-
PDF
- Split View
-
Views
-
Cite
Cite
H.E. Moss, J.M. Rodd, E.A. Stamatakis, P. Bright, L.K. Tyler, Anteromedial Temporal Cortex Supports Fine-grained Differentiation among Objects, Cerebral Cortex, Volume 15, Issue 5, May 2005, Pages 616–627, https://doi.org/10.1093/cercor/bhh163
Close - Share Icon Share
Abstract
Patients with damage to left anteromedial temporal cortex often show a striking deficit: they fail to recognize animals and other living things. This failure of recognition presents an important challenge to theories of the neural representation of conceptual knowledge. Here we propose that this lesion–behaviour association arises because polymodal neurons in anteromedial temporal cortex integrate simple features into complex feature conjunctions, providing the neural infrastructure for differentiating among objects.
Introduction
Of the many intriguing and potentially informative patterns of impairment reported in the neuropsychological literature, category-specific semantic deficits are among the most striking. Patients with this type of deficit have a disproportionate difficulty in recognizing and retrieving information about objects in specific categories or domains. The most frequently observed pattern is a deficit for living things, including plant and animal life (Warrington and Shallice, 1984; Warrington and McCarthy, 1987; Sartori and Job, 1988; Silveri and Gainotti, 1988; De Renzi and Lucchelli, 1994; Laiacona et al., 1997; Caramazza and Shelton, 1998; Moss et al., 1998; Tyler and Moss, 2001; Humphreys and Riddoch, 2003). Category-specific semantic deficits for living things are often associated with damage to anterior ventromedial regions of temporal cortex, usually involving both left and right hemispheres, and typically as a consequence of herpes simplex encephalitis (HSE) (Gainotti et al., 1995; Gainotti, 2000). However, there has been relatively little discussion of the specific role played by anteromedial temporal regions in object processing and representation, and why lesions to this area should have a more detrimental effect on the recognition of living things than on other object domains. In this paper we propose an account of the relationship between anteromedial temporal lobe damage and category-specific deficits for living things.
Research with non-human primates suggests that knowledge of objects is represented in a feature-based hierarchical system in which neurons code increasingly complex conjunctions of features from posterior to anterior regions of inferior temporal cortex. On this account, anterior ventromedial regions of temporal cortex play a crucial role in object identification, integrating information from different sensory systems into the more complex polymodal feature conjunctions that are necessary for fine-grained discrimination among similar objects (Desimone and Ungerleider, 1989; Murray and Bussey, 1999; Murray and Richmond, 2001; Bussey et al., 2002). Damasio (1989) has proposed a similar hierarchical system for object recognition in the human brain, claiming that the simple features that comprise an object (such as features depicting visual form and movement) are represented in sensory systems located in posterior regions of temporal cortex. These simple features are combined into more complex configurations in ‘convergence zones’ located in anterior temporal regions (see also Simmons and Barsalou, 2003 for a related proposal).
We recently reported an event-related functional magnetic resonance imaging (fMRI) study, which provides evidence for the role of left anteromedial structures in fine-grained discrimination (Tyler et al., 2004a). Regions of temporal cortex were activated to different degrees depending on the level of detailed information that had to be extracted from pictured objects. Areas within left anteromedial temporal cortex were activated when participants named familiar objects at the basic level (e.g. table, cat), requiring within-category differentiation. Activation centred on the perirhinal cortex, extending anteriorly and medially to include much of the fusiform gyrus, the amygdala and the hippocampus. These activations were not seen when the same pictures were named at the domain level (living or man-made), a task for which fine-grained discrimination was not necessary. In contrast, more posterior temporal regions, including the left fusiform gyrus, were equally activated across both naming tasks.
When applied to the human system, the hierarchical object-processing account (Damasio, 1989; Simmons and Barsalou, 2003) provides a potential framework in which to explain the frequent co-occurrence of category-specific deficits for living things with anteromedial temporal lesions, usually as a consequence of HSE. There is considerable evidence from the literature on category-specific semantic deficits that living things such as animals and fruit are characterized by many overlapping shared features, that are highly intercorrelated, and relatively few distinctive features (McRae et al., 1997; Devlin et al., 1998; Moss et al., 1998; Tyler et al., 2000; Tyler and Moss, 2001; McRae and Cree, 2002; Randall et al., 2004; see also Keil, 1987). For example, lions, tigers and leopards are very similar in shape and constituent parts (they are ‘structurally similar’, Humphreys et al., 1988), and also in their texture and the ways that they move. They are distinguished primarily by properties such as colour and markings. In contrast, objects within non-living categories (e.g. tools and vehicles) tend to be less similar to each other, with a greater proportion of distinctive to shared properties.
We have developed an account — the Conceptual Structure Account — in which these differences in the structure of concepts in different domains play a key role in the emergence of selective impairments for living things, primarily because their weakly correlated distinctive properties are susceptible to damage (Tyler et al., 2000; Tyler and Moss, 2001; Randall et al., 2004). Although there has been considerable debate as to whether such factors alone provide a comprehensive explanation for the full range of category-specific deficits (e.g. Caramazza and Shelton, 1998; Garrard et al., 2001; Caramazza and Mahon, 2003), there is, nevertheless, general agreement on the basic premise that living things tend to have a higher degree of within-category similarity than do non-living things (e.g. Humphreys et al., 1988; Gaffan and Heywood, 1993; Dixon et al., 1997; Lloyd-Jones and Humphreys, 1997; Tranel et al., 1997; Devlin et al., 1998; Garrard et al., 2001; Greer et al., 2001; Lamberts and Shapiro, 2002; Moss et al., 2002; although see Laws et al., 2002). The tendency for category members to be similar in the living domain applies not only to visual form properties that are directly relevant to object recognition [e.g. shape overlap (Humphreys et al., 1988) or visual features identified from drawings (Rogers et al., 2004)], but also to other kinds of non-visual semantic property that are important for the conceptual representation of an object, such as its functions and behaviours. An important source of evidence for this claim comes from property generation studies; when people are asked to generate lists of all the properties they can think of for large numbers of concepts, there is reliably greater overlap in the sets of properties given for items in living categories than non-living categories (Devlin et al., 1998; Garrard et al., 2001; McRae et al., 2002;Randall et al., 2004; Rogers et al., 2004). Naturally, the degree of similarity varies for individual items, so that there will be exceptional items in each domain that do not adhere to the general pattern. Moreover, it is possible to manipulate the degree of within-category similarity by comparing items at different levels of specificity. So, for example, the within-category similarity for vehicles is much increased if we compare items within the subordinate level category — such as different models of car. Nevertheless, the crucial point is that at comparable levels of abstraction — and especially at the psychologically salient ‘basic level’ at which people naturally name objects (e.g. lion, lettuce, car or spade, cf. Rosch et al., 1976) — members of living-things categories such as animals or fruit are more similar to each other than are members of non-living-things categories such as tools or vehicles.
The hierarchical object-processing account predicts that the high degree of within-category similarity for living things will place greater demands on the processes of integration of complex conjunctions of features and fine-grained discrimination supported by anteromedial temporal cortex. Thus, within-category distinctions for living things will be disproportionately affected by lesions to these regions.
