-
PDF
- Split View
-
Views
-
Cite
Cite
Rebeca Perez, Lars Wörmer, Peter Sass, Iris Maldener, A highly asynchronous developmental program triggered during germination of dormant akinetes of filamentous diazotrophic cyanobacteria, FEMS Microbiology Ecology, Volume 94, Issue 1, January 2018, fix131, https://doi.org/10.1093/femsec/fix131
Close - Share Icon Share
Abstract
Germination of akinetes of filamentous heterocyst-forming cyanobacteria of the order Nostocales is an essential process that ensures survival and recolonization after long periods of unfavorable conditions, as desiccation, cold and low light. We studied the morphological, physiological and metabolic changes that occur during germination of akinetes in two model species of cell differentiation, Anabaena variabilis ATCC 29413 and Nostoc punctiforme ATCC 29133, which live in different habitats. We characterized the akinete envelopes and showed their similarity to envelopes of N2-fixing heterocysts. Akinete germination started inside the envelopes and was dependent on light intensity but independent of nitrogen supply. During the germination of A. variabilis akinetes, cell division and heterocyst differentiation were highly accelerated. The energy for cell division was initially supplied by respiration of glycogen and subsequently by photosynthesis. By contrast, during germination of N. punctiforme akinetes, cell division and heterocyst differentiation were slow. During the initial 15–20 h, N. punctiforme akinetes increased in volume and some burst. Only then did intact akinetes start to divide and fully germinate, possibly fueled by nutrients released from dead akinetes. The different strategies used by these different cyanobacteria allow successful germination of dormant cells and recolonization under favorable conditions.
INTRODUCTION
Filamentous cyanobacteria of the order Nostocales and Stigonematales are found in diverse environments, such as freshwater and terrestrial biotopes. Their persistence and success in nature are attributed to their ability to form nitrogen-fixing heterocysts and resting cells called akinetes, which allow survival over long periods under unfavorable conditions. In freshwater environments worldwide, some species of Nostocales form toxic blooms, which cause environmental problems. Akinete formation equips these organisms to compete with other planktonic strains through resistance towards harsh conditions, such as drought, low nutrition and low temperatures. Only heterocyst-forming filamentous cyanobacteria form akinetes (Adams and Duggan 1999; Kaplan-Levy et al.2010; Maldener, Summers and Sukenik 2014).
Akinete differentiation has been studied in detail in two model organisms: the freshwater species Anabaena variabilis ATCC 29413 and the terrestrial species Nostoc punctiforme ATCC 29133. We have previously shown that the signals triggering akinete formation are species dependent, and that akinete formation is efficiently triggered by low light in A. variabilis and by phosphate starvation in N. punctiforme (Perez et al.2016). During akinete differentiation, the cells transiently accumulate storage compounds, namely glycogen, cyanophycin, a nitrogen storage molecule and lipid droplets. During akinete development, metabolic activities are drastically reduced to final low levels in mature akinetes. The morphology of mature akinetes considerably differs from that of vegetative cells in that the cellular volume is higher and the envelope is thicker. The envelope structure of heterocysts and akinetes of A. variabilis and N. punctiforme is partially similar, but the chemical composition of the different layers has not yet been completely resolved. The composition of the external akinete polysaccharide layer differs between A. variabilis and N. punctiforme but is structurally similar to the heterocysts of A. variabilis and Anabaena cylindrica, respectively (Cardemil and Wolk 1981). Similarly, the akinete envelope of Cyanospira rippkae contains the same glycolipids as the heterocyst envelope (Soriente et al.1993).
In the natural environment, akinetes germinate in response to various ambient stimuli, such as temperature, light and nutrient availability. For example, germination is dependent on light intensity and does not occur in the dark or in the presence of the photosynthesis inhibitor 3-(3,4-dichlorophenyl)-1,1-dimethylurea (Yamamoto 1976; Braune 1979; Fay 1988). In nature, sediment mixing and resuspension trigger germination of akinetes resting at the bottom of lakes. The conditions that trigger akinete germination in different species probably correspond to the conditions that support growth of vegetative cultures (Kaplan-Levy et al.2010).
Several studies of the germination of akinetes of Nostocales have been carried out over the last decades (Braune and Doehler 1996; Baker and Bellifemine 2000; Hori et al.2003; Moore, McGregor and Shaw 2004). In some cyanobacterial species, the germination of akinetes is an asynchronous process (Braune 1980; Miller and Lang 1968; Wildman, Loescher and Winger 1975), whereas in Nostoc sp. PCC 7524, germination of akinetes is synchronous (Sutherland et al.1985; Skill and Smith 1987). Germination begins with a reorganization of the cellular material, followed by elongation and cell division, which occur inside the akinete envelope. An increase in turgor pressure resulting from successive cell division events then leads to rupture of the envelope and emergence of the young filament. In N. punctiforme, the akinete envelope degrades during germination (Skill and Smith 1987; Sili et al.1994; Adams and Duggan 1999; Meeks and Elhai 2002; Moore, McGregor and Shaw 2004).
Despite these reports, the fine-tuning of morphological, physiological and molecular mechanisms involved in the germination of akinetes is still unclear. We aimed at elucidating the distinct changes that take place during the crucial process of germination in A. variabilis ATCC 29413 and N. punctiforme ATCC 29133, two model species for bacterial adaptation through cell differentiation.
