Aeschlimann et al. 2003; Eizaguirre et al. 2009], Salmo trutta [Forsberg et al. 2007]).
While it is clear that females of many fish species are capable of discriminating MH genes on the basis of olfactory cues, studies of male reproductive behavior have thus far failed to detect MH-based mating preferences (Forsberg et al. 2007; Neff et al. 2008). Analyses of olfactory performance in mammals indicate that females typically outperform males in most olfactory measures (e.g., detection, sensitivity, and discrimination) (reviewed in Good and Kopala 2006), and while comparable studies on fishes are rare, male and female teleosts often respond differently to olfactory cues (Lastein et al. 2006; Neff et al. 2008; Ratterman et al. 2009). Sex-specific differences in olfactory abilities might explain the lack of male MH-based mate choice observed in fishes. Alternatively, if preferences for MH cues are more pronounced in the choosy sex, the absence of male MH-based mate choice in this group could reflect the fact that all previous studies have investigated species considered to have female choice.
As behavioral studies have continued to increase in their sophistication, incorporating multiple traits and the perspectives of both sexes, there is now clear evidence that both females and males show preferences, often on the basis of very different mating cues (Candolin 2003; Ahnesjö 2010). We investigated the effects of olfactory and visual cues on mate choice decisions in the potbellied seahorse H. abdominalis, focusing on Major Histocompatibility Class II beta-chain (MHIIb)-mediated odor cues and body size, a sexually selected morphological trait (Mattle and Wilson 2009). The seahorse has a unique reproductive system, with a highly developed form of male parental care. Eggs transferred by the female during mating are brooded in a pouch on the males' abdomen, a phenomenon termed “male pregnancy” (Stölting and Wilson 2007). Previous studies on syngnathid fishes (seahorses and pipefishes) have focused on the role of phenotypic and environmental traits (e.g., body size and sex ratio) in sexual selection (e.g., Berglund et al. 2005; Mattle and Wilson 2009), but the role of olfactory cues has only recently been explored (Ratterman et al. 2009; Sundin et al. 2010; Lindqvist et al. 2011). Behavioral observations of natural populations of the potbellied seahorse H. abdominalis have shown evidence of female–female competition and male choice in this species (Wilson and Martin-Smith 2007), a widespread pattern in syngnathid fishes believed to be associated with the high levels of male parental care in this group (Wilson et al. 2003).
We used a hierarchical experimental design to investigate the role of olfactory and visual cues in mate choice decisions of male and female potbellied seahorses. We first carried out a sequential preference experiment, providing focal individuals of both sexes with olfactory cues derived from individuals differing in their MHIIb dissimilarity. A second preference experiment analyzed the combined effect of visual and olfactory cues on focal individual behavior. Finally, individual mating behavior was investigated in a large free-interaction experiment, which aimed to determine whether the sex-specific preferences observed in the first 2 experiments influence mating behavior under seminatural conditions.
MATERIALS AND METHODS
Sample population
The potbellied seahorse, H. abdominalis, is a temperate-water marine species occurring in coastal habitats around New Zealand and south-eastern Australia. Seahorses are listed under Appendix II of the United Nations Convention on the International Trade in Endangered Species, and all individuals used in our mate choice experiments originate from a large captive-bred population (Seahorse Australia, Beauty Point) derived from individuals collected from 3 Tasmanian sites (Wilson and Martin-Smith 2007). This population has a high neutral genetic diversity and is genetically indistinguishable from natural Tasmanian populations (see Supplementary Figure 1 in Bahr and Wilson 2011). In August 2006 and 2008, 6-month-old sexually mature individuals that had not previously mated were transferred to a large recirculating marine system at the University of Zurich, Switzerland, where they were kept segregated by sex prior to the experimental period to avoid potential pair bonding. Stock tanks were connected to a central seawater circulation system and contained artificial plants as holdfasts. Seahorses were fed with frozen Artemia salina and Mysis relicta ad libitum twice per day.
After 1 month of acclimatization to laboratory conditions, seahorses were anesthetized by placing them for 2–6 min in 10 l of a 55-ppm AQUI-S solution (isoeugenol; AQUI-S, Lower Hutt, New Zealand) in 33-ppt salt water. Fin clips of anesthetized animals were collected for genetic analysis, and digital pictures of animals in lateral orientation were taken for standard length measurements. Individually numbered plastic neck tags (1/8″ × 1/4″, FTF-69, Floy Tag, Seattle) were attached to seahorses using polyester thread to allow for individual identification. Tagging had no effect on the behavior or health of the animals, which was monitored daily.
Microsatellite and MHIIβ genotyping
To infer genetic diversity and parentage, we extracted whole genomic DNA from fin clips (adults) or muscle tissue (offspring) using a DNeasy 96 Tissue Kit (QIAGEN), and genotyped individuals at 4 hypervariable microsatellite loci (Habd3, Habd6, Habd7, Habd9; Wilson and Martin-Smith 2007). Basic population genetic data for these markers are presented elsewhere (Wilson and Martin-Smith 2007; Bahr and Wilson 2011). Microsatellite polymerase chain reaction (PCR) reactions were performed in a MJ DNA Engine Tetrad machine. Approximately 20 ng DNA was used in 10 μl reactions containing 1 μl 10× ThermoPol reaction buffer (NEB), 1 mM MgCl2, 200 nM primers, 0.4 μM dNTPs (Roche), and 0.4 U Taq DNA polymerase (NEB). PCR running conditions included 30 cycles of 94 °C for 30 s, 58 °C for 30 s, and 72 °C for 1 min, with a final extension at 72 °C for 30 min. For genotyping on an ABI 3730 automated sequencer (Applied Biosystems), PCR reactions were diluted 1:25 in ddH2O. From each of the independent microsatellite amplifications, 1.5–2.0 μl of the diluted products were combined in a plate containing 0.07 μl GeneScan 500 LIZ genotyping standard (Applied Biosystems), 9.93 μl HiDi Formamide (Sigma), and 4 μl ddH2O. Results were automatically scored using GeneMapper 4.0 (Applied Biosystems), and microsatellite alleles were verified by eye for each sample.