This account predicts that we should also see greater activation of anteromedial temporal regions for categories of living things compared to non-living items when healthy participants are engaged in tasks that require fine-grained discrimination among objects. We tested this hypothesis in an event-related fMRI study in which participants covertly named pictures of living or non-living things at two levels of specificity. In one condition, participants named the pictures at the basic level (e.g. lion, knife), thus requiring differentiation among members of a category (e.g. between lions and tigers within the animal category, or between knives and forks within the tool category) In the second condition, participants named the same pictures, but this time at the domain level (i.e. living, man-made). In this case, there would be no need to determine that a pictured animal, for example, was a lion rather than a leopard, but only to decide that it is a living thing rather than a man-made object. Thus, the experimental paradigm is the same as in our earlier fMRI study of object processing at basic and domain levels. In our earlier study (Tyler et al., 2004a) we included pictures of both living and non-living things, as this was required by our domain-level naming task, in which subjects had to respond ‘living’ or ‘man-made’). However, the experiment was designed only to contrast basic- and domain-level naming, and not to investigate differences across domains. Therefore, in that study, pictures in living and non-living domains were not closely matched on all relevant variables, nor were there sufficiently large numbers of items to analyse the data at category or domain level. In the current study we have included large numbers of pictures from well-defined categories in each domain, and with detailed matching of items across domains on a range of variables that affect object recognition and naming performance.
Our hypothesis concerning the role of anteromedial temporal cortex in fine-grained differentiation among objects also predicts that brain-damaged patients with semantic deficits who do not have damage to anteromedial temporal cortex, but whose lesions are restricted to more lateral temporal sites, will not necessarily show a category-specific deficit for living things. It is important to test this reverse prediction, since brain damage resulting from HSE is not limited to anteromedial temporal regions; lesions are usually large and may extend beyond the anteromedial temporal regions on which we have focused. For example, voxel-based morphometric (VBM) analysis of five HSE patients indicated common regions of abnormality in the temporal poles and lateral temporal cortex, in addition to the anteromedial temporal areas (Gitelman et al., 2001). It is therefore possible that the category-specific deficit for living things in these patients is attributable to object processing and/or representation supported by regions other than those that we have proposed to be critical. To examine this issue we compared two groups of three patients; one group had anteromedial damage following HSE, and showed the classic pattern of disproportionate impairment for living things. The behavioural pattern for these patients contrasted with a group of semantic dementia (SD) patients who also had profound semantic deficits, but for whom living things were not selectively impaired, as is usually, although not invariably, the pattern in SD (Lambon Ralph et al., 2003), although we discuss this point further in the General Discussion. We carried out detailed analyses of the lesions of these two groups of patients, with particular focus on the involvement/sparing of anteromedial temporal regions.
Materials and Methods
Neuroimaging Study
Participants
Twelve participants aged between 19 and 47 years (five males, seven females) participated in this study. All gave informed consent. The study was approved by Addenbrooke's NHS Trust Ethical Committee.
Materials
We presented 174 coloured pictures of common objects for participants to name. Of these, 110 were from the categories of animals, fruits and vegetables, tools and vehicles. These categories are representative of the living and non-living domains respectively, and have been the most thoroughly investigated in both neuropsychological and psychological studies of category-specificity. Moreover, these are the same categories as those that we have included in our earlier property norming study of 93 concepts (Moss et al., 2002; Randall et al., 2004). Although the items were not identical to those used here, there is considerable overlap. The property norm analyses demonstrate that living things are more semantically similar to each other than are non-living things: On average, only 33% and 25% of the properties listed for animals and fruit respectively were distinctive (given in response to only one or two category members), while 67% and 75% were shared (given in response to more than three categories members). In contrast, for tools and vehicles there were 47% and 63% distinctive properties respectively and only 53% and 39% shared properties. Property norms do not measure the visual similarity of pictures. However, as discussed in the Introduction, many earlier studies have consistently found greater visual similarity for animals and fruit than for tools and vehicles.
Living and non-living things were matched on concept agreement (the degree to which the picture was named consistently with the intended target object/animal name), exemplarity (the rated goodness of the picture as an instance of the intended object/animal) as well as rated familiarity and age of acquisition of the basic level name. All ratings were collected at the Centre for Speech and Language from groups of young healthy adults. Because of the natural variation in these factors across different categories (e.g. pictures of vehicles and animals tend to be more visually complex than those of fruits and tools) matching was carried out across two pairs of living/non-living categories, such that vehicles were closely matched with animals, and tools with fruits and vegetables. t-tests confirmed that there were no significant differences between the pairs of categories on any of the variables listed above (P > 0.05 in all cases).
For the domain-level naming task the names ‘living’ and ‘man-made’ were chosen as they have the same syllabic length and similar phonemic lengths (five versus six phonemes). Objects from the test categories were intermixed with objects from a wide variety of other categories (e.g. clothing, birds), which were included as fillers.
Before the experiment was run in the scanner we pre-tested with a separate group of 16 subjects to determine whether naming reaction times were comparable across the living and non-living domains. This pre-test showed that naming pictures at the domain level was faster overall (652 ms) than naming at the basic level (899 ms). However, there was no difference in basic-level naming reaction times between the matched living and non-living categories (fruit and vegetables versus tools, 914 and 919 ms respectively [F(1,58) < 1]; animals versus vehicles, 870 and 892 ms respectively [F(1,48) < 1]). Similarly, there was no difference in domain level naming times for any of the matched categories (fruit and vegetables versus tools, 646 and 664 ms respectively [F(1,58) = 2.4, P > 0.1]; animals versus vehicles, 649 and 655 ms respectively [F(1,48) < 1]).
Procedure
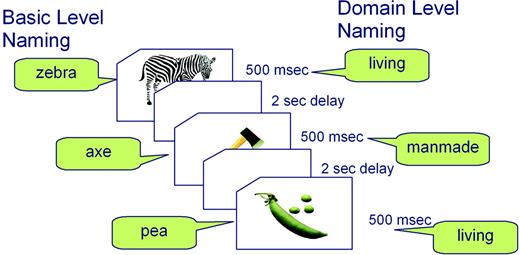
Each event consisted of a colour picture presented in the centre of a computer screen for 500 ms followed by a 2 s delay during which the participant named the picture silently. In the baseline condition, a fixation cross was presented for 500 ms followed by a 2 s delay. The pictures were presented in two blocks. Each block contained all 174 pictures and 58 baseline trials in pseudo-random order. Participants named the pictures at basic level in one block, and at domain level in the other. It was necessary to divide the basic- and domain-level naming tasks into two blocks to ensure that subjects' responses were not affected by task-switching difficulties, which we had observed to be a problem in pilot studies where the two tasks alternated more rapidly between shorter blocks. Nevertheless, within each of the two task blocks, living and non-living trials were randomly intermixed within an event-related design. The order of the two blocks was counterbalanced across participants (see Fig. 1). During the baseline trials, subjects were instructed merely to fixate on the cross. Presentation and timing of stimuli were controlled by DMDX software (Forster and Forster, 2003). Each session was preceded by a short practice session of 20 items before scanning started. Responses to all stimuli were covert to avoid excess movement in the scanner.
Diagram illustrating the experimental procedure for a subset of pictures. The same pictures were presented in two separate blocks, once for naming at the basic level and once for naming at the domain level, with block order counterbalanced over participants.
Scanning was carried out on a 3 T Bruker Medspec Avance S300 system using a gradient-echo EPI sequence (TR = 1100 ms, TE = 27.5 ms, flip angle 65°, matrix size 64 × 64, FOV 20 × 20 cm, in plane resolution 3.1 mm × 3.1 mm, 21 oblique slices angled away from the eyes, 4 mm thick, with head coils, 143 kHz bandwidth and spin echo guided reconstruction). T1-weighted scans were acquired for anatomical localization. The data were preprocessed and analysed using SPM99 software (www.fil.ion.ucl.ac.uk: Friston et al., 1995) implemented in Matlab (Mathworks Inc. Sherborn, MA). Pre-processing steps included within-subject realignment, spatial normalization of the functional images to a standard EPI template (masking regions of susceptibility artefact to reduce tissue distortion) and spatial smoothing using a Gaussian kernel of 8 mm. After pre-processing and spatial normalization, the data for each subject were modelled with the general linear model, using the canonical haemodynamic response function with temporal derivatives. Visual complexity of pictures was included as a parametric modulator. Visual complexity measures were collected from a group of 15 healthy young adults, who rated each picture on a scale of 1 (very simple) to 9 (very complex). Parameter estimate images from each subject were combined into a group random effects analysis (RFX).