MATERIALS AND METHODS
Cyanobacteria and growth conditions
Anabaena variabilis ATCC 29413 strain FD and Nostoc punctiforme ATCC 29133 were grown under continuous illumination (17–22 μmol photons m−2 s−1) at 28°C with shaking at 120 r.p.m. in the liquid medium of Allen and Arnon (1955), diluted 4-fold with water (AA/4) and supplemented with 5 mM MOPS (pH 7.5), 2.5 mM NH4Cl, 2.5 mM NaNO3 and 2.5 mM KNO3. Akinete formation was induced by transferring late-exponential cultures of A. variabilis and N. punctiforme to low-light conditions or to fresh medium devoid of phosphate (Perez et al.2016). For determination of lipids, cultures were cultivated in BG11 and BG110 media (Rippka et al.1979), because induction of heterocyst differentiation worked better in these media compared to AA/4 media.
Induction of germination
Germination of akinetes in cultures exposed to low light or starved for phosphate for 2–16 months (see Perez et al.2016) was induced by transferring akinetes to normal light conditions or by washing and transferring them to fresh medium. Germinating cells were observed with a Leica DM 2500 light microscope with an X 100/1.3 oil objective and connected to a Leica DFC420C camera (Leica Microsystems GmbH, Wetzlar, Germany).
Staining procedures for microscopy
The polysaccharide layer of heterocysts and akinete envelopes was stained with Alcian Blue. For this, 2–10 μl of Alcian blue solution [1.5% in H2O (w/v)] was added to 1 ml of a culture containing heterocysts or akinetes and incubated for 10–15 min. Polyphosphate (Ppi) bodies were stained with Neisser stain as follows. Cultures of each strain starved for phosphate for 16 months were induced to germinate by transferring to fresh phosphate-containing medium. Aliquots at different time points of germination were Neisser stained and examined under the microscope. Ppi bodies stained dark purple-black (Sukenik et al.2012). To follow the fate of the lipid envelope and cellular lipid droplets in akinetes of both strains, samples of akinete cultures at different time points of germination were fixed, stained with borondipyrromethene difluoride (BODIPY) 493/503 (Molecular Probes, ThermoFisher Scientific, Waltham, MA, USA) and examined as described in Perez et al. (2016).
After all staining procedures, cells were placed on slides covered with 1% agarose and observed by light microscopy with a Leica DM 2500 microscope connected to a Leica DFC420C camera or with a Leica DM 5500B fluorescence microscope connected to a Leica DFC420C camera, as described in Perez et al. (2016).
Time-lapse microscopy
Germination of akinetes was followed by time-lapse fluorescence microscopy under constant, high light intensities (150 μmol photons m−2 s−1). For this, akinete cultures exposed to low light for 2 months were washed four times with medium and suspended in fresh medium without a nitrogen source (see Supporting Information, Methods). Then a 10-μl aliquot of each strain was spotted on agar (1%) dissolved in medium without a nitrogen source. The agar with the culture was placed inside a small petri dish with a cover slide at the bottom. Time-lapse fluorescence micrographs were recorded using a Nikon Eclipse Ti automated microscope equipped with a Perfect Focus system (Nikon Instruments Europe BV, Amsterdam, Netherlands), an Orca Flash 4.0 camera (Hamamatsu, Photonics, Hamamatsu City, Japan) and CFI Plan-Apo DM × 100/1.45 Oil Ph3 objective (Nikon). Images were processed using the NIS elements AR software package (Nikon, Nikon Instruments Europe BV, Amsterdam, Netherlands). Images were taken at 30 min intervals for 62–89 h.
Transmission electron microscopy
For electron microscopy, mature akinetes of A. variabilis or N. punctiforme in culture were induced to germinate, and samples at different time points were fixed with glutaraldehyde and post-fixed with potassium permanganate, as described in Fiedler et al. (1998). Ultrathin sections were stained with uranyl acetate and lead citrate and examined with a Philips Tecnai10 electron microscope at 80 kV.
Analysis of the envelope glycolipids
To determine the lipid composition of heterocysts and akinetes, 50 ml aliquots of cultures grown with (BG11) and without a nitrogen source (BG110), and of cultures induced to form akinetes by low light (A. variabilis) or phosphate starvation (N. punctiforme) for 2 months were used. Lipids were extracted following the method of Winkenbach, Wolk and Jost (1972), concentrated by evaporation, dissolved in chloroform and separated by thin layer chromatography (TLC) on a thin-layer silica plate (Merck, Darmstadt, Germany). The lipids were visualized by spraying the plates with 25% H2SO4 and heating to 220°C for 1–2 min.
The glycolipids from two selected akinete TLC bands (red and blue arrows in Fig. 3c) were extracted from the scraped off silica by successive washing steps in methanol and methanol:dichloromethane (1:5, vol/vol). The obtained extract was dried under a gentle N2 stream and resuspended in 100 μl methanol:dichloromethane (9:1, vol/vol). After addition of an internal standard (C46-GTGT, glycerol tri-alkyl glycerol tetra-ether), 10% of the extract was injected onto a Waters Acquity BEHC18 column in a Dionex Ultimate 3000RS UHPLC system. The chromatographic gradient of Wörmer et al. (2013) was slightly modified to improve detection of cyanobacterial HGs. After separation, analytes were ionized by electrospray ionization and analyzed by mass spectrometry (MS) on a maXis quadrupole time-of-flight mass spectrometer (Q ToF-MS, Bruker Daltonics, Bremen, Germany). Lipids were detected in the positive ionization mode while scanning a mass-to-charge (m/z) range from 150 to 2000. MS2 scans were obtained in the data-dependent mode: for each MS full scan up to three MS2 experiments, targeting the most abundant ions, were performed. Active exclusion limits the times a given ion is selected for fragmentation (3 times every 0.5 min) and thus allowed us to also obtain MS2 data of less abundant ions. Lipids were identified by monitoring exact masses of possible parent ions (present as either H+, NH4+ or Na+ adducts) in combination with characteristic fragmentation patterns, as outlined by Sturt et al. (2004), Bauersachs et al. (2009) and Wörmer et al. (2012).
