-
PDF
- Split View
-
Views
-
Cite
Cite
Anne Harduin-Lepers, Rosella Mollicone, Philippe Delannoy, Rafael Oriol, The animal sialyltransferases and sialyltransferase-related genes: a phylogenetic approach, Glycobiology, Volume 15, Issue 8, August 2005, Pages 805–817, https://doi.org/10.1093/glycob/cwi063
Close - Share Icon Share
Abstract
The animal sialyltransferases are Golgi type II transmembrane glycosyltransferases. Twenty distinct sialyltransferases have been identified in both human and murine genomes. These enzymes catalyze transfer of sialic acid from CMP-Neu5Ac to the glycan moiety of glycoconjugates. Despite low overall identities, they share four conserved peptide motifs [L (large), S (small), motif III, and motif VS (very small)] that are hallmarks for sialyltransferase identification. We have identified 155 new putative genes in 25 animal species, and we have exploited two lines of evidence: (1) sequence comparisons and (2) exon–intron organization of the genes. An ortholog to the ancestor present before the split of ST6Gal I and II subfamilies was detected in arthropods. An ortholog to the ancestor present before the split of ST6GalNAc III, IV, V, and VI subfamilies was detected in sea urchin. An ortholog to the ancestor present before the split of ST3Gal I and II subfamilies was detected in ciona, and an ortholog to the ancestor of all the ST8Sia was detected in amphioxus. Therefore, single examples of the four families (ST3Gal, ST6Gal, ST6GalNAc, and ST8Sia) have appeared in invertebrates, earlier than previously thought, whereas the four families were all detected in bony fishes, amphibians, birds, and mammals. As previously hypothesized, sequence similarities among sialyltransferases suggest a common genetic origin, by successive duplications of an ancestral gene, followed by divergent evolution. Finally, we propose predictions on these invertebrates sialyltransferase-related activities that have not previously been demonstrated and that will ultimately need to be substantiated by protein expression and enzymatic activity assays.
Introduction
Sialyltransferases are a subset of glycosyltransferases that use CMP-Neu5Ac as an activated sugar donor to catalyze the transfer of sialic acid residues to terminal nonreducing positions of oligosaccharide chains of glycoproteins and glycolipids. They catalyze the formation of different linkages (α2–3, α2–6, and α2–8) and differ in their acceptor specificities (for reviews, see Harduin-Lepers et al., 1995, 2001; Takashima et al., 2002a,b).
All vertebrate sialyltransferases have a similar architecture. They are type II transmembrane glycoproteins that predominantly reside in the trans-Golgi compartment. They have a short N-terminal cytoplasmic tail, a unique transmembrane domain, and a stem region of variable length from 20 to 200 amino acids followed by a large C‐terminal catalytic domain. The vertebrate sialyltransferase amino acid sequences described up to date show overall limited sequence identity (from 15 to 57% for human sialyltransferases), but share four peptide conserved motifs called the sialylmotifs: L (large), S (small) (Drickamer, 1993; Livingston and Paulson, 1993), motif III (Jeanneau et al., 2004), and motif VS (very small) (Geremia et al., 1997; Jeanneau et al., 2004). These motifs are involved in the formation of essential disulfide bonds and are implicated in the recognition of both donor and acceptor substrates (Datta and Paulson, 1995; Datta et al., 1998) and in the catalytic activity (Jeanneau et al., 2004). The sialylmotifs are hallmarks for the identification of eukaryotic sialyltransferase genes (Harduin-Lepers et al., 2001).
Sialyltransferase genes are made up of multiple exons, and as it has been reviewed recently, they are widely dispersed in human (Harduin-Lepers et al., 2001) and mouse genomes (Takashima et al., 2003). Genome sequencing programs offer a new route into understanding multigene families both within a single species and across different species. All the animal sialyltransferases belong to the same CAZy 29 glycosyltransferase family (Coutinho et al., 2003) that is based on the detection of common modules in protein sequences. The evolution of complex organisms has been associated with the generation of gene families by successive duplications of an initial relatively small set of ancestral genes. Through this process, followed by subsequent mutation, duplication and exon shuffling between gene families, genes have evolved both discrete and partially redundant functions with the other family members.
Although sialic acid residues are detected mainly in the deuterostome lineage (vertebrates, ascidians, echinoderms), sialyltransferases enzymatic activities have only been documented in mammals, birds, amphibians, bony fishes, and very recently, in Drosophila melanogaster (Koles et al., 2004). By contrast, the evidence for sialylation in plants and protostomes (annelids, arthropods, and mollusks) has been scarce and controversial.
In this study, we have used the data available from various genome-sequencing programs to start to build a foundation for understanding the evolution of the animal sialyltransferase family. As a result, many novel sialyltransferases genes were found. The purpose of this article is to analyze the sialyltransferase sequences that are, as previously mentioned (Harduin-Lepers et al., 2001; Kaneko et al., 2001), secondary to many gene duplications, which occurred early in vertebrate evolution. In addition, we provide evidence that the appearance of the four main sialyltransferase families has occurred among the ancestors of the present invertebrates.