Patient Lesion Analysis
Participants
Participants were three patients with category-specific deficits for living things as a consequence of HSE and three with a clear diagnosis of semantic dementia — a progressive disorder of semantic memory, with relative preservation of other linguistic and cognitive faculties (e.g. Snowden et al., 1989; Hodges et al., 1992). Case histories for the three HSE patients (RC, JBR and WL) can be found in our earlier papers, where these patients have been described in detail (Bunn et al., 1998; Moss et al., 1998; Tyler et al., 2002). Case descriptions for the three semantic dementia patients (EK, BS and JT) can also be found elsewhere (Jefferies et al., 2004; Tyler et al., 2004b). Table 1 presents background cognitive test results, together with two measures of semantic knowledge for each patient. On the Mini-mental State Examination (Folstein et al., 1975), all patients scored within the mild to moderate cognitive impairment range. On the Ravens' Progressive Matrices test (Raven et al., 1976), a measure of non-verbal problem solving, only one patient (JT) showed evidence of significant impairment. Digit span (using the subtest from the Wechsler Memory Scale–Revised: Wechsler, 1987) varied from the 18th to the 52nd percentile equivalents (JBR and BS respectively). We also present results from two tests of semantic knowledge, the Pyramids and Palm Trees test (Howard and Patterson, 1992) and a within semantic category word-to-picture matching test (Moss et al., 1998). Normal, healthy individuals typically score at ceiling on both tests, but all six patients were markedly impaired, consistent with profound semantic memory deficits. Finally, Table 1 includes the patients' scores on several of the subtests of the Cortical Vision Screening Test (James et al., 2001), showing none of them have an impairment of shape, colour or size discrimination, and on the minimal feature test of the Birmingham Object Recognition Battery (Riddoch and Humphreys, 1993), showing intact object constancy. These results suggest that none of the patients has any degree of pre-categorical visual processing deficit.
Background neuropsychological data for patients
| . | . | Herpes simplex encephalitis (HSE) . | . | . | Semantic dementia (SD) . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
. | Kax . | RC . | JBR . | WL . | EK . | BS . | JT . | ||||
| Age | 43 | 47 | 70 | 61 | 68 | 67 | |||||
| MMSEa | 30 | 24 | 23 | 19 | 23 | 27 | 23 | ||||
| Ravens' matrices | 36 | 33 | 35 | 36 | 33 | 32 | 24 | ||||
| Digit Spanb | 7 | 5 | 6 | 7 | 7 | 5 | |||||
| Pyramids Palm Trees (%)c | 74 | 83 | 81 | 54 | 73 | 64 | |||||
| Word–picture match (%) | 78 | 78 | 84 | 89 | 85 | 77 | |||||
| BORBd Minimal Feature Match | 25 | 24 | 88 | 25 | 25 | 24 | 24 | ||||
| CORVISTe | |||||||||||
| Shape discrimination | 8 | 8 | 8 | 8 | 8 | 8 | 8 | ||||
| Size discrimination | 2 | 2 | 1 | 2 | 2 | 1 | 2 | ||||
| Shape detection | 8 | 7 | 8 | 8 | 7 | 8 | 8 | ||||
| Hue discrimination | 4 | 4 | 4 | 4 | 4 | 4 | 4 | ||||
| . | . | Herpes simplex encephalitis (HSE) . | . | . | Semantic dementia (SD) . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
. | Kax . | RC . | JBR . | WL . | EK . | BS . | JT . | ||||
| Age | 43 | 47 | 70 | 61 | 68 | 67 | |||||
| MMSEa | 30 | 24 | 23 | 19 | 23 | 27 | 23 | ||||
| Ravens' matrices | 36 | 33 | 35 | 36 | 33 | 32 | 24 | ||||
| Digit Spanb | 7 | 5 | 6 | 7 | 7 | 5 | |||||
| Pyramids Palm Trees (%)c | 74 | 83 | 81 | 54 | 73 | 64 | |||||
| Word–picture match (%) | 78 | 78 | 84 | 89 | 85 | 77 | |||||
| BORBd Minimal Feature Match | 25 | 24 | 88 | 25 | 25 | 24 | 24 | ||||
| CORVISTe | |||||||||||
| Shape discrimination | 8 | 8 | 8 | 8 | 8 | 8 | 8 | ||||
| Size discrimination | 2 | 2 | 1 | 2 | 2 | 1 | 2 | ||||
| Shape detection | 8 | 7 | 8 | 8 | 7 | 8 | 8 | ||||
| Hue discrimination | 4 | 4 | 4 | 4 | 4 | 4 | 4 | ||||
Mini-Mental State Examination.
Maximum forward digit span.
Howard and Patterson (1992) — mean performance on picture and word stimuli.
Birmingham Object Recognition Battery (Riddoch and Humphreys, 1993).
Cortical Vision Screening Test (James et al., 2001).
Background neuropsychological data for patients
| . | . | Herpes simplex encephalitis (HSE) . | . | . | Semantic dementia (SD) . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
. | Kax . | RC . | JBR . | WL . | EK . | BS . | JT . | ||||
| Age | 43 | 47 | 70 | 61 | 68 | 67 | |||||
| MMSEa | 30 | 24 | 23 | 19 | 23 | 27 | 23 | ||||
| Ravens' matrices | 36 | 33 | 35 | 36 | 33 | 32 | 24 | ||||
| Digit Spanb | 7 | 5 | 6 | 7 | 7 | 5 | |||||
| Pyramids Palm Trees (%)c | 74 | 83 | 81 | 54 | 73 | 64 | |||||
| Word–picture match (%) | 78 | 78 | 84 | 89 | 85 | 77 | |||||
| BORBd Minimal Feature Match | 25 | 24 | 88 | 25 | 25 | 24 | 24 | ||||
| CORVISTe | |||||||||||
| Shape discrimination | 8 | 8 | 8 | 8 | 8 | 8 | 8 | ||||
| Size discrimination | 2 | 2 | 1 | 2 | 2 | 1 | 2 | ||||
| Shape detection | 8 | 7 | 8 | 8 | 7 | 8 | 8 | ||||
| Hue discrimination | 4 | 4 | 4 | 4 | 4 | 4 | 4 | ||||
| . | . | Herpes simplex encephalitis (HSE) . | . | . | Semantic dementia (SD) . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
. | Kax . | RC . | JBR . | WL . | EK . | BS . | JT . | ||||
| Age | 43 | 47 | 70 | 61 | 68 | 67 | |||||
| MMSEa | 30 | 24 | 23 | 19 | 23 | 27 | 23 | ||||
| Ravens' matrices | 36 | 33 | 35 | 36 | 33 | 32 | 24 | ||||
| Digit Spanb | 7 | 5 | 6 | 7 | 7 | 5 | |||||
| Pyramids Palm Trees (%)c | 74 | 83 | 81 | 54 | 73 | 64 | |||||
| Word–picture match (%) | 78 | 78 | 84 | 89 | 85 | 77 | |||||
| BORBd Minimal Feature Match | 25 | 24 | 88 | 25 | 25 | 24 | 24 | ||||
| CORVISTe | |||||||||||
| Shape discrimination | 8 | 8 | 8 | 8 | 8 | 8 | 8 | ||||
| Size discrimination | 2 | 2 | 1 | 2 | 2 | 1 | 2 | ||||
| Shape detection | 8 | 7 | 8 | 8 | 7 | 8 | 8 | ||||
| Hue discrimination | 4 | 4 | 4 | 4 | 4 | 4 | 4 | ||||
Mini-Mental State Examination.