To test for MH-dependent mate choice in the potbellied seahorse, we assessed MH class II beta-chain gene diversity by sequencing the complete peptide-binding region (exon 2, 273 bp) in all individuals (Bahr and Wilson 2011). Sequence data were aligned and verified by eye in BioEdit v.7.0.9 (Hall 1999). Allelic phase of heterozygote sequences was inferred using a Bayesian statistical method implemented in PHASE v.2.1 using the default program settings (Stephens and Donnelly 2003).
Variables measured
Individual neutral genetic diversity was calculated as described in Aparicio et al. (2007), weighting the contribution of each locus according to its allelic variability. Genetic parentage in experiment 3 was assessed through the comparison of potential parent and offspring genotypes using the likelihood-based method of Cervus v3.03 (Kalinowski et al. 2007), with a strict confidence cutoff of 95%. Both parents of each offspring were considered to be unknown, but were assumed to be present in the population of 25 male and female seahorses included in the breeding population. A maximum of 1 mismatch was allowed in each triplet of candidate parents and offspring, to allow for the presence of germline mutation. MHIIb allele identity was used to verify microsatellite results (data not shown).
To assess MHIIb diversity, we calculated the number of amino acid differences between exon 2 alleles carried by each individual using Mega v.4.0.2 (Tamura et al. 2007). This variable was consequently termed “intraindividual MHIIb distance,” while the average allelic difference between individuals was recorded as “MHIIb dissimilarity.” MHIIb dissimilarity between male and female seahorses consequently reflects the average expected dissimilarity in their offspring (Landry et al. 2001; Neff et al. 2008).
We also investigated the effect of body size on behavioral preferences in experiments 2 and 3. The standard length of each seahorse was calculated with tpsDig v2.12 (Rohlf 2010) using digital pictures and arbitrary landmarks along the lateral body axis, from the tip of the snout to the tip of the tail.
Experiment 1—olfactory cues
Our first experiment was conducted daily between May and July 2009, investigating MHIIb-based olfactory preferences in male and female seahorses using a sequential mate choice design. For each focal individual, 3 different olfactory stimuli, differing in their MHIIb dissimilarity to the focal individual, were used. Twenty-five focal females were used with 18 different male stimuli and 25 focal males with 17 different female stimuli. Animals were used as stimulus individuals in 3, 6, or 9 trials, in a balanced design, which ensured that stimulus animals were used an equal number of times as similar, intermediate, and dissimilar stimuli. There was no evidence that focal individuals showed a consistent preference (either for or against) for specific stimulus individuals (Supplementary Table 1).
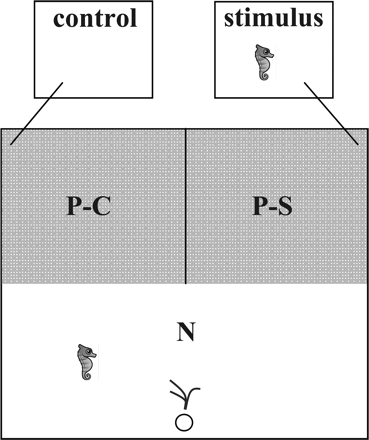
We used 2 experimental tanks (55 × 55 × 75 cm, circa 210 l; Figure 1) located in the same room as stock tanks and connected to the same seawater system. Choice tanks were divided into 3 sections (Figure 1), including a neutral zone (half of the aquarium) and 2 preference zones (each one quarter of the aquarium). An artificial plant, identical to those in the stock tanks, was provided as a holdfast shelter next to the outflow in the center of the neutral zone. The 2 preference zones were separated by a solid divider, preventing the exchange of water and chemical cues between them. Stimulus water came from a separate 40-l tank maintained at a temperature identical to the test tank, into which the stimulus individual was acclimatized for 14–16 h before the experimental trial. The focal individual was transferred to the test tank at the same time. A 40-l tank without a stimulus animal served as a control. Stimulus and control tanks were connected to test tanks via 2-mm diameter silicone tubing, and water flow (1 ml/s) was initiated at the start of each behavioral trial. Test, stimulus, and stock tanks were illuminated by overhead fluorescent lighting. Water parameters were kept constant throughout the experimental period (temperature 19.5 ± 0.3 °C, pH 8.5 ± 0.3, salinity 33.9 ± 0.6 ppt), and the light regime was set to 14:10 hours (light:dark). Animals were not fed during the settling period or trial.
Experimental design of experiment 1, showing preference zones (stimulus P-S and control P-C), the neutral zone (N), as well as locations of the water outflow (circle) and artificial holdfast.