Measurement of photosynthesis and respiration
Photosynthetic oxygen evolution of germinating akinetes was measured in vivo using a Clark-type oxygen electrode (Hansatech DW1, King's Lynn, Norfolk, UK). Light (55 μmol photons m−2 s−1) was provided by a high-intensity white light source (Hansatech L2). Oxygen evolution was measured at constant and controlled room temperature (∼20°C). Akinetes in cultures starved of phosphate for 2 months were induced to germinate by washing and transferring to fresh medium containing phosphate; 2-ml aliquots were taken after 0, 6, 12, 24, 30, 48 and 72 h of germination and measured. Respiratory activity was determined by measuring O2 consumption in the dark. Chlorophyll a in cultures was determined by the method of Mackinney (1941).
RESULTS
The akinete envelopes of Anabaena variabilis and Nostoc punctiforme
The akinete envelope of both Anabaena variabilis and Nostoc punctiforme is thick and multi-layered (Perez et al.2016). During germination, this rigid envelope must either open or disintegrate to release the newly formed small filament. To better understand this process, we first characterized the akinete envelope of A. variabilis and N. punctiforme in more detail using various microscopy and chemical approaches.
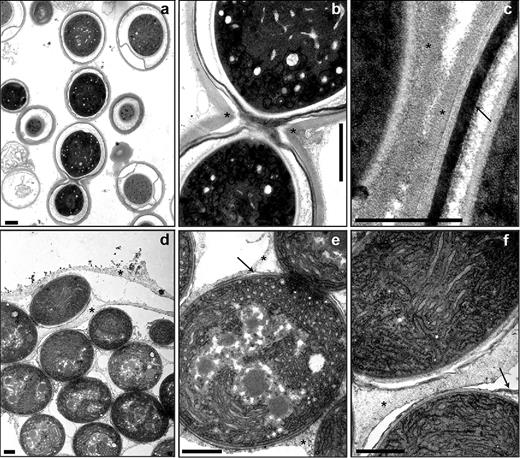
In electron micrographs of chemically fixed akinetes, most of the mature akinetes of A. variabilis were completely detached from the neighboring cells of the filament, but in a few cases, two or more mature akinetes with a fully developed envelope were still connected (Fig. 1a and b). At higher magnification (Fig. 1c), several layers were resolved; the outermost layer looked very similar to the homogeneous polysaccharide layer of the heterocyst envelope (Wolk, Ernst and Elhai 1994). Underneath the outermost layer, one or two darkly staining layers were present that appeared similar to the laminated heterocyst glycolipid layer (Winkenbach, Wolk and Jost 1972; Fiedler et al.1998). More diffusely contrasted layers were deposited between the outer membrane of the cell wall and the innermost lipid layer, which has been described earlier as a mucilaginous layer (Braune 1980; Perez et al.2016). The chemical nature of this layer has not yet been determined.
Ultrastructure of akinetes and their envelopes. Akinetes of A. variabilis in cultures exposed to low light (a–c) and of N. punctiforme induced by phosphate starvation for 60 days (d–f). Transmission electron micrographs of akinetes (a and d), connected akinetes (b and e) and akinete envelopes (c–f). Asterisk, exopolysaccharide layer; arrow, glycolipid layer. Bars, 1 μm (a, b, d, e and f) and 500 nm (c).
Many mature akinetes of N. punctiforme appeared in filaments (Fig. 1d–f). However, envelope structures were already present inside the septa of adjacent akinetes. The akinetes usually remained embedded in a common extracellular matrix, presumably composed of exopolysaccharides. A thin laminated layer underneath this envelope was observed in several mature akinetes of N. punctiforme and is presumably composed of glycolipids (Fig. 1e and f and compare with Perez et al. (2016)).
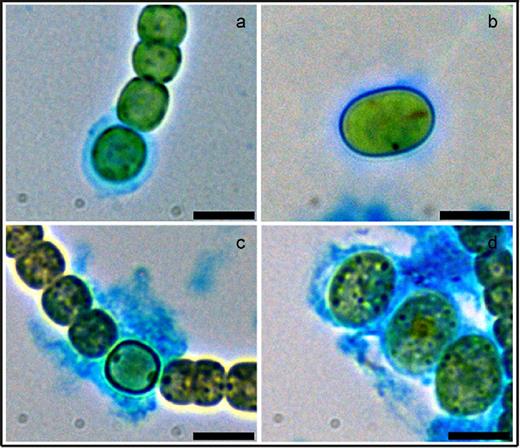
To confirm the presence of an exopolysaccharide layer, akinetes of both species were stained with Alcian blue. This dye is frequently used to detect the polysaccharide layer of heterocysts (e.g. Maldener, Hannus and Kammerer 2003). A layer around akinetes of each species stained positively (Fig. 2b and d), which indicated the presence of a layer composed of polysaccharides similar to those of heterocysts of the same species (Fig. 2a and c).