Maximum forward digit span.
Howard and Patterson (1992) — mean performance on picture and word stimuli.
Birmingham Object Recognition Battery (Riddoch and Humphreys, 1993).
Cortical Vision Screening Test (James et al., 2001).
Behavioural Tasks: Methods, Materials and Procedure
Each patient was tested on a set of three behavioural tasks which probed knowledge of living and non-living objects in different ways, to establish whether there was a disproportionate deficit for living things relative to non-living objects.
Basic Level Picture Naming
Patients were asked to name colour photographs of living and non-living things. This task, identical to the basic-level naming condition used with healthy subjects in the imaging study, requires recognition of the pictured object and differentiation from other similar objects in order to generate the correct name. The living things were made up of animals (20) and fruit (16) and the non-living things of vehicles (20) and tools (16). Pictures were matched for visual complexity, imageability and familiarity across domains (see Bunn et al., 1998, for details of the picture set).
Property Verification
This task provided a measure of conceptual knowledge from verbal input. Questions about the properties of a set of 40 living (animals and fruit) and 40 non-living (vehicles and tools) were devised, equally divided between true and false trials, perceptual and functional attributes and distinctive and shared properties. Concepts and properties were matched across the living and non-living domains for familiarity, property salience and distinctiveness (see Moss et al., 2002, for further details of this task). Accuracy of response (true/false) was averaged across properties in living and non-living domains for each patient.
Category Fluency
This task measures the patients' ability to generate names for concepts within the living and non-living domains. Each patient was asked to list as many animals, fruit, tools and vehicles as they could within 1 min.
Lesion Analysis Procedure
Statistical lesion analysis was carried out in SPM99 (www.fil.ion.ucl.ac.uk: Friston et al., 1995) and involved the comparison of single patient images with a group of control images. The images were pre-processed by spatially normalizing them in SPM99 with both linear (12 affine transformations i.e. translations, rotations, zooms and shears in x, y, z directions) and non-linear (7 × 8 × 7 basis functions) transformations. Medium regularization was used to constrain the non-linear part of the algorithm and effectively penalize unlikely deformations that might have resulted from lesions (Ashburner and Friston, 1999). The images were then skull stripped by masking each one with the standard SPM brain mask. Since we did not aim to discover inter-individual skull variations, removing the skull from the images speeded the computation. Finally the images were smoothed with a 10 mm isotropic Gaussian kernel to account for inter-individual anatomical variations and also to render the data normally distributed. The global mean of each image was included as a confounding covariate in the analysis to account for effects of global signal differences between scans (Stamatakis and Tyler, 2003). The anatomical T1-weighted images for the HSE patients and the controls were acquired on a 2 T Siemens MRI scanner (TR = 500 ms, TE = 4 ms, flip angle 21°, matrix 256 × 224, 1 × 1 × 1.5 mm3 voxels). The SD patients were compared to controls in a separate model following the same methodology. The SD patients and equivalent control scans were acquired on a Siemens 1.5 T scanner (TR = 540 ms, TE = 15 ms, flip angle 90°, matrix 144 × 256, 1.2 × 0.9 × 6 mm3 voxels). Group deficits were obtained by conducting a conjunction analysis for each of the two groups of patients.
Results
Neuroimaging Study
A comparison between basic- and domain-level naming in our earlier object-naming study (Tyler et al., 2004a), revealed activations in the anteromedial temporal cortex and more specifically in the left perirhinal and entorhinal cortices. The current experiment investigated activation in these a priori defined regions (Tyler et al., 2004a) and consequently the results were thresholded at P = 0.001, uncorrected (Bailey et al., 1991).
We are aware that susceptibility artefacts are a cause for concern in anteromedial brain regions. However, the potential problems associated with these areas are either (i) total lack of signal or (ii) signal reduction associated with reduced sensitivity in the detection of BOLD responses (Lipschutz et al., 2001). These limitations are likely to lead to an overall underestimation of activation in these regions, but they do not interact with the differences across conditions in any way that would undermine our results. Nevertheless, we apply stringent quality control to our imaging data by visually and statistically inspecting them for artefacts before we use them in any analysis. Moreover, our earlier study (Tyler et al., 2004a) and those of other research groups have also reported activations in the same areas (Pihlajamäki et al., 2001; Bartha et al., 2003; Jackson and Schacter, 2004).
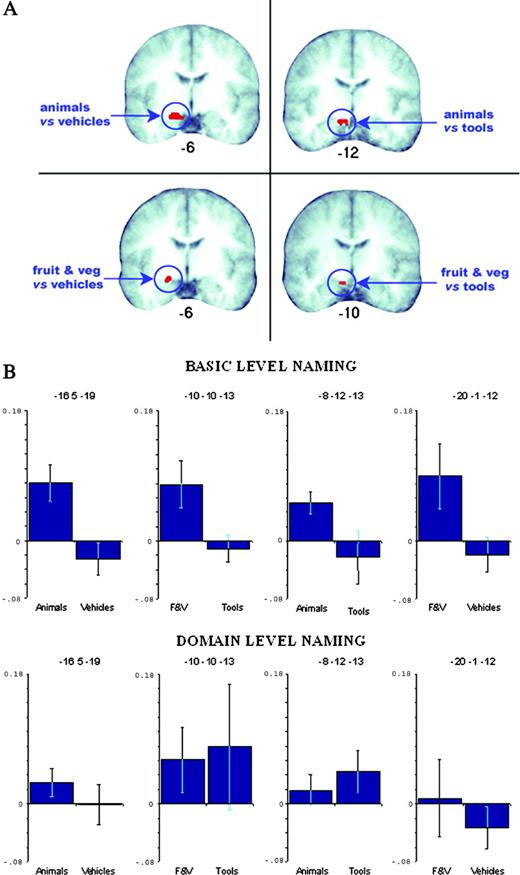
The analysis here focused on the direct contrasts between the matched categories: animals versus vehicles, fruits and vegetables versus tools. We predicted that in each case, the living-things category would generate more activation than the artefact category in anteromedial regions for the basic-level naming task, but that there would be no such difference when the same pictures were named at the domain level. The results confirmed our predictions. Naming animals at a basic level produced a cluster of activation in entorhinal (BA 28) and dorsal entorhinal (BA 34) cortex (peak Talairach coordinates –16 5 –19), over and above the activation produced by naming vehicles at a basic level (see Fig. 2). Similarly, naming fruits and vegetables at a basic level produced more activation in the entorhinal cortex (BA 28) than naming tools at a basic level (peak coordinates –10 –10 –13). There were no significant differences between any of the categories for domain-level naming.
Results of the fMRI study. (A) Comparisons of basic-level naming contrasts are shown superimposed on the mean T1 anatomical image of the participants in this study. Animals versus vehicles resulted in a cluster in the parahippocampal area BA 28, also extending anteriorly to BA34. Animals versus tools produced a cluster in BA28. Fruit and vegetables versus vehicles resulted in a cluster in BA 34 and finally fruit and vegetables versus tools resulted in a cluster in BA 28. Talairach y-coordinates are shown underneath each anatomical slice where left = left. Slices were selected on the basis of maximum cluster extent in this plane. (B) Plots showing percentage signal change (plus standard error bars) at the activation peaks observed for basic-level naming. Plots shown for basic- and domain-level naming. F&V = fruits and vegetables.