Results
The ST3Gal family
All the described enzymes of this family transfer Neu5Ac residues in α2,3-linkage to terminal galactose (Gal) residues found in glycoproteins or glycolipids. In this ST3Gal family, the ST3Gal I and II subfamilies use exclusively the type 3 oligosaccharide structure Galβ1-3GalNAc-R, whereas the ST3Gal III, IV, V, and VI use the oligosaccharide isomers Galβ1-3/4Glc(NAc)-R. Within this last group, the ST3Gal V subfamily uses exclusively the lactosyl-ceramide (Galβ1-4Glcβ1-Cer) as an acceptor substrate giving rise to the synthesis of the ganglioside GM3.
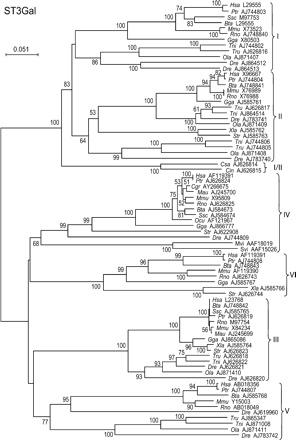
The two main branches of the phylogenetic tree of the ST3Gal family clearly separate ST3Gal I and II from ST3Gal III, IV, V, and VI subfamilies (Figure 1). In the first branch, the determination of subfamily-specific positions for the ST3Gal I and II clearly confirmed 12 gene products in the ST3Gal I subfamily (>60% ST3Gal I specific conserved positions) and 16 gene products in the ST3Gal II subfamily (>70% ST3Gal II specific conserved positions) (Figure 2), but two invertebrate hypothetical proteins (from the sea squirts Ciona intestinalis and Ciona savignyi) were identified that we could not ascribe to either of the ST3Gal I or ST3Gal II subfamilies, because they contained half of the positions specific for ST3Gal I and half of the positions specific for ST3Gal II (between 45 and 55%). The phylogenetic tree of the ST3Gal family is in good agreement with these findings and suggests that the branching of these two urochordate potential sialyltransferases occurred before the duplication at the origin of the present ST3Gal I and II subfamilies that are present in all the vertebrates studied. Therefore, we propose that the two ciona genes constitute a new group of orthologs of the common ancestor present before the split of ST3Gal I and II subfamilies that we will call ST3Gal I/II (Figure 1).
Neighbor-joining phylogenetic tree of the 77 sialyltransferases of the ST3Gal family. One hundred and fifty-six of the 235 positions (60%) were selected in seven G-BLOCKS. Bootstrap values were calculated from 500 replicates, and values >50% are reported at the left of each divergence point. The scale bar represents the number of substitutions per site for a unit branch length. The two urochordate genes from Ciona savignyi (Csa AJ626814) and Ciona intestinalis (Cin AJ626815) are orthologs to the common ancestor (ST3Gal I/II) present before the split of ST3Gal I and II subfamilies.
ClustalW alignment of the peptide sequences of the sialylmotif regions of the ST3Gal I and II subfamilies. White letters with dark gray background represent conserved positions specific from ST3Gal I, and black letters on pale gray background represent conserved positions specific from ST3Gal II. The total number of subfamily-specific conserved positions is represented in the last column preceded by the relative proportions (%) of ST3Gal I and II specific positions, for each protein. The position of the sialylmotifs [L (large), S (small), motif III, and motif VS (very small)] is indicated in the first line of each subfamily. The two urochordate genes from Ciona savignyi (Csa AJ626814) and Ciona intestinalis (Cin AJ626815), flanked by horizontal thick lines, cannot be classified in either of the two subfamilies because they have similar proportions of ST3Gal I and II specific conserved positions (between 45 and 55%, bold characters).
No other intermediate genes were found among the other members of the ST3Gal family (ST3Gal III, IV, V, or VI), and they all could be clearly classified in one of the four subfamilies by both methods: classical phylogeny (Figure 1) and the determination of the relative proportion of subfamily-specific positions (see online supplement data). The relative positions of the duplication events at the origin of each of the four subfamilies, of this second branch, cannot be unequivocally defined, because of the low bootstrap values at the root of the subfamilies, but a genomic organization unique for ST3Gal V suggests that this subfamily might be different from the other three (Figure 3A). This last observation is in good agreement with the fact that the ST3Gal V subfamily is the only one able to use Galβ1-4Glcβ1-Cer (lactosylceramide), whereas the other three subfamilies of this branch use Galβ1-3/4GlcNAc-R as an acceptor substrate.
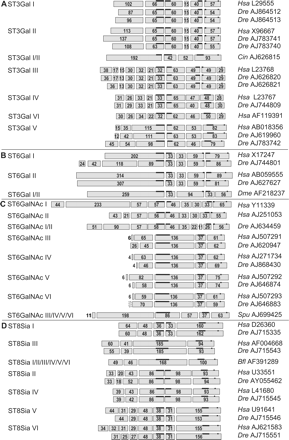
Schematic showing the genomic organization of sialyltransferase genes. Coding exons are represented by rectangles with their relative sizes in amino acids indicated within. Each subfamily is indicated on the left side and the accession number in EMBL/GenBank on the right side. In the ST3Gal family (A), a similar genomic organization was found for the ST3Gal I and II subfamilies, and a similar genomic organization was also found for the ST3Gal III, IV, and VI subfamilies. The genomic organization of ST3Gal V is unique and different from its closest neighbors, the ST3Gal III, IV, and VI subfamilies. Two copies of Danio rerio genes are present in ST3Gal I, II, and III subfamilies. (ST3Gal I: Dre AJ864512, Dre AJ864513; ST3Gal II: Dre AJ783740, Dre AJ783741; and ST3Gal III: Dre AJ626820, Dre AJ626821). In the ST6Gal family (B), the ST6Gal I/II Dme AF218237 gene shares a similar genomic organization with the ST6Gal I and II subfamilies. In the ST6GalNAc family (C), the ST6GalNAc I/II Dre AJ634459 gene shares a similar genomic organization with ST6GalNAc I and II subfamilies and the ST6GalNAc III, IV, V, and VI subfamilies share also similar genomic organization. In the ST8Sia family (D), the ST8Sia I/II/III/IV/V/VI from amphioxus (Bfl AF391289) has similar genomic organization with the ST8Sia III subfamily. The ST8Sia II and IV subfamilies share similar genomic organization, and finally, the ST8Sia V and VI subfamilies share also similar genomic organization.