Focal individuals were simultaneously presented with stimulus water flow from an individual of the opposite sex (preference zone P-S) and control water (preference zone P-C). Three independent trials for each of the focal individuals were used to test absolute preferences for 3 different stimuli differing in MHIIb dissimilarity. Experimental trials were fully randomized, and focal individuals were retested after a period of at least 3 days (range: 3–56 days). The side of the compartment for the stimulus water, the grouping of individuals, and experimental tank were randomized for each trial. The behavior of focal individuals was recorded using an overhead camera (ABUS, Wetter, Germany) throughout the experimental trials, which were conducted within 1 h of the onset of artificial illumination, the time at which seahorses are reproductively most active (Mattle and Wilson 2009). After each trial, seahorses were held in stock tanks, separated into experimentally experienced and inexperienced animals.
Statistical analysis—experiment 1
Preliminary experiments using color tracers demonstrated that stimulus water reached the neutral zone of the test tank within 5–7 min (data not shown). Consequently, the behavior of focal seahorses was analyzed for 1 h (3600 s), starting 5 min after the onset of the waterflow. From video records, we scored the time the focal fish spent with its full head length in either preference zone. Preference was quantified as the relative proportion of time spent in the preference zone with stimulus water (P-S) compared with the total time spent in both preference zones (P-S + P-C). Consequently, a value above 0.5 indicates a preference for the stimulus. Preference could only be calculated if the focal animal entered at least one of the preference zones, and a large proportion of experimental trials were excluded (44% for females and 25% for males), due to the low activity of the focal individual, a common observation in this species (Mattle and Wilson 2009). Focal individuals with preference scores from at least 2 of 3 trials were included in the analysis (8 females and 18 males). For each of these individuals, preference scores for MH-similar and MH-dissimilar stimuli were then compared in order to quantify individual behavior (paired data structure). If a focal individual showed a preference in all 3 trials, the 2 trials with the most divergent stimuli were included in the analysis. MHIIb-dissimilarity values between the stimulus and focal individual, as well as the difference in MHIIb dissimilarity between stimuli are provided in Supplementary Table 2.
We tested the influence of MHIIb dissimilarity on individual preferences, measured as a proportion ranging from 0 to 1 (see above). We also calculated the coefficient of determination, measured as the proportion of variation in preference scores explained by MHIIb dissimilarity, by comparing the difference in preference scores for MHIIb-dissimilar and -similar stimuli relative to their MHIIb dissimilarity. The genetic distance between MHIIb-similar and -dissimilar stimuli did not differ significantly between the sexes (Supplementary Table 2). Preference scores were arcsine transformed when analyzed with one-sample t-tests. Nonparametric tests were used unless otherwise indicated, as even minor deviations from normality and homoscedasticity in small data sets can lower the power of parametric tests, increasing the Type I error rate (Erceg-Hurn and Mirosevich 2008). All statistical analyses were performed in SPSS 17.0 (Chicago, IL, USA), with the exception of power analyses on two-tailed Wilcoxon signed-rank tests, which were conducted in G*Power 3.1.2 (Faul et al. 2007).
Experiment 2—olfactory and visual cues
In our second experiment, we tested the relative importance of olfactory and visual cues in individual preference. This experiment was originally designed to test body size preferences in H. abdominalis (for details on experimental design, see Mattle and Wilson 2009), but the presence of both visual and olfactory cues in this experimental design allowed for post hoc tests of MHIIb-based mate choice. In this study, 30 male and female seahorses were offered a dichotomous choice between a large and a small partner, without considering relative MHIIb dissimilarity. MH class II beta gene diversity of the individuals involved in this experiment was subsequently assessed as outlined above. The seahorses used here were a separate cohort of individuals introduced into the lab in August 2006.
Statistical analysis—experiment 2
Trials were analyzed for MHIIb-based mate choice if the 2 stimuli differed in their MHIIb dissimilarity to the focal individual. As in experiment 1, a large number of focal seahorses showed low activity levels in this experiment, failing to enter either preference zone (females 48% and males 23%), yielding a total of 15 trials for focal females and 18 for males.
We tested for the impact of MHIIb dissimilarity on individual preferences. Preference was calculated as for experiment 1 as the time spent with the MHIIb-dissimilar stimulus divided by the total time spent with both stimuli. As in experiment 1, the genetic distance between MHIIb-similar and -dissimilar stimuli did not significantly differ between the sexes (Supplementary Table 2). Preference scores were arcsine transformed when analyzed with one-sample t-tests. MHIIb-dissimilarity values between stimulus and focal individuals, as well as differences in MHIIb dissimilarity between stimuli are provided in Supplementary Table 2. The difference in MHIIb dissimilarity between stimuli in experiment 2 was less than that for experiment 1 (experiment 1: mean difference in MHIIb dissimilarity ± standard deviation (SD) = 2.89 ± 1.71 amino acids; experiment 2: 2.09 ± 1.63 amino acids; Supplementary Table 2).
We also tested for body size–based preferences in this experiment, following Mattle and Wilson (2009). Focal female (21.0 ± 1.4 cm, mean ± SD) and male (20.4 ± 1.8 cm) seahorses did not significantly differ in size (Mann–Whitney U-Test: U = 122.0, n1 = 15, n2 = 18, P = 0.638). The average size difference between stimuli was significantly larger in female trials compared with that for male trials (females: mean standard length difference ± SD = 3.3 ± 0.6 cm; males: mean ± SD = 2.5 ± 0.5 cm; Mann–Whitney U-Test: U = 41.5, n1 = 15, n2 = 18, P = 0.001).