Visualization of the exopolysaccharide layer of heterocysts and akinetes. Bright-field images of Alcian-blue-stained heterocysts (a and c) and akinetes (b and d) of A. variabilis (a and b) and N. punctiforme (c and d). Bars, 3 μm.
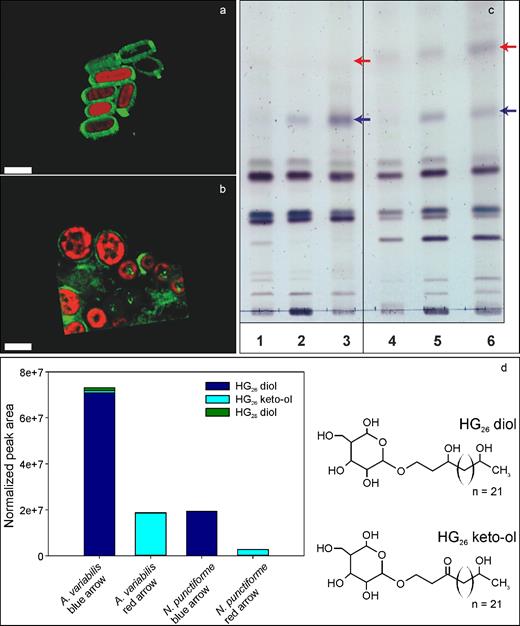
We further investigated the lipids of the akinete envelopes by staining with the fluorescent dye BODIPY, which can be used for staining neutral lipids in vivo (Peramuna and Summers 2014; Perez et al.2016). In micrographs of akinetes of A. variabilis, a defined envelope around the akinetes was strongly stained, with a higher intensity at the poles of the oval-shaped akinetes (Fig. 3a). By contrast, N. punctiforme akinetes were only weakly stained (Fig. 3b), which indicated a thin envelope layer, in line with the electron micrographs (Fig. 1e and f).
The glycolipid layer of akinete envelopes. Visualization of the lipid layer of akinetes of (a) A. variabilis and (b) N. punctiforme stained in vivo with the green fluorescent dye BODIPY in cultures starved of phosphate for 2 months. Shown are overlaid BODIPY fluorescence and autofluorescence 3-D images. Bars, 5 μm. (c) Thin-layer chromatogram of lipids from different cell types of A. variabilis (lanes 1–3) and N. punctiforme (lanes 4–6); vegetative filaments grown with NO3− (lanes 1 and 4); filaments containing heterocysts grown without NO3− (lanes 2 and 5); A. variabilis akinetes induced by low light (lane 3) and N. punctiforme induced by phosphate starvation (lane 6) for 2 months. Arrows, expected occurrence of heterocyst glycolipids. (d) Peak areas of the different HGs detected and normalized to the internal standard added, and molecular structure of the two major HGs: HG26 diol and HG26 keto-ol.
We then analyzed the chemical composition of the akinete lipid layer of both species. Lipid extracts of cultures containing only vegetative cells (grown with NO3−), filaments containing heterocysts (starved of NO3− for 48 h) and mature akinetes were separated by thin-layer chromatography. Compared to the lipid pattern of vegetative cells, two additional lipids were detected in the heterocyst and akinete samples of both species, which migrated identically (Fig. 3c, blue and red arrows). The chemical composition of the separated akinete lipids was analyzed by HPLC-MS. Several glycolipids known from heterocysts (HG), namely HG26-diol, HG26-keto-ol, and the minor component HG28-diol, were identified in the akinete lipids of A. variabilis (Fig. 3d). In akinete lipids of N. punctiforme, HG26-diol and a minor amount of HG26-keto-ol were present (Fig. 3d). In addition, diglycosyl diacylglycerol (2G-DAG), a lipid widely distributed in all photosynthetic organisms (Murata and Siegenthaler 1998), and two unidentified lipid classes were detected in extracts of the TLC bands of A. variabilis and N. punctiforme (not shown).
Induction and kinetics of akinete germination and heterocyst formation
Germination of akinetes was induced by shifting mature akinete cultures back to standard growth conditions. Three to six hours after shifting an akinete culture of A. variabilis to standard light conditions, cells began to divide inside the envelope of many akinetes. Cells in akinetes of N. punctiforme also began to divide 3–9 h after transferring to fresh standard medium. However, akinete germination of both organisms was asynchronous; at much later time points, akinetes were still present and germination was still observed for up to 2–3 days.
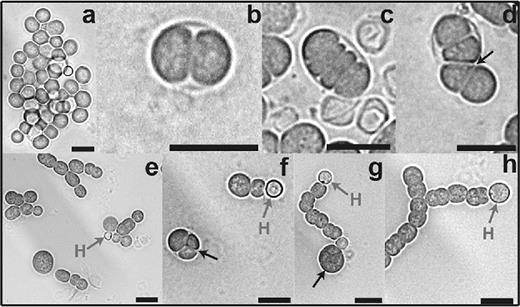
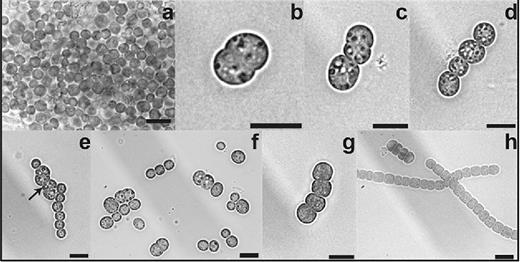
We followed the germination kinetics of single akinetes over 72 h (A. variabilis) or 89 h (N. punctiforme) using time-lapse microscopy (Supporting information: A. variabilis videos 1 and 2; N. punctiforme videos 3–6). In A. variabilis, successive rapid cell division events resulted in formation of a short filament, which penetrated the akinete envelope at one pole. In many cases, one end of the short filaments remained attached to the ruptured envelope (Fig. 4d; videos 1 and 2). Irregular filaments were observed after 24 h, which resulted from the aberrantly placed early cell division planes (Fig. 4d, f and g; video 1).