Two sialyltransferase genes were found in virus pathogenic for rabbits (Mvi AAF18019 and Svi AQAF15026). These genes encode α2,3-sialyltransferase enzymes that have been shown to have catalytic activity toward the oligosaccharide Galβ1-3/4GlcNAc-R (Jackson et al., 1999). They belong to the ST3Gal IV subfamily (Figure 1), but they probably result from a retrotransposition followed by a horizontal transfer from the rabbit host to the virus, because the two are monoexonic sialyltransferase genes, and several complete genomes of the same family of virus have no other sialyltransferase genes detectable.
Two sialyltransferase pseudogenes with several premature stop codons, and with a continuous DNA sequence devoid of introns, were found in human chromosome 4 (§Hsa AJ865084) and chimpanzee chromosome 3 (§Ptr AJ865085) (Figure 2). These two pseudogenes were ascribed to the ST3Gal I subfamily, but were not included in the phylogeny analysis.
The ST6Gal family
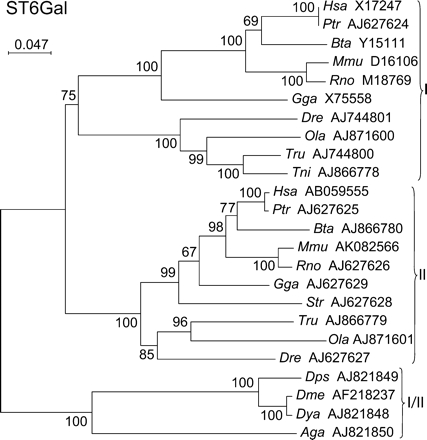
The enzymes of this family comprise only two subfamilies, ST6Gal I and II, that both use the Galβ1-4GlcNAc-R as the acceptor substrate. Members of both subfamilies were found in all vertebrates from fish to man (Figure 4). An ST6Gal cDNA cloned from D. melanogaster (Koles et al., 2004) suggested that the ST6Gal family was present in insects. This fact is strongly supported by the finding of ST6Gal putative genes in two other flies, Drosophila yakuba (Dya AJ821848) and Drosophila pseudoobscura (Dps AJ821848), and in the mosquito Anopheles gambiae (Aga AJ821850). All these insect potential sialyltransferases branch out from the tree, before the split of ST6Gal I and II subfamilies, illustrating that the duplication at the origin of the present ST6Gal I and II subfamilies occurred after the separation of insects from the common evolutionary trunk, and before the appearance of vertebrates. This was confirmed by the determination of the proportion of subfamily-specific positions in the 2 × 2 clustal alignments (Figure 5), which show that the four insect gene products cannot be ascribed to either of ST6Gal I or II subfamilies, because they contain between 48 and 61% specific positions of both subfamilies. However, they belong to the ST6Gal family, because they are more closely related to this family than to any of the other sialyltransferase families, they constitute a new group ortholog to the common ancestor (ST6Gal I/II) that was present before the split of ST6Gal I and II subfamilies (Figure 4).
Neighbor-joining phylogenetic tree of the 24 sialyltransferases of the ST6Gal family. Two hundred and seventy-five of the 338 positions (81%) were selected in 11 G-BLOCKS. Bootstrap values were calculated from 500 replicates, and values >50% are reported at the left of each divergence point. The scale bar represents the number of substitutions per site for a unit branch length. The four insect sialyltransferases from Drosophila pseudoobscura (Dps AJ821849), Drosophila melanogaster (Dme AF218237), Drosophila yakuba (Dya AJ821848), and Anopheles gambiae (Aga AJ821850) are orthologs to the common ancestor (ST6Gal I/II) present before the split of ST6Gal I and II subfamilies.
ClustalW alignments of the sialylmotif regions of the ST6Gal I and II subfamilies. White letters with dark gray background represent conserved positions specific from ST6Gal I, and black letters on pale gray background represent conserved positions specific from ST6Gal II. The total number of subfamily-specific conserved positions is represented in the last column preceded by the relative proportions (%) of ST6Gal I and II specific positions for each protein. The position of the sialylmotifs [L (large), S (small), motif III, and motif VS (very small)] is indicated in the first line of each subfamily. The four insect sialyltransferases from Drosophila pseudoobscura (Dps AJ821849), Drosophila melanogaster (Dme AF218237), Drosophila yakuba (Dya AJ821848), and Anopheles gambiae (Aga AJ821850), flanked by thick horizontal lines, cannot be classified in either of the two subfamilies, because they have similar proportions of ST6Gal I and II specific conserved positions (between 48 and 61%, bold characters).