A Generalized Linear Model (GZLM) was used to test the nonadditive effects of MHIIb dissimilarity and body size on preference scores. In contrast to the previous analyses, in which preference was calculated for either the large or the MHIIb-dissimilar stimulus, variables used in the GZLM were calculated independent of stimulus size and MHIIb dissimilarity. Preference was instead calculated for stimulus 1, the stimulus used in the left compartment of the experimental tank, which might be small or large, and MHIIb similar or dissimilar. MHIIb dissimilarity and body size were calculated as the difference between stimulus 1 and 2. We used a Poisson distribution and a natural log-link function, with the time spent with stimulus 1 as the dependent variable and the natural logarithm of the time spent with both stimuli as an offset variable (Garson 2011). Sex was set as fixed factor, and size and MHIIb difference were included as covariates. Chi-square statistics were calculated to evaluate model effects using a likelihood ratio test; all other settings were fixed at SPSS 17.0 defaults. We used a scale weight variable to correct for data overdispersion (variance > mean) (Garson 2011).
Experiment 3—free interaction
In a third experiment, seahorses were allowed to freely interact and mate over a period of 4 months (February–June 2010), to investigate whether mate choice under seminatural conditions was influenced by MHIIb and/or body size cues. We established a breeding population of 25 males and 25 females, randomly chosen from our captive-bred population. Of these individuals, 36 were derived from experiment 1, which had been carried out 9 months earlier. Seahorses were transferred to a 750-l aquarium (73 × 69 × 148 cm), which was connected to the same seawater circulation system as stock tanks. Shelters and holdfasts were provided using rocks, pipes, and plastic plants to create a complex habitat. Water parameters were kept constant at the same values as in experiment 1, and seahorses were fed frozen artemia and mysids twice per day. Seahorses have been shown to mate monogamously within broods (Kvarnemo et al. 2000; Wilson and Martin-Smith 2007), and we sampled an average of 5 offspring (range 1–14) from each clutch for genetic analysis of parentage. No male mortality was observed during the experimental period, but 5 females died during the 4-month experiment. These females were significantly smaller than the surviving females (Mann–Whitney U-Test: U = 14.0, n1 = 5, n2 = 20, P = 0.014). While only one of these females reproduced during the trial period, we included all 5 females in subsequent analyses, as the majority of these individuals were available for mating for at least half of the experimental period. An analysis excluding these females produced similar results for MHIIb, but influenced our analysis of body size, due to the small size of these individuals (data not shown). While body size analyses conducted without these individuals were nonsignificant, males exhibited a pattern of size-based mate choice consistent with that observed in the full data set (Wilcoxon signed-rank test: n = 17, Z = −1.634, P = 0.102).
Statistical analysis—experiment 3
Neutral genetic diversity was high in the experimental population (homozygosity: mean = 0.1, range 0–0.5; (as calculated in Huchard et al. 2010), leading to low average levels of relatedness (mean = 0.05, range 0–1). Consequently, no mate choice for unrelated or diverse partners could be detected (data not shown). Parentage of seahorse offspring was inferred using 4 neutral microsatellite loci, as described above. The relationship between mating number and the interbrood interval (measured as the time between successive clutches produced by the same individual) was investigated using a general linear model (GLM), with individual identity included as a random factor, and sex and mating number (1–5) included as fixed factors.
Digital pictures of all adults were taken at the conclusion of the experiment. Male and female seahorses in our experimental population were similar in size (females: mean ± SD = 23.09 ± 1.91 cm, males: mean ± SD = 22.44 ± 1.92 cm; Mann–Whitney U-Test: U = 240.5, n1 = 25, n2 = 25, P = 0.162). To test for size-based mate choice, we compared the median size difference between an individual and its mating partner(s) (size mate − size focal individual) relative to its median size difference to all individuals of the opposite sex (size potential mate − size focal individual). This second measure reflects the expected size difference under random mating.
The seahorses used in this experiment carried 16 of the 17 previously characterized MHIIb alleles (no Hiab-DAB-E2*07) (Bahr and Wilson 2011) plus 2 additional alleles (GenBank: JN398459–460). Of the 50 individuals, 9 individuals (18%) were homozygous for the MHIIb gene. Male and female intraindividual MHIIb distance did not differ significantly (females: mean ± SD = 6.80 ± 3.15 amino acids, males: mean ± SD = 5.48 ± 4.28 amino acids; Mann–Whitney U-Test: U = 271.5, n1 = n2 = 25, P = 0.421). We used an individual-based approach to test for MH-based mate choice, comparing each individual's median MHIIb dissimilarity with its mating partners to its median MHIIb dissimilarity to all individuals of the opposite sex, the expected pattern of MHIIb dissimilarity under random mating.
Finally, we tested whether mating success (the number of successful matings per individual) was influenced by intraindividual MH distance and body size, using a GZLM. We used a multinomial distribution with a cumulative negative log–log link function, which is well suited for distributions with many low values (Garson 2011). Model selection was based on Akaike information criterion (AIC) values, with low AIC values indicating a better model fit (Garson 2011). Initial analyses included multinomial, negative binomial, and tweedie distributions with different link functions. We used mating success as the dependent variable, sex as a fixed factor, and MHIIb distance and body size as covariates. Chi-square statistics were calculated using a likelihood ratio test to evaluate model effects.
RESULTS
Experiment 1—olfactory cues
Are MHIIb-dissimilar stimuli preferred compared with MHIIb-similar individuals?