Progress of akinete germination in A. variabilis. Germination of mature akinetes was induced by returning cultures to normal light conditions and observed after 0 (a), 3 (b) 6 (c and d), 9 (e), 24 (f) and 48 h (g and h): mature akinetes at 0 h (a); cell division inside the akinete envelope between 3 and 24 h (b–f), small filament emerging at 6 h (d) and short filament with terminal heterocyst between 9 and 48 h (e–h). H, heterocyst. Black arrow, irregular plane of division. Bars, 10 μm.
Since media lacking a nitrogen source were used, we could also follow heterocyst formation during the germination process. In A. variabilis, short filaments with a terminal heterocyst were observed after 9 h (Fig. 4e). In each recovering filament of three germinating akinetes (video 2), heterocyst differentiated from single cells; differentiation started at about 24 h with a transient increase in autofluorescence, which was completely lost in the mature heterocysts. The red autofluorescence of cyanobacteria comes from the photosynthetic antenna pigments of photosystem II, the phycobiliproteins, which are degraded in mature heterocysts of A. variabilis and N. punctiforme to avoid photosynthetic oxygen formation. Interestingly, some akinetes showed an intense autofluorescent spot, which disappeared in the course of germination. At the end of the process, long filaments with typical vegetative autofluorescence were observed (videos 1 and 2).
The fate of N. punctiforme akinetes during germination (Fig. 5a–h) differed from that of A. variabilis akinetes. The akinetes typically appeared in filaments. After 12 h, some of them lysed quickly in less than 30 min; in the time-lapse videos, it looked as if the cells exploded. Most of the remaining akinetes changed their shape from round to oval before they constricted the cell division septum in the middle plane (Fig. 5c and f). Video 6 shows two akinetes; one burst after 12 h, and the other started to divide after 30 h and formed a new filament. Video 4 shows germination of several attached akinetes in a filament, with some cells bursting between 9 and 12 h. This bursting of single akinetes of N. punctiforme was regularly observed. As in A. variabilis, irregular filaments were observed, which resulted from the aberrantly placed early cell division planes. We could also follow heterocyst formation during the germination process since media lacking a nitrogen source were used (video 3). The first cell division events were visible between 15 and 17 h, and heterocyst development started after approximately 50 h. Interestingly, several granules transiently appeared during germination.
Progress of akinete germination in N. punctiforme. Germination of mature akinetes was induced by washing and transferring the akinete cultures to fresh medium with phosphate and observed after 0 (a), 3 (b and c), 6 (d), 9 (e), 24 (f) and 48 h (g and h): mature akinetes at 0 h (a) and 24 h (f), cell division of akinetes between 3 and 24 h (b–f) and long filaments at 48 h (h). Black arrow, irregular plane of division. Bars, 10 μm.
Morphological changes during germination
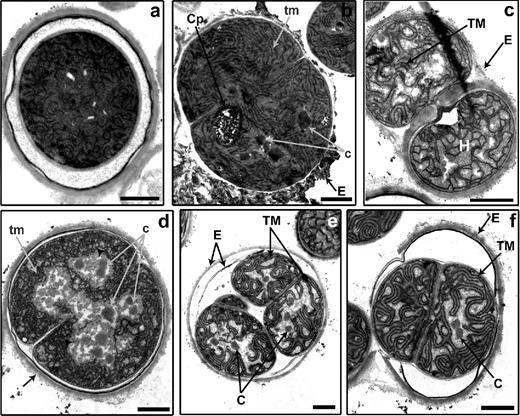
We investigated the ultrastructure of akinetes at different stages of germination using transmission electron microscopy (TEM). At the beginning of the process, dividing akinetes of A. variabilis were characterized by reconstitution of thylakoid membranes and carboxysomes, a polyhedral body containing the ribulose-1,5-bisphosphate carboxylase/oxygenase, and in some cases by cyanophycin granules (Fig. 6b–d). When germination was allowed under nitrogen-deplete conditions, heterocysts were observed at early time points (e.g. Fig. 6c at 6h). The exopolysaccharide layer of the akinete envelope was still present and enclosed a vegetative cell and a morphologically fully developed heterocyst. At the end of the germination process, the cells showed the typical vegetative cell ultrastructure, with fully developed thylakoid membranes and carboxysomes, and the absence of reserve granules (Fig. 6e and f). The original multi-layered akinete envelope was still present and covered the growing filament until the akinetes ruptured and the short filament emerged (Fig. 6f).
Morphological changes during akinete germination in A. variabilis. Transmission electron micrographs of akinetes at 3 (a), 6 (b and c) and 48 h (d–f) after germination, induced by light. c, initial carboxysomes; C, carboxysome; Cp, cyanophycin; E, akinete envelope; H, heterocyst; tm, undeveloped thylakoid membranes; TM, thylakoid membranes; arrowheads, glycogen. Bars, 1 μm.