For completeness, we also carried out additional contrasts between the non-matched living and non-living categories. The pattern of results was the same as for the matched categories. Naming animals at a basic level also produced significantly more activation in the entorhinal cortex (BA 28) than naming tools at a basic level (peak coordinates –8 –12 –13), while naming fruit and vegetables at a basic level produced significantly more activation in the dorsal entorhinal cortex (BA 34) than naming vehicles at a basic level (peak coordinates –20 –1 –12). Again, these categories did not show any differences in the domain-naming task.
Finally we compared naming of living things with naming of artefacts, averaged across categories. This contrast produced a cluster of significant activation in entorhinal cortex (BA 28) during basic-level naming (peak = –18 –1 –10). No differences were found for domain-level naming.
In order to understand better the differing patterns of activation between living things and artefacts at the two levels of naming, we plotted percentage signal change at each of the category comparison peaks described above. As shown in Figure 2B, we found consistently more activation during naming of living things relative to artefacts, but only for basic-level naming. During domain-level naming, activation was not differentiated as a function of the category from which the stimuli were drawn. While the plots are, overall, in agreement with our prediction that only basic-level naming will recruit anteromedial temporal cortex (as this condition requires more fine-grained discrimination), we found that at one activation peak (–10 –10 –13) there was more activation for tools during domain naming relative to basic naming. Although this observation cannot easily be incorporated within our theoretical framework, we note that there is a disproportionately high level of variability across subjects for domain naming of tools relative to that found for all other categories and conditions. However, we must conclude on the basis of these data that our claim is more consistently supported by the visually ‘complex’ categories (animals and vehicles).
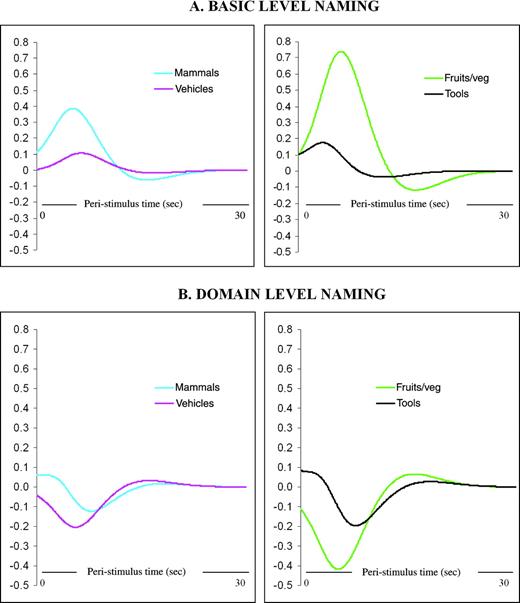
As a further comparison of the response to living things and artefact categories during basic- and domain-level naming, we fitted the response (in terms of percentage signal change relative to baseline) against peristimulus time (PST) at the activation peaks for the matched category comparisons. Figure 3, which shows time-course plots for a representative participant, supports the main analyses. For basic-level naming, there is a clear, positive peak for the two living-things categories, but the response for both artefact categories is relatively small. For domain-level naming, responses are negative and relatively small, and there is no clear differentiation in the response size among the living-things and artefact categories.
Time-course data: fitted responses for a typical participant shown in terms of percent signal change (relative to baseline) against peristimulus time (PST) at basic-level naming activation peaks for matched category comparisons (animals versus vehicles and fruits and vegetables versus tools). Responses shown for (A) basic-level naming and (B) domain level naming.
It is also important to establish whether the experimental contrasts gave rise to any other significant activations in regions of the brain outside the anteromedial temporal areas that were the focus of the study. To determine this, we checked for significant clusters of activation at a corrected level of P < 0.05 (the more conservative alpha level being appropriate here, since in this case we did not have an a priori hypothesis of an effect in any specific region). There were no significant areas of activation, apart from one small cluster in the left putamen for the basic-level fruit and vegetables minus vehicles contrast (–26 2 5), a region usually associated with the control of sensorimotor functions, although research in monkeys has also linked this area with decision-making processes (Merchant et al., 1997).
Patient Behavioural Data
Data for the patients on each of the behavioural tasks are summarized in Table 2 and data for the two groups in each task are plotted in Figure 4.
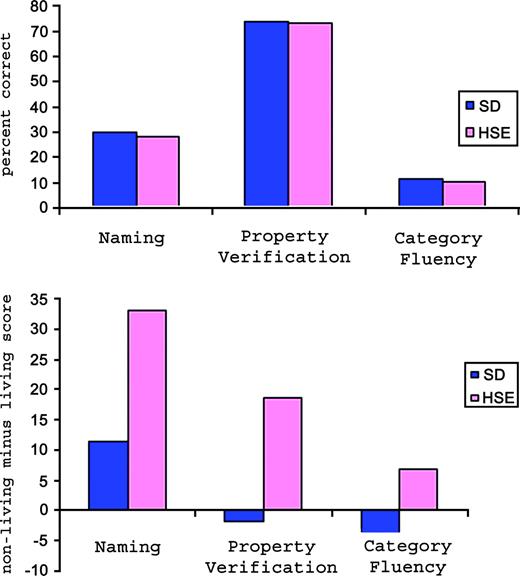
Evidence from clinical cases. Graph in upper panel shows the overall accuracy of HSE and SD patients in three semantic tasks (scores for category fluency are actual number of correct responses rather than percent correct). Graph in lower panel shows the difference between scores on living and non-living things in behavioural tasks for HSE and SD patients.
Patient results for living versus non-living things in semantic tasks
| . | Herpes simplex encephalitis (HSE) . | . | . | . | Semantic dementia (SD) . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | RC . | JBR . | WL . | Mean . | EK . | BS . | JT . | Mean . | ||||||||
| Naming (% correct) | ||||||||||||||||
| Living | 10.5 | 22.5 | 8.15 | 13.7 | 20 | 40 | 7.145 | 22.4 | ||||||||
| Non-living | 43 | 55.5 | 41.5 | 46.7 | 28 | 61.25 | 12.5 | 33.9 | ||||||||
| Difference | 32.5a | 33a | 33.35a | 33.0 | 8 | 21.25 | 5.355 | 11.5 | ||||||||
| Property verification (% correct) | ||||||||||||||||
| Living | 53.5 | 74 | 67 | 64.8 | 70 | 84 | 69 | 74.3 | ||||||||
| Non-living | 74.5 | 92.5 | 83.5 | 83.5 | 74.5 | 83.5 | 60 | 72.7 | ||||||||
| Difference | 21a | 18.5a | 16.5a | 18.7 | 4.5 | −0.5 | −9 | −1.7 | ||||||||
| Category fluency (number correct) | ||||||||||||||||
| Living | 6 | 10 | NA | 8.0 | 17 | 17 | 3 | 12.3 | ||||||||
| Non-living | 13 | 17 | NA | 15.0 | 10 | 14 | 2 | 8.7 | ||||||||
| Difference | 7 | 7 | NA | 7.0 | −7 | −3 | −1 | −3.7 | ||||||||
| . | Herpes simplex encephalitis (HSE) . | . | . | . | Semantic dementia (SD) . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | RC . | JBR . | WL . | Mean . | EK . | BS . | JT . | Mean . | ||||||||
| Naming (% correct) | ||||||||||||||||
| Living | 10.5 | 22.5 | 8.15 | 13.7 | 20 | 40 | 7.145 | 22.4 | ||||||||
| Non-living | 43 | 55.5 | 41.5 | 46.7 | 28 | 61.25 | 12.5 | 33.9 | ||||||||
| Difference | 32.5a | 33a | 33.35a | 33.0 | 8 | 21.25 | 5.355 | 11.5 | ||||||||
| Property verification (% correct) | ||||||||||||||||
| Living | 53.5 | 74 | 67 | 64.8 | 70 | 84 | 69 | 74.3 | ||||||||
| Non-living | 74.5 | 92.5 | 83.5 | 83.5 | 74.5 | 83.5 | 60 | 72.7 | ||||||||
| Difference | 21a | 18.5a | 16.5a | 18.7 | 4.5 | −0.5 | −9 | −1.7 | ||||||||
| Category fluency (number correct) | ||||||||||||||||
| Living | 6 | 10 | NA | 8.0 | 17 | 17 | 3 | 12.3 | ||||||||
| Non-living | 13 | 17 | NA | 15.0 | 10 | 14 | 2 | 8.7 | ||||||||
| Difference | 7 | 7 | NA | 7.0 | −7 | −3 | −1 | −3.7 | ||||||||
Difference score (living minus non-living) is significant at P < 0.01 (χ2 test). Accuracy scores are shown as percentage correct for ease of comparison of performance over tasks with different numbers of items. Analyses were carried out over raw scores.