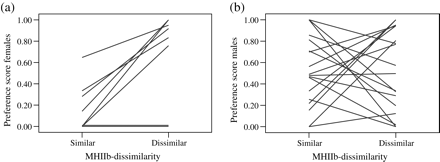
Female seahorses showed significantly stronger preferences for MHIIb-dissimilar stimuli than for MHIIb-similar cues (preference [similar]: median = 0.07, preference [dissimilar]: median = 0.88; Wilcoxon signed-rank test: n = 8, Z = −2.201, P = 0.028; Figure 2a), while males showed similar preferences for MHIIb-similar and -dissimilar stimuli (preference [similar]: median = 0.49, preference [dissimilar]: median = 0.54; Wilcoxon signed-rank test: n = 18, Z = 0.000, P = 1.000; Figure 2b). Females and males differed significantly in their preferences for MHIIb odor cues (Mann–Whitney U-Test, U = 111.0, n1 = 8, n2 = 18, P = 0.030). The power to detect the difference in female preference scores was high (power = 0.88), despite the small sample size. The coefficient of determination (r2) was estimated as 0.270 for females and 0.062 for males, indicating that MHIIb dissimilarity explains a higher proportion of variation in female preferences than that for males.
Experiment 1—preference scores of (a) female (n = 8) and (b) male (n = 18) seahorses for MHIIb-similar and -dissimilar stimuli. A score of 0.5 indicates that the focal individual spent equal time on the stimulus and control sides of the tank, whereas scores <0.5 show a preference for the control and scores >0.5 indicate a preference for the stimulus.
Is the stimulus zone preferred compared with the control zone?
A comparison of the time spent in the stimulus zone relative to the total time spent in both preference zones (P-S + P-C) indicated that females avoided the smell of MHIIb-similar stimuli (one-sample t-test: t7 = −3.663, P = 0.008; Figure 2a), but did not show a preference for MHIIb-dissimilar stimuli compared with the control (P-C) (t7 = 0.935, P = 0.381), a pattern influenced by 2 individuals who did not enter the stimulus zone (P-S) during their trials (Figure 2a). After excluding these individuals, a significant preference for MHIIb-dissimilar stimuli was also detected (t5 = 6.274, P = 0.002). In contrast, males showed no preference for either preference zone (female stimuli vs. control) (MHIIb-similar trials: t17 = 0.412, P = 0.685; MHIIb-dissimilar trials: t17 = 0.309, P = 0.761; Figure 2b).
Experiment 2—olfactory and visual cues
Individuals likely evaluate multiple cues when making mate choice decisions (Candolin 2003). In experiment 2, focal individuals were simultaneously presented with both olfactory and visual cues from stimulus animals. Fifteen female and 18 male trials were included, after excluding trials in which both stimulus individuals had identical MHIIb-dissimilarity values.
Do MHIIb dissimilarity and/or body size influence preference?
A GZLM for preference, including MHIIb dissimilarity, standard length, and sex as main effects (AIC = 38.32), fit the data significantly better than an intercept-only model (omnibus test: likelihood ratio [LR] chi square = 10.96, df = 3, P = 0.012). The addition of 2-way interactions (AIC = 40.92) did not improve the fit of the model (LR test, main effects vs. main effects + 2-way interactions: chi square = 1.224, df = 3, P = 0.747). None of these interactions explained a significant proportion of the variance in preference (sex × MHIIb difference: P = 0.960; sex × size difference: P = 0.938; MHIIb difference × size difference: P = 0.289), and a three-way interaction of sex, MHIIb difference, and size difference was also nonsignificant in the fully parameterized model (P = 0.054). The main-effects model shows a significant positive effect of body size, but in contrast to the results of experiment 1, no effect of MHIIb dissimilarity on individual preferences. Model effects, along with parameter estimates, are provided in Table 1.
Experiment 2—GZLM results for the effects of MHIIb dissimilarity and body size on male (n = 18) and female (n = 15) preferences in Hippocampus abdominalis
| Factor | Model effects | Parameter estimates | ||
| LR chi square | P value | B | Odds ratio | |
| Sex | 0.005 | 0.943 | 0.019 | 1.019 |
| Stimuli − difference in body size | 5.815 | 0.016 | 0.110 | 1.116 |
| Stimuli − difference in MHIIb dissimilarity | 1.612 | 0.204 | 0.066 | 1.068 |
| Factor | Model effects | Parameter estimates | ||
| LR chi square | P value | B | Odds ratio | |
| Sex | 0.005 | 0.943 | 0.019 | 1.019 |
| Stimuli − difference in body size | 5.815 | 0.016 | 0.110 | 1.116 |
| Stimuli − difference in MHIIb dissimilarity | 1.612 | 0.204 | 0.066 | 1.068 |
Experiment 2—GZLM results for the effects of MHIIb dissimilarity and body size on male (n = 18) and female (n = 15) preferences in Hippocampus abdominalis
| Factor | Model effects | Parameter estimates | ||
| LR chi square | P value | B | Odds ratio | |
| Sex | 0.005 | 0.943 | 0.019 | 1.019 |
| Stimuli − difference in body size | 5.815 | 0.016 | 0.110 | 1.116 |
| Stimuli − difference in MHIIb dissimilarity | 1.612 | 0.204 | 0.066 | 1.068 |
| Factor | Model effects | Parameter estimates | ||
| LR chi square | P value | B | Odds ratio | |
| Sex | 0.005 | 0.943 | 0.019 | 1.019 |
| Stimuli − difference in body size | 5.815 | 0.016 | 0.110 | 1.116 |
| Stimuli − difference in MHIIb dissimilarity | 1.612 | 0.204 | 0.066 | 1.068 |
Are larger individuals preferred?