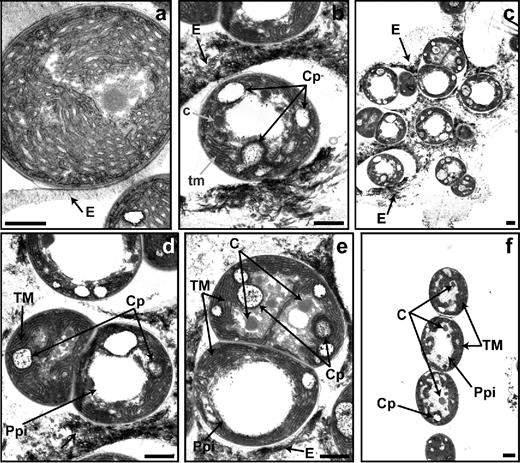
In a single sample of N. punctiforme that was prepared after 48 h of induction, several phases of akinete germination and fully developed short filaments of vegetative cells with the typical ultrastructure were observed by TEM (Fig. 7a–f). In A. variabilis, germination occurred inside a defined envelope, but in N. punctiforme, several germinating akinetes were embedded in an extracellular matrix that seemed to dissolve during the germination process. Occasionally, empty envelopes were also detected (Fig. 7c; Fig. S1, Supporting Information). Several cyanophycin granules transiently appeared during germination (compare Fig. 7c–e with Fig. 7f). Furthermore, large electron-translucent granules were observed in dividing cells as well as in some cells of the small filament; these granules might be Ppi bodies (Fig. 7d–f; see below). Inner membranes and carboxysomes regenerated during germination.
Morphological phases of akinete germination in N. punctiforme. Transmission electron micrographs of akinetes at 0 (a) and 48 h (b–f) of germination induced by transferring the akinetes to fresh media with phosphate. c, initial carboxysomes; C, carboxysome; Cp, cyanophycin; E, akinete envelope; tm, undeveloped thylakoid membranes; TM, thylakoid membranes; Ppi, polyphosphate bodies. Bars, 1 μm.
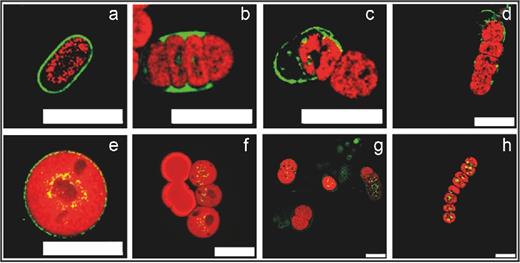
To elucidate the fate of the lipid layer, different stages of germinating akinetes were stained with BODIPY. In A. variabilis, the green fluorescent lipid layer remained attached to the newly formed short filament during the first stages of germination (Fig. 8a–d). In N. punctiforme akinetes, the faintly stained lipid layer disappeared during akinete germination (Fig. 8e and g). In fluorescent images of BODIPY-stained akinetes, intracellular lipid droplets were observed in the newly formed short filaments of N. punctiforme, but not in A. variabilis (Fig. 8).
Visualization of the akinete envelope during germination. The glycolipid layer of the akinete envelope of A. variabilis (a–d) and N. punctiforme (e–h) was stained in vivo with the green fluorescent dye BODIPY. Akinetes at 0 h (a and e) of germination and cell division during asynchronous germination (b–d and f–h). Shown are overlaid BODIPY fluorescence (green) and autofluorescence (red) images.
To determine whether the electron-translucent granules in N. punctiforme were Ppi bodies and whether A. variabilis also contained Ppi bodies during germination, we stained germinating akinetes with Neisser stain (Fig. 9). Mature akinetes of both N. punctiforme and A. variabilis do not contain Ppi bodies, as shown previously (Perez et al.2016). Dark, large spots corresponding to Ppi bodies were detected at 6 and 30 h of germination (Fig. 9b–c, f and g) and in almost all vegetative cells of the newly formed short filaments of both species (Fig. 9d and h).
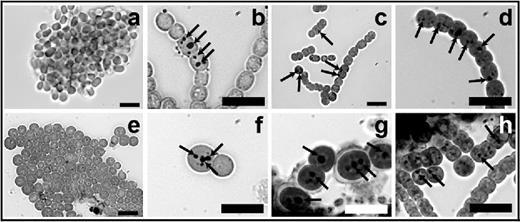
Occurrence of Ppi bodies during germination. Akinetes in A. variabilis (a–d) and N. punctiforme (e–h) cultures starved for phosphate for 16 months were washed and transferred to fresh media with phosphate. Samples were taken and stained with Neisser stain at 0 (a and e), 6 (b and f), 30 (c and g) and 72 h of germination (d and h). Ppi bodies (arrows) stained darkly. Bars, 10 μm.