Patient results for living versus non-living things in semantic tasks
| . | Herpes simplex encephalitis (HSE) . | . | . | . | Semantic dementia (SD) . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | RC . | JBR . | WL . | Mean . | EK . | BS . | JT . | Mean . | ||||||||
| Naming (% correct) | ||||||||||||||||
| Living | 10.5 | 22.5 | 8.15 | 13.7 | 20 | 40 | 7.145 | 22.4 | ||||||||
| Non-living | 43 | 55.5 | 41.5 | 46.7 | 28 | 61.25 | 12.5 | 33.9 | ||||||||
| Difference | 32.5a | 33a | 33.35a | 33.0 | 8 | 21.25 | 5.355 | 11.5 | ||||||||
| Property verification (% correct) | ||||||||||||||||
| Living | 53.5 | 74 | 67 | 64.8 | 70 | 84 | 69 | 74.3 | ||||||||
| Non-living | 74.5 | 92.5 | 83.5 | 83.5 | 74.5 | 83.5 | 60 | 72.7 | ||||||||
| Difference | 21a | 18.5a | 16.5a | 18.7 | 4.5 | −0.5 | −9 | −1.7 | ||||||||
| Category fluency (number correct) | ||||||||||||||||
| Living | 6 | 10 | NA | 8.0 | 17 | 17 | 3 | 12.3 | ||||||||
| Non-living | 13 | 17 | NA | 15.0 | 10 | 14 | 2 | 8.7 | ||||||||
| Difference | 7 | 7 | NA | 7.0 | −7 | −3 | −1 | −3.7 | ||||||||
| . | Herpes simplex encephalitis (HSE) . | . | . | . | Semantic dementia (SD) . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | RC . | JBR . | WL . | Mean . | EK . | BS . | JT . | Mean . | ||||||||
| Naming (% correct) | ||||||||||||||||
| Living | 10.5 | 22.5 | 8.15 | 13.7 | 20 | 40 | 7.145 | 22.4 | ||||||||
| Non-living | 43 | 55.5 | 41.5 | 46.7 | 28 | 61.25 | 12.5 | 33.9 | ||||||||
| Difference | 32.5a | 33a | 33.35a | 33.0 | 8 | 21.25 | 5.355 | 11.5 | ||||||||
| Property verification (% correct) | ||||||||||||||||
| Living | 53.5 | 74 | 67 | 64.8 | 70 | 84 | 69 | 74.3 | ||||||||
| Non-living | 74.5 | 92.5 | 83.5 | 83.5 | 74.5 | 83.5 | 60 | 72.7 | ||||||||
| Difference | 21a | 18.5a | 16.5a | 18.7 | 4.5 | −0.5 | −9 | −1.7 | ||||||||
| Category fluency (number correct) | ||||||||||||||||
| Living | 6 | 10 | NA | 8.0 | 17 | 17 | 3 | 12.3 | ||||||||
| Non-living | 13 | 17 | NA | 15.0 | 10 | 14 | 2 | 8.7 | ||||||||
| Difference | 7 | 7 | NA | 7.0 | −7 | −3 | −1 | −3.7 | ||||||||
Difference score (living minus non-living) is significant at P < 0.01 (χ2 test). Accuracy scores are shown as percentage correct for ease of comparison of performance over tasks with different numbers of items. Analyses were carried out over raw scores.
Naming
All six patients were severely impaired in the naming test. As shown in Figure 4, there was no difference in their overall naming ability. However, the contrast between living and non-living domains revealed a clear difference between the two groups. All the HSE patients were significantly poorer at naming living things than non-living things [RC, χ2(2) = 7 , P < 0.01; WL, χ2(2) = 8.9, P < 0.01; JBR, χ2(2) = 7, P < 0.01], whereas there was no such domain difference for the SD patients (χ2 < 1 in all cases). Patients BS and JT did not complete the full naming test; however, the subsets of items over which their scores are computed contained equal numbers of living and non-living things, and were representative of the total set.
Property Verification
As for the naming test, there was a clear distinction in the pattern of responses for the HSE and SD patients. Overall accuracy was similar for the two groups, at ∼70% correct, but the effect of semantic domain was very different. All three HSE patients made significantly more errors in verifying the properties of living things than non-living things [RC, χ2(2) = 15.79, P < 0.001; JBR, χ2(2) = 17.49, P < 0.001; WL, χ2(2) = 9.8, P < 0.01], while there was no such domain effect for the SD patients (χ2 < 1 in all cases).
Category Fluency
Both the HSE patients who have done this test (RC and JBR) were able to generate many more exemplars for the non-living categories than for the living categories. The SD patients did not show this living-things disadvantage.
In summary, on all three tasks, HSE patients had significantly more difficulty with concepts in the living domain (including fruit and vegetables and animals) than the non-living domain (tools and vehicles), whereas the SD patients showed no evidence of a selective deficit for living things, in spite of an overall semantic impairment.
Patient Lesion Analysis
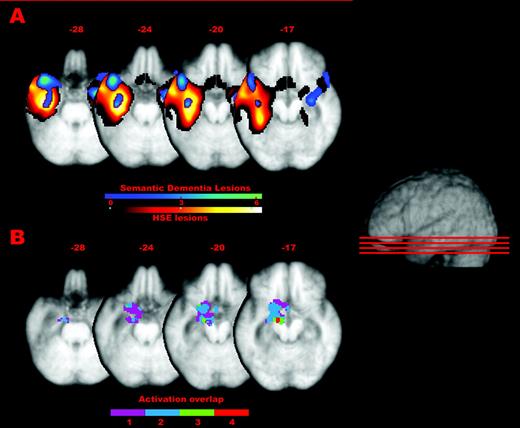
Figure 5A shows the common lesions for the HSE and SD groups superimposed on the mean patient T1 scan. These lesions were detected by comparing each patient to a control group and conducting conjunction analyses in the two groups. The collective damage for the SD patients centred on the left temporal pole and included the middle temporal gyrus and the anterior fusiform gyrus. The damage for the HSE group was larger than for the SD patients and extended from the anterior to the most posterior aspects of the temporal cortex. Anterior-laterally it included the inferior, middle and superior temporal gyri, and anterior-medially it extended into the left parahippocampal areas including the fusiform, entorhinal, dorsal entorhinal and perirhinal cortices. Additionally, there was greater ventricular enlargement in the left hemisphere in the HSE patients than in the SD patients. The anterior-medial areas damaged in the HSE patients are the ones we found to be more activated for basic- than domain-level naming in our earlier study (Tyler et al., 2004a) and to be especially important for the naming of living things at the basic level in the current fMRI study. Figure 5B shows an overlap of the activations for the living minus non-living categories (animals versus vehicles, animals versus tools, fruit and vegetables versus vehicles, and fruit and vegetables versus tools) in the basic-level naming task, superimposed on the mean patient brain at the same slices as shown in Figure 5A, allowing a comparison between the extent of the lesions in the two groups and the peak activations in the fMRI study.