Female seahorses showed no preference with respect to body size (preference [large]: 0.60 ± 0.38; one-sample t-test (H0 = 0.5): t14 = 0.969, P = 0.349), whereas males showed a significant preference for large females relative to small individuals (preference [large]: 0.71 ± 0.27; t17 = 3.286, P = 0.004).
Are MHIIb-dissimilar stimuli preferred?
As in the GZLM, neither female nor male seahorses showed a significant preference for MHIIb-dissimilar stimuli relative to similar individuals (females—preference (dissimilar): 0.49 ± 0.40, one sample t-test (H0 = 0.5): t14 = −0.254, P = 0.803; males—preference (dissimilar): 0.58 ± 0.33, t17 = 1.066, P = 0.301).
Experiment 3—free interaction
In a third experiment, we tested whether the preferences inferred in the first 2 experiments influence mate choice decisions under seminatural conditions. Over the course of the 4-month experiment, 38 matings were detected via genetic analysis of parentage, only 2 of which were from the same 2 parents, leading to 36 different mother–father pairs. A total of 15/25 females (60%) and 17/25 males (68%) reproduced during this period (Figures 3 and 4). Mating success of females ranged from 0 to 5 (1.52 ± 1.58, mean ± SD), while male mating success varied from 0 to 4 matings (1.52 ± 1.33) (Figures 3 and 4). Females had up to 4 different male partners, and males had a maximum of 3 different female partners, with both sexes showing a decreasing interbrood interval with successive matings (full-model GLM (AIC = 613.89): mating number: F4,59 = 2.704, P = 0.039; sex: F1,59 = 0.005, P = 0.943; mating number × sex: F3,59 = 0.222, P = 0.881). The best fit model (mating number only; AIC = 606.65) also showed a negative relationship between mating number and interbrood interval (F4,63 = 2.855, P = 0.031). Sex-specific variance in mating success did not differ significantly (variance females = 2.51, males = 1.76; Levene's test: W1,48 = 0.677, P = 0.415). Males released broods over as many as 4 consecutive days. Interbrood intervals were ≥13 days for females and ≥25 days for males, indicating a higher potential reproductive rate (measured as the maximum number of offspring that can be produced per time unit) of females under experimental conditions, in contrast to the pattern observed in other seahorse species (Vincent 1992; Masonjones and Lewis 2000). Our data confirm within-brood monogamy in H. abdominalis (Wilson and Martin-Smith 2007) and between-brood polygamy within a breeding season (Woods 2000), even under the high-density experimental conditions of our experiment.
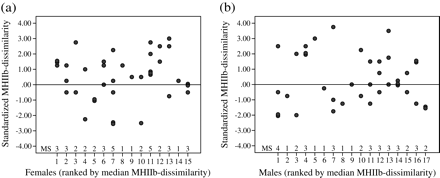
Experiment 3—standardized MHIIb dissimilarities of (a) (n = 15) and (b) male (n = 17) seahorses during mating. Standardized MHIIb dissimilarity = MHIIb dissimilarity of a mating event − median MHIIb dissimilarity to all available mates (expectation under random mating). Scores > 0 indicate that mating partners were more MHIIb dissimilar than the random expectation, while scores < 0 indicate that partners were more similar. MS = mating success.
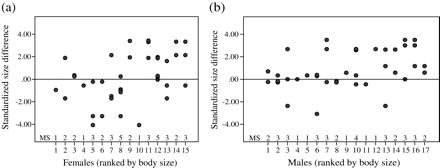
Experiment 3—size of mated (a) female (n = 15) and (b) male (n = 17) seahorses. Standardized size difference = (size mate − size focal individual) − median (sizes of all available mates − size focal individual). Scores > 0 indicate that mating partners were larger than the random expectation, while scores < 0 indicate matings with smaller than expected individuals. MS = mating success.
Is mating random with respect to MHIIb dissimilarity?
Females tended to mate more often with MHIIb-dissimilar males than expected under random mating (mean standardized MHIIb dissimilarity, 95% confidence interval: 0.507, 0.033–0.978), consistent with the results of experiment 1, although this effect was not significant (Wilcoxon signed rank test: n = 15, Z = −1.822, P = 0.068, Figure 3a), while males mated randomly with respect to MHIIb dissimilarity (0.380, −0.148, −0.898), consistent with the results of experiments 1 and 2 (n = 17, Z = −0.630, P = 0.529, Figure 3b).
Is mating random with respect to body size?
Males mated significantly more often with large females than expected under random mating (Mean Standardized Size Difference, 95% Confidence Interval: 0.932, 0.360–1.504), consistent with the results of experiment 2, (Wilcoxon signed rank test: n = 17, Z = −2.898, P = 0.004; Figure 4b), whereas female seahorses showed no pattern of size-based mate choice (−0.165, −0.784–0.456) (Wilcoxon signed rank test: n = 15, Z = −0.398, P = 0.691; Figure 4a).
Is mating success influenced by MH and body size?
We were also interested to see if individual mating success is influenced by size and/or intraindividual MHIIb distance. A GZLM using mating success as the dependent variable with main effects and 2-way interactions (AIC= 159.34) explained the data significantly better than the null model (Omnibus test: LR chi square = 25.36, df = 6, P < 0.001). Model effects, along with parameter estimates, are provided in Table 2.