Metabolic activities during germination
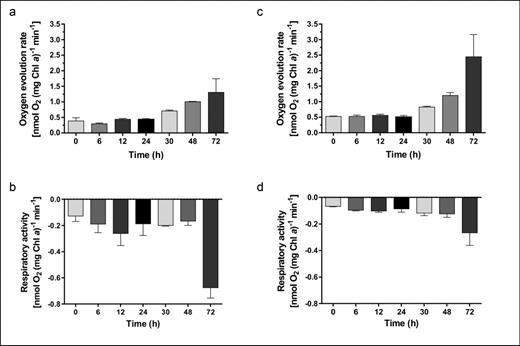
We measured the photosynthetic oxygen release and oxygen consumption in the dark (respiration) during germination of akinetes of both species. In contrast to the rapid recovery of autofluorescent pigments during germination observed by time-lapse microscopy, the photosynthetic activity in both species only started to increase after 24 h (Fig. 10a and c). Nevertheless, the O2-evolution rates fit with the reorganization of the thylakoid membranes observed in electron micrographs (Fig. 6e and f; Fig. 7e and f). In germinating cultures of A. variabilis, respiration slightly increased during the first 12 h; it then remained at the same or a slightly lower level until 48 h, and then increased threefold at 72 h, where it reached the O2-evolution rate of vegetative cells (Fig. 10b; compare Perez et al. (2016)). By contrast, in germinating cultures of N. punctiforme, the O2-evolution rate only slightly increased from 6 to 48 h, followed by a stronger increase at 72 h (Fig. 10d).
Metabolic activities during germination. Photosynthetic oxygen evolution (a and c) and respiration (b and d) of A. variabilis (a–b) and N. punctiforme (c–d) cultures with mature akinetes induced to germinate by washing and transferring to fresh medium were measured in vivo using a Clark-type oxygen electrode over 72 h. Respiratory activity was measured as oxygen consumption in the dark. Chl a, Chlorophyll a. The data are the means of at least three independent samples ±SD.
DISCUSSION
The success of cyanobacteria of the order Nostocales in nature is attributed to, among others, their ability to form dormant cells (akinetes) under unfavorable environmental conditions. These cells are able to germinate to produce a new filament of vegetative cells when conditions become favorable. The essential process of germination is poorly understood, and this study provides the first detailed characterization of akinete germination in Anabaena variabilis and Nostoc punctiforme at the levels of morphology and metabolism.
Survival of the dormant cell requires substantial changes in the envelope. In previous studies, we have shown that A. variabilis akinetes have a multi-layered envelope, while N. punctiforme akinetes have a bi-layered envelope (Perez et al.2016). Here, we analyzed the composition of the envelope in more detail using techniques used successfully for heterocysts and compared the structures of the two cell types. Our analysis clearly showed similarities between akinete envelopes and typical heterocyst exopolysaccharide and glycolipid layers (Fig. 1a–f). The positive Alcian blue staining (Fig. 2a–d) implied that the exopolysaccharides of both cell types are composed of similar sugars with acidic groups, which confirms the earlier studies of A. variabilis and A. cylindrica by Cardemil and Wolk (1981). Another similarity between the heterocysts and akinetes of A. variabilis is the putative polysaccharide exporter HepA, which is required for the normal deposition of these layers (Leganés, Fernández-Piñas and Wolk 1994).
We identified three glycolipids characteristic for heterocysts and that are ubiquitous in the heterocysts formed by many cyanobacteria (Gambacorta et al.1998; Wörmer et al.2012; Bauersachs et al.2014). These HGs have previous been reported in akinetes only in Cyanospira rippkae, in an akinete suspension obtained after centrifugation of an old culture (Soriente et al.1993). Our results provide strong evidence that HGs are not exclusive to heterocysts but are also present in akinetes. This observation supports the hypothesis that akinetes were evolutionary precursors of heterocysts (Wolk, Ernst and Elhai 1994).
During germination, cell division took place inside the akinete envelope. We questioned how the short filament is released from the envelope. In A. variabilis, the new filament emerged from the akinete envelope, which was laterally opened with the lipid layer still attached to the terminal cell, i.e. to the original ‘mother’ cell of the filament. This indicates that the akinete envelope is not degraded during filament release but is instead probably ruptured by high pressure due to successive cell division events, as previously suggested by Moore, McGregor and Shaw (2004) and Kaplan-Levy et al. (2010). In N. punctiforme, the envelope rapidly degraded and was not detected after germination (Figs 7f and 8f). However, the degradation of the akinete envelope of N. punctiforme depends on the stage of development, as indicated by defined envelopes observed during the germination of a 3-year-old akinete culture (Fig. S1, Supporting Information). Polar degradation of the akinete envelope and emergence of a young filament have often been observed, e.g. in Nostoc sp. PCC 6720, C. rippkae and Cylindrospermopsis raciborskii (Skill and Smith 1987; Sili et al.1994; Moore, McGregor and Shaw 2004).
Many environmental conditions, such as temperature, light and nutrient availability, trigger akinete germination (Maldener, Summers and Sukenik 2014). Like akinete induction from vegetative cell cultures (Perez et al.2016), germination of mature akinetes of A. variabilis and N. punctiforme is highly asynchronous (Fig. 4a–h and Fig. 5a–h). We observed that an increase in light intensity rapidly triggered germination of A. variabilis, similar to previous reports on germination of A. cylindrica and A. variabilis Kützing (Yamamoto 1976; Braune 1979). Transfer of mature akinetes to fresh medium containing phosphate induced germination of N. punctiforme. This was also the case in nitrogen-deficient medium, which showed that a nitrogen source was not needed for germination. In this case, the filaments formed heterocysts for N2 fixation. Phosphate and light but not nitrogen are also required for akinete germination of Anabaena circinalis and Nodularia spumigena (van Dok and Hart 1997; Huber 1985).