(A) Common lesions for the HSE and SD groups superimposed the mean patient T1 scan. These lesions were detected by comparing each patient to a control group and conducting conjunction analyses in the two groups (conjunction T scores are shown in the colour bars). (B) Overlap of the activations for the living minus non-living categories (animals versus vehicles, animals versus tools, fruit and vegetables versus vehicles, and fruit and vegetables versus tools) in the basic-level naming task, superimposed on the mean patient brain at the same slices as shown in (A). The colour bar in (B) indicates the degree of overlap between the four different sets of activations (1 minimum to 4 maximum overlap).
As can also be seen from Figure 5, there is also some right temporal damage in the semantic dementia group. This is attributable to one patient, BS. Although his damage extends into the right anteromedial temporal regions, he does not show any consistent difference across the semantic tasks when compared with the other two SD patients for whom there is no obvious right anteromedial damage. Together with the fact that the critical category-specific activations for basic level naming in the fMRI study were found in the left temporal cortices only, this suggests that the fine-grained differentiation and integration of features in object processing is confined to left perirhinal regions and does not involve homologous right hemisphere areas.
General Discussion
The results of the fMRI study and the relationship between the behaviour and lesion sites for the patients provide converging evidence that the left anteromedial temporal cortex plays a crucial role in the differentiation of objects. The current results are also consistent with the prediction that items in different domains typically place varying processing demands on these differentiation processes, due to differences in their conceptual structure. Specifically, members of living-things categories tend to be more similar to each other, with a greater proportion of highly correlated shared properties and relatively few distinctive properties, while non-living objects have a higher proportion of distinctive to shared information and so are more easily distinguished from each other. There are other potential differences between living and living things that could lead to differences in overall activation. For example, we may have richer semantic representations overall for living things, leading to greater activation, or living things may be more attention-demanding than non-living things. However, most such accounts would predict increased activation for living things in both domain- and basic-level naming tasks, rather than the observed interaction between naming level and domain. Moreover, the similar reaction times for naming living and non-living things at the basic level that we found in behavioural pre-test confirms that the pictures and names were well matched on the variables that could affect object recognition and naming response difficulty in other ways, such as visual complexity of pictures, name frequency and so on. This suggests that the increased left anteromedial temporal activation for naming living things at the basic level was indeed due to greater integration and differentiation demands. Interestingly, this was not reflected in longer reaction times for naming living things in the behavioural task, at least for healthy young adult subjects. However, we would predict that with greater pressure to respond quickly/and or degraded pictorial stimuli, that such a reaction time disadvantage would emerge for living things (cf. Randall et al., 2004).
Our results show not only that left anteromedial temporal cortex is activated to a greater extent when healthy individuals named living things than non-living things, but also that damage to these regions produces selective deficits for living things. The contrast between the HSE and SD patients enables us to be more confident in attributing the selective problem for living things to anteromedial damage, rather than other more lateral temporal regions, which were lesioned in both groups. Although the three semantic dementia patients were impaired on semantic tasks, they did not show markedly disproportionate problems for living relative to non-living things.
Damage to the neural infrastructure for integration and differentiation processes within left anteromedial temporal cortex provides a plausible account of category-specific deficits for living things, within the broader framework of a hierarchical object processing system. It is also consistent with detailed patterns of impaired and preserved function for patients showing such a deficit; they are typically able to identify living things at the category and domain level and to correctly verify semantic features that are shared across the category, but they fail on within-category differentiation tasks, such as basic-level naming, word–picture matching and verifying distinctive properties (Moss et al., 1998; Tyler and Moss, 2001; Moss et al., 2002). The contrast between impaired specific within-category discrimination and intact domain-level knowledge in both the patient data and the different patterns of activations for basic- and domain-level naming we have observed in the neuro-imaging studies suggest that anteromedial temporal cortices are process driven rather than stimulus specific. A process-driven view of medial temporal lobe function is also consistent with evidence from the memory literature that anteromedial temporal structures are involved either in the time-limited consolidation of newly acquired memory representations prior to their storage in more lateral temporal regions (e.g. Alvarez and Squire, 1994) or else act as indexers or pointers to information held elsewhere (e.g. Nadel and Moscovitch, 1997). With respect to object processing, our data demonstrate that structurally similar categories of animals and fruits do not invariably recruit this region: for tasks that do not require within-category discrimination, there are no differences in anteromedial activation between living and non-living things, and no category-specific deficits in patients with anteromedial temporal damage.
Although in this experiment we have focused on visual object recognition, we do not mean to suggest that the role of the anteromedial temporal cortex is limited to the visual modality. As well as being the endpoint of the visual object processing stream from simple to complex conjunctions of visual features, a key claim of the hierarchical object processing account (in both the non-human primate work and in the Simmons and Barsalou model of the human system) is that polymodal neurons in perirhinal cortex integrate information across a range of sensory modalities. Indeed, in the human system this region may also allow the emergence of non-sensory, fact-like knowledge and associations among objects (Murray and Richmond, 2001; Simmons and Barsalou, 2003). Therefore, we can view the anteromedial cortex as being critical to semantic processing and not just visual object recognition, as it supports the combination of many types of features into meaningful multimodal conceptual representations. The flipside of this point is that discrimination of visual forms will not necessarily engage the perirhinal cortex if they do not require integration with non-visual features or if they can be resolved on the basis of simple visual features alone (cf. Murray and Richmond, 2001).
This proposed role of anteromedial temporal cortex in category-specific semantic deficits builds on our earlier research, in which we have investigated the implications of damage to conceptual representations within a unitary semantic system. As summarized above, our Conceptual Structure Account highlights the systematic differences in conceptual structure between concepts in the living and non-living domains, in terms of distinctiveness, intercorrelation and overall number of features. We claim that the distinctive features of living things are particularly vulnerable to damage because they are weakly intercorrelated with other features. In contrast, the distinctive properties of non-living artifacts tend to be more robust to damage because they are often supported by mutual intercorrelation. Loss of distinctive information for living things leads to difficulty in distinguishing between them — hence, a category-specific deficit for living things. A potential limitation of the Conceptual Structure Account, however, is that these representational differences between living and non-living things may not be powerful enough to be the sole basis of the very severe category-specific deficits seen in HSE patients — such as the three described in the current paper. Recently, several studies have questioned whether differences in patterns of intercorrelation of features are as consistent or as marked as would seem necessary to account for category-specific deficits (Garrard et al., 2001, McRae and Cree, 2002). Moreover, if conceptual representations are at least partially captured in networks of neurons within the inferior temporal lobes, then the Conceptual Structure Account predicts that all patients with inferior temporal lesions should show category-specific deficits for living things. The fact that patients with SD clearly have inferior temporal atrophy, yet do not typically have marked selective impairments for living things, as we have shown in the current study (see also Lambon Ralph et al., 2003), seems to undermine the Conceptual Structure Account. However, a detailed analysis of SD profiles reveals a mild but quite consistent tendency for living things to be somewhat more impaired than non-living things, especially in tasks where distinctive, visual features are critical, although such differences may be small in individual patients (H.E. Moss and L. K. Tyler, in preparation). Nevertheless, in general SD patients do not typically show the clear-cut deficits for living things that are characteristic of HSE. We suggest that this difference in the magnitude of the domain effect may be explained by the presence or absence of extensive damage to the anteromedial regions, centring on perirhinal and entorhinal cortices.