Experiment 3—GZLM results for the effects of individual MHIIb distance and body size on mating success in Hippocampus abdominalis (n = 50)
| Factor | Model effects | Parameter estimates | ||
| LR chi square | P value | B | Odds ratio | |
| Sex | 1.618 | 0.203 | 4.912 | 135.860 |
| Body size | 0.107 | 0.743 | 0.071 | 1.074 |
| MHIIb distance | 1.101 | 0.294 | −0.815 | 0.443 |
| Sex × body size | 2.851 | 0.091 | −0.286 | 0.751 |
| Sex × MHIIb distance | 14.503 | <0.001 | 0.296 | 1.344 |
| Body size × MHIIb distance | 1.264 | 0.261 | 0.031 | 1.032 |
| Factor | Model effects | Parameter estimates | ||
| LR chi square | P value | B | Odds ratio | |
| Sex | 1.618 | 0.203 | 4.912 | 135.860 |
| Body size | 0.107 | 0.743 | 0.071 | 1.074 |
| MHIIb distance | 1.101 | 0.294 | −0.815 | 0.443 |
| Sex × body size | 2.851 | 0.091 | −0.286 | 0.751 |
| Sex × MHIIb distance | 14.503 | <0.001 | 0.296 | 1.344 |
| Body size × MHIIb distance | 1.264 | 0.261 | 0.031 | 1.032 |
Experiment 3—GZLM results for the effects of individual MHIIb distance and body size on mating success in Hippocampus abdominalis (n = 50)
| Factor | Model effects | Parameter estimates | ||
| LR chi square | P value | B | Odds ratio | |
| Sex | 1.618 | 0.203 | 4.912 | 135.860 |
| Body size | 0.107 | 0.743 | 0.071 | 1.074 |
| MHIIb distance | 1.101 | 0.294 | −0.815 | 0.443 |
| Sex × body size | 2.851 | 0.091 | −0.286 | 0.751 |
| Sex × MHIIb distance | 14.503 | <0.001 | 0.296 | 1.344 |
| Body size × MHIIb distance | 1.264 | 0.261 | 0.031 | 1.032 |
| Factor | Model effects | Parameter estimates | ||
| LR chi square | P value | B | Odds ratio | |
| Sex | 1.618 | 0.203 | 4.912 | 135.860 |
| Body size | 0.107 | 0.743 | 0.071 | 1.074 |
| MHIIb distance | 1.101 | 0.294 | −0.815 | 0.443 |
| Sex × body size | 2.851 | 0.091 | −0.286 | 0.751 |
| Sex × MHIIb distance | 14.503 | <0.001 | 0.296 | 1.344 |
| Body size × MHIIb distance | 1.264 | 0.261 | 0.031 | 1.032 |
The fitted model shows a significant interaction between sex and intraindividual MHIIb distance (Table 2), indicating that the importance of individual MHIIb diversity on mating success differs between male and female seahorses. Sex-specific effects of body size were not significant in this analysis (P = 0.091, Table 2).
Female mating success decreased significantly with increasing intraindividual MHIIb distance (2-tailed Spearman's correlation: n = 25, r = −0.400, P = 0.048), while male mating success increased significantly with MHIIb distance (n = 25, r = 0.460, P = 0.021). Intraindividual MHIIb distance was significantly higher in males carrying rare allelic variants (Spearman's correlation: MHIIb distance vs. population frequency of the rarest allele in each individual—males: r = 0.446, P = 0.025; females: r = 0.084, P = 0.689).
Mating success of female seahorses was positively correlated with body size (2-tailed Spearman's correlation: n = 25, r = 0.412, P = 0.041). Mating success of males, in contrast, was unrelated to size (2-tailed Spearman's correlation: n = 25, r = 0.019, P = 0.928).
DISCUSSION
Using a hierarchical experimental design, we have shown that male and female seahorses differ in their preferences for morphological and olfactory traits. Despite these differences, mate choice decisions in the potbellied seahorse appear to be influenced by both sexes, with females mating with MHIIb-dissimilar partners and males mating more frequently with large-bodied females.
Female mate choice for MH
Female seahorses prefer MHIIb-dissimilar males over MHIIb-similar individuals when presented with olfactory cues, a preference which is also evident in realized mating behavior. In contrast, we found no evidence of MH-based preferences in male seahorses, despite evidence of male mate choice in this species (Wilson and Martin-Smith 2007). Our results suggest that female-mediated sexual selection on MH genes in H. abdominalis likely contributes to the high MHIIb diversity observed in this species (Bahr and Wilson 2011). Consistent with theoretical expectations (Wakeland et al. 1990), males carrying divergent MHIIb alleles had higher mating success, a pattern that could reflect female preference for males carrying diverse and/or rare MHIIb alleles, or condition-related benefits associated with these allelic variants. Surprisingly, females carrying divergent MHIIb variants had lower than expected mating success, a counterintuitive pattern that requires further investigation. Overall, these results are consistent with previous studies on teleosts, which indicate that females, but not males, use MHIIb-based olfactory cues during mate choice (Forsberg et al. 2007; Neff et al. 2008).