Single-akinete time-lapse microscopy of A. variabilis revealed unusually rapid cell division events and heterocyst differentiation (Figs 4e and 6c; videos 1 and 2). Heterocysts of Nostoc sp. PCC 7524, Nostoc sp. PCC 6720, Anabaena sp. PCC 7937 and Cyanospira capsulata also differentiate quickly in the absence of a nitrogen source during germination (Sutherland et al.1985; Skill and Smith 1987; Sili et al.1994). The rapid start of cell division and heterocyst development indicates that the required enzymes are already present or are synthesized quickly. This situation is similar to the resuscitation of chlorotic cells of the unicellular Synechocystis sp. PCC 6803, which immediately activates respiration to gain energy from glycogen catabolism and to reinstall the machinery for protein synthesis and nitrate assimilation (Klotz et al.2016). This concept is also supported by the high amount of glycogen and DNA in mature akinetes of A. variabilis, which could facilitate rapid cell division during germination (Perez et al.2016; Perez 2016). By contrast, rapidly germinating akinetes of N. punctiforme were rare (Fig. 5b–e; videos 3–6). Its cell cycle is slower than that of A. variabilis and could be due to the lack of the storage molecule glycogen as a source of energy during differentiation (Perez et al.2016; Fig. 7b). Our frequent observation of bursting single akinetes implies that the nutrients released from these dead akinetes promote germination of others. A weakened cell wall could explain why some akinetes were not able to withstand the increase in turgor during germination, which resulted in the lysis of these cells.
A. variabilis akinetes lacked cyanophycin granules, yet they were able to germinate. These observations are in line with an earlier assumption that cyanophycin is not a direct nitrogen source for protein synthesis and therefore is not essential for germination (Sutherland et al.1985). The granules transiently accumulated during the germination of N. punctiforme (videos 3–6) could correspond to lipid droplets, as shown by BODIPY staining, but it cannot be ruled out that they consist of polyhydroxybutyrate, which has previously been reported for this species (Ansari and Fatma 2016). Polyhydroxybutyrate accumulates in some unicellular cyanobacteria during nitrogen starvation, but its function under starvation has not yet been resolved (Hauf et al.2015; Damrow, Maldener and Zilliges 2016; Klotz et al.2016). The lipid droplets became larger towards the end of germination (Fig. 8e–h) implying that lipid droplets are not used as carbon or energy source during germination.
We detected Ppi granules towards the end of the germination process in the young filament in both species (Fig. 9d and h). Ppi formation is required for and coordinated with cell cycle exit in Pseudomonas aeruginosa (Racki et al.2017). Ppi could therefore have a mediator function between metabolic states and the cell cycle during germination of akinetes.
This study provides, to our knowledge, the first characterization of photosynthetic and respiratory activities during akinete germination of A. variabilis and N. punctiforme. The recovery of photosynthetic activity correlated well with the regeneration of thylakoid membranes (Fig. 10a and c; Fig. 6e and f; Fig. 7f). Occasionally, we observed an intense autofluorescent spot in akinetes of A. variabilis, which disappeared during germination (video 2). A high autofluorescence was also detected in germinating akinetes of N. punctiforme, which decreased towards the end of the process (videos 3–6). The intense autofluorescence could be from dissociated phycobiliproteins (the antennae of photosystem II), which reassemble during reactivation of photosynthesis (see also Reuter et al. (1994)). From the time course of recovery of respiration in A. variabilis, we suggest that stored glycogen is metabolized at the beginning of the process, to support cell division with energy. The role of glycogen as a substrate for respiration has been previously shown for Synechocystis sp. PCC 6803 (Gründel et al.2012). Similarly, at the beginning of the resuscitation program of this cyanobacterium, the chlorotic cells suppressed photosystem II activity, activated respiration of glycogen to gain energy and later re-activated the photosynthetic capacity (Klotz et al.2016). In contrast to A. variabilis, the respiration rate of germinating akinetes of N. punctiforme weakly increased during the first 48 h (Fig. 10d), which is in line with the lack of glycogen as a nutrient storage compound. An increase in respiration occurred only after photosynthesis had recovered and organic compounds were produced.
Altogether, this study provides the first detailed characterization of akinete germination in A. variabilis and N. punctiforme, and demonstrates that this developmental program is highly asynchronous. Germination was triggered by light and nutrient availability but did not require a nitrogen source, which reflects the diazotrophic lifestyle of these cyanobacteria. The accelerated cell division and heterocyst differentiation of A. variabilis during germination implies regulation at the level of cell-cycle control, which deserves further research. Together with our previous work on akinete formation in N. punctiforme and A. variabilis (Perez et al.2016), this study of germination clearly shows the differences between the two filamentous cyanobacterial species, which occupy different habitats in nature. Finally, the results provide a solid base for future investigations at molecular and regulatory levels.
SUPPLEMENTARY DATA
Supplementary data are available at FEMSEC online.
Acknowledgements
We thank Professor Karl Forchhammer for fruitful discussions, Claudia Menzel and Carolina Nishi for the technical assistance, Ritu Garg and Filipp Oesterhelt for assistance with the time-lapse experiment and Oliver Betz for TEM access. We are indebted to Karen Brune for critically reading the manuscript.
FUNDING
This work was supported by the RTG 1708 ‘Molecular principles of bacterial survival strategies’ and by the Collaborative Research Center (CRC) 766, both granted by the German Research Council (Deutsche Forschungsgemeinschaft, DFG).
Conflict of interest. None declared.