Our proposal is that the conceptual structure of living things concepts has two consequences. First, as we have claimed on the Conceptual Structure Account, there will tend to be a greater loss of distinctive features when the semantic system is damaged. This will be sufficient to give rise to the pattern seen in semantic dementia, in which there is a general semantic impairment, with living things tending to be slightly more disadvantaged than non-living things. Second, living things place greater demands on the processes of integration of features and differentiation among objects, even in the intact brain. The hierarchical object-processing account (e.g. Murray and Bussey, 1999) claims that the critical neuroanatomical basis for feature integration and discrimination is the anteromedial temporal cortex. Hence, this area should be taxed relatively more in the identification of living than non-living things — a prediction that was supported in the fMRI study. When the anteromedial temporal region is damaged, such that differentiation processes are compromised, living things will be impaired disproportionately. When there are large lesions extending both to anteromedial and more lateral and posterior temporal sites, as in the HSE patients reported here, there will be a ‘double whammy’ effect, such that living things are hampered, both by a loss of distinctive information and by the differentiation deficit, giving rise to a severe impairment for living relative to non-living things. Consistent with the basic claims of our earlier studies, both these consequences flow directly from the structure of living-things concepts. Thus we have extended our original Conceptual Structure Account by incorporating claims about the neuroanatomical basis of feature integration and differentiation that arise from research into hierarchical object processing streams in non-human primates as well as humans.
An important caveat to this proposal is that it is a claim about neuroanatomical–functional associations, rather than about the defining characteristics of all patients in a specific clinical group. In general, patients with SD do not have the extensive anteromedial lesions that are typical of HSE. This is clearly shown in the lesion analysis of the three SD patients in our sample, for whom atrophy was confined to more lateral areas, at least in the left hemsiphere. In these cases, we expect any living things deficits to be mild. Strong correlations between SD severity and the degree of anterolateral atrophy have been observed (e.g. Simons et al., 1999; Mummery et al., 2000). A recent study by Galton et al. (2001) identified the left fusiform gyrus as the region most highly correlated with performance on visual and verbal semantic association tests, with additional more extensive left temporal areas, including temporal pole and inferior/middle temporal gyri, associated with object naming. However, the involvement of the perirhinal and entorhinal areas in SD remains unclear. In cases of SD where these regions are implicated (and specifically on the left) we would predict a greater category-specific effect for living things than for those patients in which no such damage appears to be present. Two SD patients with significant category-specific deficits for living things have been reported in the literature, of whom one had medial temporal involvement, consistent with this prediction (Barbarotto et al., 1995) and one did not (Lambon Ralph et al., 2003). The latter patient had bilateral temporal atrophy but did not have more medial damage than the five other SD patients with whom he was compared. However, in order to address this issue fully, it will be necessary to carry out detailed lesion–behaviour analyses over groups of SD patients, to assess the correlation between the disadvantage for living things across a range of tasks with the degree of atrophy in left anteromedial versus more lateral temporal regions.
In summary, we propose that damage to the processes of integration of features and differentiation among objects supported by anteromedial temporal cortex provides a plausible account of the marked category-specific deficits for living things typically associated with HSE. This processing deficit may have its effects over and above any loss of weakly correlated distinctive properties from the conceptual representations of living things. By itself, the correlational structure of living things concepts may give rise to weaker asymmetries between living and non-living things, such as those seen in the context of SD, in which the anteromedial temporal regions are spared, or at least less extensively damaged.
We thank Ciro Morgese for his help with the imaging study, and the radiographers at the WBIC for their assistance. This work was supported by an MRC [UK] programme grant to L.K.T. J.M.R. was supported by a research fellowship from Peterhouse, Cambridge.
References
Alvarez P, Squire LR (
Ashburner J, Friston KJ (
Bailey DL, Jones T, Friston KF, Colebatch JG, Frackowiak RSJ (
Barbarotto R, Capitani E, Spinnler H, Trivelli C (
Bartha L, Brenneis C, Schocke M, Trinka E, Köylü B, Trieb T, Kremser C, Jaschke W, Bauer G, Poewe W, Benke T (
Bunn EM, Tyler LK, Moss HE (
Bussey TJ, Saksida LM, Murray EA (
Caramazza A, Shelton JR (
Caramazza A, Mahon BR (
Damasio AR (
De Renzi E, Lucchelli F (
Desimone R, Ungerleider LG (
Devlin J, Gonnerman L, Andersen E, Seidenberg M (
Dixon M, Bub DN, Arguin M (
Folstein M, Folstein S, McHugh PR (
Forster KI, Forster JC (
Friston KJ, Holmes AP, Worsley KJ, Poline J-B, Frith CD, Frackowiak RSJ (
Gainotti G (
Gainotti G, Silveri MC, Daniele A, Giustolisi L (
Galton C, Patterson K, Graham K, Lambon Ralph MA, Williams G, Antoun N, Sahakian BJ, Hodges JR (
Gaffan D, Heywood C (
Garrard P, Lambon Ralph MA, Hodges JR, Patterson K (
Gitelman DR, Ashburner J, Friston J, Tyler LK, Price CJ (
Greer MJ, van Casteren M, McLellan SA, Moss HE, Rodd J, Rogers TT, Tyler LK (
Hodges JR, Patterson K, Oxbury S, Funnell E (
Humphreys GW, Riddoch MJ, Quinlan P (
Humphreys GW, Riddoch JM (
Jackson O, Schacter DL (
James M, Plant GT, Warrington EK (
Jefferies E, Patterson K, Jones RW, Bateman D, Lambon Ralph MA (
Keil F (
Laiacona M, Capitani E, Barbarott R (
Lamberts K, Shapiro L (
Lambon Ralph MA, Patterson K, Garrard P, Hodges JR (
Laws KR, Gale TM, Frank R, Davey N (
Lipschutz D, Friston KJ, Ashburner J, Turner R, Price CJ (
Lloyd-Jones TJ, Humphreys GW (
McRae K, Cree GS (
McRae K, de Sa VR, Seidenberg MS (
Merchant H, Zainos A, Hernández A, Salinas E, Romo R (
Moss HE, Tyler LK, Durrant-Peatfield M, Bunn EM (
Moss HE, Tyler LK, Devlin J (
Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RSJ, Hodges JR (
Murray EA, Bussey TJ (
Murray EA, Richmond BJ (
Nadel L, Moscovitch M (
Pihlajamäki M, Tanila H, Hänninen T, Könönen M, Mikkonen M, Jalkanen V, Partanen K, Aronen H, Soininen H (
Randall B, Moss HE, Rodd J, Greer M, Tyler LK (
Raven J, Raven JC, Court JH (
Rogers TT, Lambon Ralph MA, Garrard P, Bozeat S, McClelland JL, Hodges JR, Patterson K (
Rosch E, Mervis CB, Gray WD, Johnson DM, Boyes-Braem P (
Sartori G, Job R (
Silveri MC, Gainotti G (
Simmons K, Barsalou LW (
Simons JS, Graham KS, Hodges JR (
Snowden JS, Goulding PJ, Neary D (
Stamatakis EA, Tyler LK (
Talairach J, Tournoux P (
Tranel D, Logan CG, Frank RJ, Damasio AR (
Tyler LK, Moss HE (
Tyler LK, Moss HE, Durrant-Peatfield M, Levy JP (
Tyler LK, de Mornay-Davies P, Anokhina R, Longworth C, Randall B, Marslen-Wilson WD (
Tyler LK, Stamatakis EA, Acres K, Bright P, Abdallah S, Rodd JM, Moss HE (
Tyler LK, Stamatakis EA, Jones RW, Bright P, Acres K (
Warrington EK, McCarthy RA (
Author notes
1Department of Experimental Psychology, University of Cambridge, Cambridge, UK and 2Wolfson Brain Imaging Unit, University of Cambridge, Cambridge, UK