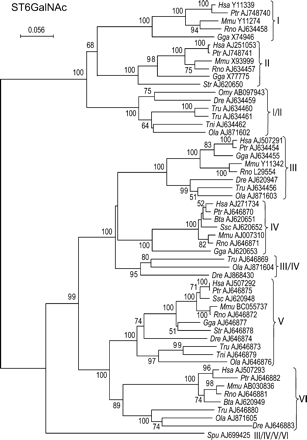
The ST6GalNAc family
The enzymes of this family catalyze the transfer of Neu5Ac residues in α2–6 linkage to the N-acetylgalactosamine (GalNAc) residues found in O-glycosylproteins (ST6GalNAc I, II, and IV) or found in glycolipids (ST6GalNAc III, V, and VI). Interestingly, ST6GalNAc I and II catalyze the transfer of Neu5Ac onto Galβ1-3GalNAc peptides (sialylated or not), and their activity greatly depends on the peptide moiety, whereas ST6GalNAc III, IV, V, and VI exhibit a more restricted substrate specificity, only utilizing sialylated acceptor substrates (Neu5Acα2-3Galβ1–3GalNAc-R), found either in glycoproteins or glycolipids such as GM1b. In good agreement with these different substrate specificities, the phylogenetic tree of the ST6GalNAc family shows two main branches, the first containing ST6GalNAc I and II and the second containing ST6GalNAc III, IV, V, and VI subfamilies (Figure 6).
Neighbor-joining phylogenetic tree of the 55 sialyltransferases of the ST6GalNAc family. One hundred and seventy-two of 247 positions (70%) were selected in eight G-BLOCKS. Bootstrap values were calculated from 500 replicates, and values above 50% are reported on the left of each divergence point. The scale bar represents the number of substitutions per site for a unit branch length. Six genes from the bony fish Oncorhynchus mykiss (Omy ABO97943), Danio rerio (Dre AJ634459), Takifugu rubripes (Tre AJ634460 and Tre AJ634461), Tetraodon nigroviridis (Tni AJ634462), and Oryzias latipes (Ola AJ871602) are orthologs to the common ancestor (ST6GalNAc I/II) present before the split of ST6galNAc I and II subfamilies. The gene from the sea urchin Strongylocentrotus purpuratus (Spu AJ699425) is ortholog to the common ancestor (ST6GalNAc III/IV/V/VI) present before the separation of ST6GalNAc III, IV, V, and VI subfamilies. Three genes from the bony fish T. rubripes (Tru AJ646869), O. latipes (Ola AJ871604), and D. rerio (Dre AJ868430) branch out just before the split of ST6GalNAc III and IV with a nonsignificant low bootstrap (<50%). This fact plus the lack of other ST6GalNAc IV genes from bony fish suggest that these three sialyltransferase sequences belong to the bony fish ST6GalNAc IV subfamily.
Several hypothetical sialyltransferases of this family had intermediate proportions of subfamily conserved specific amino acids and could not be clearly ascribed to any of the six subfamilies. In the first branch of the ST6GalNAc family, the sialyltransferases of five species of bony fish: Oncorhynchus mykiss (Omy AB097943), Danio rerio (Dre AJ634459), Takifugu rubripes (Tru AJ634460 and Tru AJ634461), Tetraodon nigroviridis (Tni AJ634462), and Oryzias latipes (Ola AJ871602) branch out before the split of ST6GalNAc I and II subfamilies and have between 26 and 74% specific conserved positions of both subfamilies (online supplement data), suggesting that they are orthologs of the ancestor (ST6GalNAc I/II) present before the split of ST6GalNAc I and II. This allows us to assign the duplication at the origin of these two subfamilies after the appearance of fish and before the appearance of amphibians, because the two ST6GalNAc I and II subfamilies were clearly identified in amphibians, birds, and mammals.
In the second branch of the ST6GalNAc family containing the remaining four subfamilies, the sea urchin Strongylocentrotus purpuratus putative sialyltransferase gene (Spu AJ699425) is clearly branching out before the occurrence of the duplications at the origin of the four subfamilies and contains 42 and 58% of subfamily ST6GalNAc III and IV specific conserved positions (Figure 7). A similar result was obtained with the subfamily-specific conserved positions of ST6GalNAc V and VI (45 and 55%, online supplement data), suggesting that the S. purpuratus gene is an ortholog to the common ancestor (ST6GalNAc III/IV/V/VI) present before the separation of these four subfamilies.
ClustalW alignments of the sialylmotif regions of ST6GalNAc III and IV subfamilies. White letters with dark gray background represent conserved positions specific from ST6GalNAc III, and black letters on pale gray background represent conserved positions specific from ST6GalNAc IV. The total number of subfamily-specific conserved positions is represented in the last column, preceded by the relative proportions (%) of ST6GalNAc III and IV subfamily-specific positions, for each protein. The position of the sialylmotifs [L (large), S (small), motif III, and motif VS (very small)] is indicated in the first line of each subfamily. The gene from the sea urchin Strongylocentrotus purpuratus (Spu AJ699425) flanked by horizontal thick lines, could not be classified in either of the two subfamilies, because it has similar proportions of ST6Gal III and IV specific conserved positions (41 and 59%, bold characters). The three genes from bony fish Oryzias latipes (Ola AJ871604), Takifugu rubripes (Tru AJ646869), and Danio rerio (Dre AJ868430) also have intermediate values of subfamily-specific positions, but they probably belong to the ST6GalNAc IV (see Discussion and Figure 6).