While the results of experiments 1 and 3 support female-based preferences for MHIIb in the seahorse, no MH-based preferences were detected in experiment 2, where olfactory and visual cues were presented together. These results might reflect differences in the design of this experiment, which was aimed at studying the effects of body size and included stimulus animals whose MHIIb dissimilarity was more similar than in experiment 1 (see MATERIALS AND METHODS). While a divider separated odors produced by the stimulus animals, this divider did not extend into the choice compartment (Figure 1 in Mattle and Wilson 2009), something which may have affected the focal individual's ability to distinguish odor cues. A follow-up experiment, using a similar tank design to that used in experiment 1, would be worthwhile to explore the significance of these results.
Male seahorses showed no evidence of MHIIb preference in any of our 3 experiments. Indeed, males showed no obvious preference for females relative to control water on the basis of olfactory cues (experiment 1), suggesting that male H. abdominalis cannot detect, or do not use, sex-specific odor cues during mating. A lack of male-based olfactory discrimination of sex has also been observed in a close relative of the seahorse, the sex-role reversed Syngnathus typhle (Sundin et al. 2010; Lindqvist et al. 2011; but see Ratterman et al. 2009 on Syngnathus scovelli). Further investigations of male olfactory preferences in other sex-role reversed species would be beneficial in order to determine the conditions under which they use olfactory signals. Olfactory cues might be less important for males than visual signals during mate choice, if fecundity selection favors individuals who mate with large females.
To our knowledge, evidence of male olfactory preferences for MH-based odor cues is restricted to a small number of studies on humans and mice (Wedekind and Furi 1997; Penn and Potts 1999) and has never been detected in any fish species. While such a pattern may reflect differences in the olfactory capabilities of males and females, this may also stem from the sex-specific production of MHIIb odor cues in this group. Recent work on Gasterosteus aculeatus suggests that a MH-independent signal essential for MH detection is produced exclusively by males in this species, effectively preventing the detection of MH cues produced by females (Milinski et al. 2010). If MH-associated signals exhibit a similar expression pattern in other species, these accessory compounds could provide one potential mechanism by which MH odor cues could be detected and used in a sex-specific fashion.
Male mate choice for body size
The results of our free interaction experiment indicate that male preferences for large-bodied females (experiment 2: Mattle and Wilson 2009) are realized under seminatural conditions. Males mated more frequently with large females than expected by chance, whereas females showed no size-based mating pattern, suggesting that sexual selection may act more strongly on female than male body size in this species. Female mating success increased with body size, likely reflecting the combined effects of male size-based preferences, increased potential reproductive rate (Vincent 1990; Woods 2007), and/or the competitive benefits of large-bodied females. These results might help to explain the observation of female-biased sexual size dimorphism in natural populations of H. abdominalis (Martin-Smith and Vincent 2005; Wilson and Martin-Smith 2007). An investigation of a natural population of the Western Australian seahorse H. subelongatus found that mated females were significantly larger than unmated individuals, but detected no evidence of differential mating with respect to size in males (Kvarnemo et al. 2007), a result consistent with that observed for H. abdominalis in our study.
Mutual mate choice
The potbellied seahorse is considered to be sex-role reversed based on observations of strong female–female competition and male choice in natural populations of this species (Wilson and Martin-Smith 2007). Our study suggests that both sexes actively influence mate choice decisions in H. abdominalis, with sexual selection simultaneously favoring large females and MH-dissimilar males, a pattern of mutual mate choice based on sex-specific mating cues. These results highlight how the preferences of both sexes may influence mate choice behavior, and call into question the traditional dichotomous view of a choosy and a competitive sex (see also Berglund et al. 2005; Ahnesjö 2010). Theoretical models predict the evolution of mutual mate choice in species in which relative parental investment is comparable between the sexes (Kokko and Johnstone 2002), results supported by recent empirical studies that show that mutual mate choice occurs more frequently than previously expected, especially in monogamous species where both parents make a considerable investment in reproduction (see Supplementary Data in Hooper and Miller 2008).
CONCLUSION
Using a combination of mate choice experiments and observations of matings in a free-interaction mating arena, we have demonstrated that male and female seahorses show distinct mating preferences, both of which are realized under seminatural conditions. MHIIb diversity influences female mate choice and male mating success in the seahorse, while male mate choice and female mating success are dependent on body size, leading to mutual mate choice in this species.
Our study demonstrates how the integration of multiple mating cues and both male and female perspectives in behavioral research can result in a more nuanced appreciation of mate choice and sexual selection. Studies such as this are part of an increasing body of research challenging the traditional dichotomous view of the sexes in terms of sex roles, with a choosy and a competitive sex, and call into question models of sexual selection based on such assumptions. We have provided important evidence for how sex-specific preferences for 2 key traits may influence reproductive outcomes, but these are only two of what are likely a large number of traits influencing mate choice decisions in this system. Future work should investigate the multivariate interactions between such traits and, at the same time, explore the implications of mutual mate choice and multimodal integration on standard models of sexual selection.
SUPPLEMENTARY MATERIAL
Supplementary material can be found at http://www.beheco.oxfordjournals.org/.
FUNDING
Funding for this work was provided by the University of Zurich.
We thank Marco Bahr for his help with analyzing video records and Iris Eigenmann, Maely Gauthier, Jan Knott, Kai Stölting, Alexandra Wegmann, Jasmin Winkler, and Stephan Zehnder for assistance with animal care. We are also grateful to Claus Wedekind for suggestions on data analyses, and to Ingrid Ahnesjö, Malte Andersson and Jacek Radwan for helpful comments on the manuscript. Animal husbandry and experimental procedures were approved by the Veterinäramt Zürich (Permit 103/2008).