All the remaining gene products could be clearly ascribed to one of the four subfamilies of this branch with the exception of three (Tru AJ646869, Ola AJ871604, and Dre AJ868430) that had intermediate proportions of subfamily-specific amino acid positions for ST6GalNAc III and IV, and branched out just before the ST6GalNAc III and IV split, but with a very low bootstrap (not shown because it is lower than the threshold of 50%) (Figure 6).
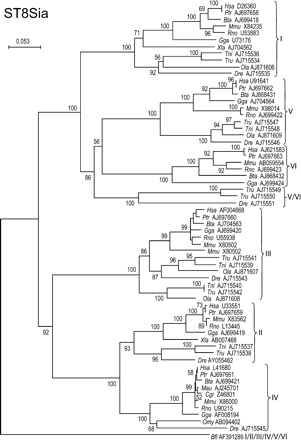
The ST8Sia family
Enzymes of this ST8Sia family mediate the transfer of Neu5Ac residues in α2,8-linkage to other Neu5Ac residues found in glycoproteins and glycolipids. The two main branches of this family tree contain three subfamilies each: ST8Sia I, V, and VI in the first branch and ST8Sia II, III, and IV in the second branch (Figure 8). Three fish potential sialyltransferases of the first branch had a proportion of subfamily-specific conserved positions intermediate between ST8Sia V and ST8Sia VI (Figure 9), and they apparently branch out before the split of ST8Sia V and VI, but with a low bootstrap (56%) (Figure 8).
Finally, one hypothetical protein from the cephalochordata Branchiostoma floridae (Bfl AF391289) had intermediate values of subfamily-specific conserved positions in all the 2 × 2 alignments of the ST8Sia family (Figure 9) and appeared at the root of this family tree, suggesting that it is an ortholog to the common ancestor present before the occurrence of the duplication events that lead to the emergence of the six subfamilies of ST8Sia (Figure 8).
Neighbor-joining phylogenetic tree of the 64 sialyltransferases of the ST8Sia family. Two hundred and four of the 292 positions (70%) were selected in 10 G-BLOCKS. Bootstrap values were calculated from 500 replicates, and values >50%, are reported at the left of each divergence point. The scale bar represents the number of substitutions per site for a unit branch length. The cephalochordate sialyltransferase sequence from Branchiostoma floridae (Bfl AJ715545) branched out at the root of the tree and is ortholog to the common ancestor present before the emergence of the six subfamilies of the ST8Sia family. Three genes from bony fish Takifugu rubripes (Tru AJ715549 and Tru AJ715550) and Danio rerio (Dre AJ715551), branched out before the split of ST8Sia V and VI subfamilies, but the low bootstrap (56%) and the lack of other bony fish sialyltransferases in the ST8Sia VI subfamily suggest that they are genes of the ST8Sia VI subfamily.
ClustalW alignment of the sialylmotif regions of the ST8Sia V and VI subfamilies. White letters with dark gray background represent conserved positions specific from ST8Sia V, and black letters on pale gray background represent conserved positions specific from ST8Sia VI. The total number of subfamily-specific conserved positions is represented in the last column, preceded by the relative proportions (%) of ST8Sia V and ST8Sia VI specific positions, for each protein. The position of the sialylmotifs [L (large), S (small), motif III, and motif VS (very small)] is indicated in the first line of each subfamily. The cephalochordate gene from Branchiostoma floridae (Bfl AJ715545) cannot be classified in either of the two subfamilies, because it contains the same proportion of ST8Sia V and VI subfamily-specific positions (50%). Three genes from bony fish Takifugu rubripes (Tru AJ715549 and Tru AJ715550) and Danio rerio (Dre AJ715551) have also similar proportions of subfamily-specific positions (between 43 and 57%), but they probably belong to the ST8Sia VI subfamily (see Discussion and Figure 8).
Discussion
Phylogeny calculations are based on the differences among sequences, as opposed to the percentages of conserved positions that are directly related to similarities among subfamily-specific conserved positions. However, these two approaches are complementary and point toward the same conclusions.
Sialyltransferase activities have been detected in prokaryotes (Gilbert et al., 1996, 2000; Yamamoto et al., 1998; Shen et al., 1999; Hood et al., 2001; Jones et al., 2002), but these activities are carried out by enzymes devoid of the four sialylmotifs, and therefore, they are not directly related to the eukaryote sialyltransferases. Three Arabidopsis thaliana and three Oryza sativa sialyltransferase-like sequences are present in the CAZy family 29 of glycosyltransferases. They contain the sialylmotifs L and S, but the last two motifs (III and VS) are difficult to identify, and, even if the presence of sialylated structures has been reported in plants (Shah et al., 2003), their hypothetical sialyltransferase activity is still a matter of debate (Séveno et al., 2004). In contrast, all the known animal sialyltransferases have the four sialylmotifs suggesting that they have evolved by successive duplications from a common ancestral gene followed by divergent evolution, similar to the evolutionary model proposed for the fucosyltransferases (Oriol et al., 1999).
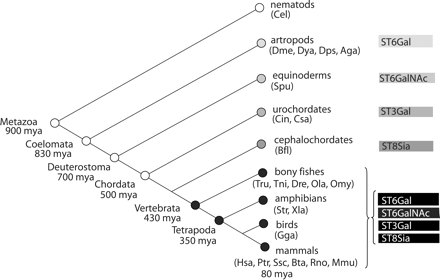
As Figure 10 illustrates, the four main animal families of sialyltransferases were detected in invertebrates, but none of these invertebrate genes could be ascribed to a specific sialyltransferase subfamily, suggesting that the duplication events at the origin of the present 20 subfamilies of sialyltransferases had occurred early in the vertebrate lineage, before the emergence of amphibians because many subfamilies are detected in bony fish. No Neu5Ac and no sialyltransferase-like sequences were found in the genomes of nematodes (Caenorhabditis elegans), suggesting that the first precursor gene of the animal sialyltransferase series might be the ST6Gal from insects, and no other ST6Gal genes were found in other invertebrates. In a similar way, the first precursor gene of the ST6GalNAc family was found in S. purpuratus, and no other ST6GalNAc genes were found in other invertebrates. The first precursor gene of the ST3Gal family was found in ciona, and no other ST3Gal genes were found in other invertebrates. The first precursor gene of the ST8Sia family was found in B. floridae (amphioxus), and no other genes of the ST8Sia family were found in other invertebrates (Figure 10). All these findings suggest that the sialyltransferases have appeared earlier in evolution than previously thought (Schauer, 1982; Varki, 1993; Angata and Varki, 2002). In addition, there is an apparent trend for the appearance of the sialyltransferase activities, starting with α2,6 and followed by α2,3 and finally α2,8 linkages, but we can not define a more precise order, probably because we have not yet found many missing links of this chain. This is particularly evident for the ST8Sia family, because the enzymes of this family catalyze the transfer of Neu5Ac residues on the terminal Neu5Ac of sialylated acceptors, and therefore, other α2,3 and/or α2,6 sialyltransferase activities have to be present in amphioxus to built the corresponding sialylated acceptors for this hypothetical ST8Sia enzyme. Another possibility would be a potential multifunctional nature of these sialyltransferases that would be capable of catalyzing the formation of various α2,3, α2,6, or α2,8 linkages as described for a Neisseiria meningitidis sialyltransferase (Wakarchuk et al., 2001).
Scheme of the divergent evolutionary model of the four families of sialyltransferases. The first detectable member of each of the four main families of sialyltransferases is shown, suggesting a general trend for the appearance of sialyltransferase activities: starting with α2,6, followed by α2,3, and finally, α2,8 linkages. However, some links are probably missing in the invertebrate portion of this chain. In contrast, the four families of sialyltransferases were found in vertebrates and most of the duplications, giving rise to the present 20 subfamilies of sialyltransferases, have probably occurred very early in the vertebrate lineage, because most subfamily-specific proteins were already detected in bony fishes.
Traditionally, the animal sialyltransferase superfamily has been divided into four families: ST3Gal, ST6Gal, ST6GalNAc, and ST8Sia according to the linkage formed and the acceptor substrate used by each enzyme (Harduin-Lepers et al., 2001). In this study, molecular phylogeny suggests a correlation between the acceptor substrate specificity, the amino acid sequence, and the genomic organization of the animal sialyltransferase genes.
The mammalian ST3Gal I and II show a narrow acceptor substrate specificity by using exclusively the Galβ1-3GalNAc disaccharide sequence found onto O‐glycosylproteins and glycolipids (Giordanengo et al., 1997). The ciona ST3Gal I/II orthologs to the common precursor of ST3Gal I and II subfamilies may have similar substrate specificities.
As it has been demonstrated for other gene families (Robinson-Rechavi et al., 2001; Abi-Rached et al., 2002; McLysaght et al., 2002), large gene duplication events appear to have occurred early after fish radiation. In good agreement with this concept, D. rerio has two genes from each of the ST3Gal I, II, III, V (Figure 3A) and ST6GalNAc I/II subfamilies (Figures 3C and 6). There are two genes of ST3Gal I (Figure 1), ST6GalNAc I/II (Figure 6), ST8Sia III, and ST8Sia VI subfamilies (Figure 8) in T. rubripes, and there are two genes of ST3Gal I (Figure 1) and ST8Sia III (Figure 8) subfamilies in T. nigroviridis. These observations raise the possibility of additional new enzymatic specificities in fish sialyltransferases, because new unusual gangliosides have been found in fish (Y. Guerardel, personal communication). In addition, further studies are necessary to investigate the specificity of these new sialyltransferases, because besides Neu5Ac, other sialic acids such as Neu5Gc and KDN are present in high amounts in fish glycoconjugates (Inoue et al., 1996).
The ST6Gal family is the simplest animal sialyltransferase family, containing only two subfamilies, ST6Gal I and II, and both use the Galβ1-4GlcNAc-R as an acceptor substrate in mammals. Before the identification of a sialyltransferase gene in D. melanogaster, the evidence for the presence of sialic acid in insects was scarce and controversial (for a review, see Schauer, 2000; Marchal et al., 2001). Now the D. melanogaster cDNA has been cloned and its enzymatic specificity toward Gal(NAc)β1-4GlcNAc-R demonstrated (Koles et al., 2004), suggesting that this protein has some enzymatic properties in common with the two ST6Gal subfamilies
Previous studies using recombinant enzymes, based on in vitro assays, have shown that the mammalian ST6GalNAc I and II subfamilies have a narrow acceptor substrate specificity requiring a GalNAc residue O-linked to a peptide as an acceptor substrate (Ikehara et al., 1999; Kurosawa et al., 2000; Samyn-Petit et al., 2000; Marcos et al., 2004). Therefore, we can speculate that the fish ST6GalNAc I/II sialyltransferases are involved in the sialylation of the core GalNAc residue of mucin-type O‐glycosylproteins.
In the second branch of the ST6GalNAc family that encompasses the ST6GalNAc III, IV, V, and VI subfamilies, a new gene product named ST6GalNAc III/IV/V/VI, ortholog to the common ancestor present before the separation of the four subfamilies, has been identified in the sea urchin S. purpuratus. This hypothetical enzyme may also share common enzymatic properties with the subfamilies using the trisaccharide Neu5Acα2-3Galβ1-3GalNAc as an acceptor substrate, which are found either in glycopeptides of mucin-type O-glycosylproteins or in the α series of gangliosides such as GD1α. Although it is difficult to predict the enzymatic specificity of this ortholog of a common ancestor, one can assume that it does also use the trisaccharide Neu5Acα2-3Galβ1-3GalNAc as an acceptor substrate, independently of the aglycone moiety. It thus could be involved in the biosynthesis of the sialosphingolipids identified in sea urchin (Kochetkov et al., 1976), or in the biosynthesis of the oligo/polyNeu5Gc chains O‐linked via GalNAc residues of the polypeptide chain of the egg receptor for sperm in the same species (Kitazume et al., 1996).
The fish ST6GalNAc sialyltransferases, Tru AJ646869, Ola AJ871604, and Dre AJ864430, are probably fish orthologs of the ST6GalNAc IV subfamily that have evolved from the common ancestor of ST6GalNAc III and IV at a slower rate, than its paralog genes found in the fish ST6GalNAc III subfamily, because there are no other fish ST6GalNAc IV genes, and the apparent branching out before the split of ST6GalNAc III and IV has a very low bootstrap (Figure 6). These new fish ST6GalNAc IV genes could be involved either in the sialylation of mucin-type O‐glycosylproteins or in the biosynthesis of gangliosides, which are both expressed in fishes .
As hypothesized above for the ST6GalNAc III and IV, the fish Tru AJ715549, Tru AJ715550, and Dre AJ715551 are probably the fish orthologs of the ST8Sia VI subfamily that have evolved from a common ancestor of ST8Sia V and VI, with a slower rate than its paralog genes found in the fish ST8Sia V subfamily, because there are no other fish ST8Sia VI genes, and the apparent branching out before the split of ST8Sia V and VI has a low bootstrap (Figure 8).
In the ST8Sia family, a sialyltransferase gene was found in the cephalochordata B. floridae that is an ortholog to the common ancestor of all the ST8Sia subfamilies. However, to our knowledge, there is no data concerning the presence of sialylated structures in cephalochordata or about the presence of sialyltransferase activity in these species. The ST8Sia family contains two groups of subfamilies exhibiting common enzymatic properties. One branch contains ST8Sia II, III, and IV, which are known as polysialyltransferases involved in the elongation of linear chains of sialic acids found mainly in glycoproteins, whereas the known enzymes of the other branch mediate the transfer of a unique sialic acid residue onto specific sialylated substrates driving the synthesis of di-Sia structures. This last branch includes ST8Sia I (GD3 synthase), ST8Sia V (GT3 synthase), and ST8Sia VI, which transfer only one sialic acid residue to form GD3, GT3, or O-linked disialyl sequences, respectively.
Materials and methods
Nomenclature
In this study, we consider all the animal sialyltransferases sharing the four sialylmotifs as a super family containing four main families according to the substrate and the sugar linkage made (i.e., ST3Gal, ST6GalNAc, ST8Sia, and ST6Gal). The first three contain six subfamilies each (I to VI) and the last one two subfamilies (I and II). Sialyltransferases are named according to Tsuji et al. (1996).
Sialyltransferase genes present in databanks
Human DNA and protein sequence were retrieved from the literature. Ortholog protein sequences from other species were searched with gaped-BLAST and PSI-BLAST (Altschul et al., 1997) with default parameters until convergence. Sixty-five complete open reading frames containing the four sialylmotifs and the transmembrane domain were retrieved from different animal species. Transmembrane domains were predicted by PHD-htm for each hypothetical protein (Roost et al., 1995).
New sialyltransferase genes
Two different strategies were followed for the searches of new putative sialyltransferase genes: espressed sequence tag (EST) assembly and genomic reconstruction. The other animal EST databanks (nonhuman, nonmouse) were searched by TBLASTN with the known sialyltransferases. EST contigs for each gene of each animal species were built with cap3 (Huang and Madan, 1999) and LALIGN (Huang and Miller, 1991). The different general and species specific genomic banks (WGS) (Table I) were also searched by TBLASTN with the known sialyltransferases. Putative exons were identified, and the best intron/exon boundaries were searched by using the Internet Drosophila melanogaster site (Table I). Identification of gene and determination of gene structure were carried out with the use of the specialized Internet sites (Table I), following the AG/GT rule. The EST contig sequences and the genomic sequences corresponding to each hypothetical enzyme of each species were compared, to correct eventual splicing errors, and the open reading frames of the complete new sialyltransferases containing the four sialylmotifs and the transmembrane domain were submitted to European Molecular Biology Laboratory (EMBL) (online supplement data, Supplementary Table II). One hundred and fifty-five sialyltransferases were obtained by this double approach. Overall the 65 sialyltransferases present in databanks and the 155 new sialyltransferases, described in this article, constitute the pool of 220 proteins from 25 different animal species used for phylogeny. Survey of the databanks was completed in January 2005.
URL sites consulted for the in silico search of new sialyltransferase genes
| Databanks . | URL . |
|---|---|
| General banks | |
| CAZy | http://afmb.cnrs-mrs.fr/CAZY/index.html |
| DDBJ | http://www.ddbj.nig.ac.jp/search/blast-e.html |
| NCBI | http://www.ncbi.nlm.nih.gov/BLAST/ |
| UK MRC HGMP-RC | http://www.hgmp.mrc.ac.uk/Registered/Menu/ |
| TIGR | http://tigrblast.tigr.org/tgi/ |
| Ensembl | http://www.ensembl.org |
| PHD-htm | http://pbil.ibcp.fr/htm/index.php |
| JGI | http://genome.jgi-psf.org |
| Animal species-specific banks | |
| IMCB fugu genome project | http://www.fugu-sg.org/Blast2.htm |
| Takifugu rubripes | http://fugu.hgmp.mrc.ac.uk/blast/ |
| Tetraodon nigroviridis | http://www.genoscope.cns.fr/ |
| Oryzias latipes | http://dolphin.lab.nig.ac.jp/medaka/index.php |
| Ciona savignyi | http://www.broad.mit.edu/annotation/ciona/ |
| Ciona intestinalis | http://genome.jgi-psf.org/cgi-bin/runAlignment?db=ciona4 |
| Ciona intestinalis | http://ghost.zool.kyoto-u.ac.jp/indexr1.html |
| Drosophila melanogaster | http://www.fruitfly.org/seq_tools/splice.html |
| Anopheles gambiae | http://www.genoscope.cnrs.fr/ |
| Databanks . | URL . |
|---|---|
| General banks | |
| CAZy | http://afmb.cnrs-mrs.fr/CAZY/index.html |
| DDBJ | http://www.ddbj.nig.ac.jp/search/blast-e.html |
| NCBI | http://www.ncbi.nlm.nih.gov/BLAST/ |
| UK MRC HGMP-RC | http://www.hgmp.mrc.ac.uk/Registered/Menu/ |
| TIGR | http://tigrblast.tigr.org/tgi/ |
| Ensembl | http://www.ensembl.org |
| PHD-htm | http://pbil.ibcp.fr/htm/index.php |
| JGI | http://genome.jgi-psf.org |
| Animal species-specific banks | |
| IMCB fugu genome project | http://www.fugu-sg.org/Blast2.htm |
| Takifugu rubripes | http://fugu.hgmp.mrc.ac.uk/blast/ |
| Tetraodon nigroviridis | http://www.genoscope.cns.fr/ |
| Oryzias latipes | http://dolphin.lab.nig.ac.jp/medaka/index.php |
| Ciona savignyi | http://www.broad.mit.edu/annotation/ciona/ |
| Ciona intestinalis | http://genome.jgi-psf.org/cgi-bin/runAlignment?db=ciona4 |
| Ciona intestinalis | http://ghost.zool.kyoto-u.ac.jp/indexr1.html |
| Drosophila melanogaster | http://www.fruitfly.org/seq_tools/splice.html |
| Anopheles gambiae | http://www.genoscope.cnrs.fr/ |
The initial searches were performed between 2000 and 2004, and they were all updated by a last search performed in January 2005.
URL sites consulted for the in silico search of new sialyltransferase genes
| Databanks . | URL . |
|---|---|
| General banks | |
| CAZy | http://afmb.cnrs-mrs.fr/CAZY/index.html |
| DDBJ | http://www.ddbj.nig.ac.jp/search/blast-e.html |
| NCBI | http://www.ncbi.nlm.nih.gov/BLAST/ |
| UK MRC HGMP-RC | http://www.hgmp.mrc.ac.uk/Registered/Menu/ |
| TIGR | http://tigrblast.tigr.org/tgi/ |
| Ensembl | http://www.ensembl.org |
| PHD-htm | http://pbil.ibcp.fr/htm/index.php |
| JGI | http://genome.jgi-psf.org |
| Animal species-specific banks | |
| IMCB fugu genome project | http://www.fugu-sg.org/Blast2.htm |
| Takifugu rubripes | http://fugu.hgmp.mrc.ac.uk/blast/ |
| Tetraodon nigroviridis | http://www.genoscope.cns.fr/ |
| Oryzias latipes | http://dolphin.lab.nig.ac.jp/medaka/index.php |
| Ciona savignyi | http://www.broad.mit.edu/annotation/ciona/ |
| Ciona intestinalis | http://genome.jgi-psf.org/cgi-bin/runAlignment?db=ciona4 |
| Ciona intestinalis | http://ghost.zool.kyoto-u.ac.jp/indexr1.html |
| Drosophila melanogaster | http://www.fruitfly.org/seq_tools/splice.html |
| Anopheles gambiae | http://www.genoscope.cnrs.fr/ |
| Databanks . | URL . |
|---|---|
| General banks | |
| CAZy | http://afmb.cnrs-mrs.fr/CAZY/index.html |
| DDBJ | http://www.ddbj.nig.ac.jp/search/blast-e.html |
| NCBI | http://www.ncbi.nlm.nih.gov/BLAST/ |
| UK MRC HGMP-RC | http://www.hgmp.mrc.ac.uk/Registered/Menu/ |
| TIGR | http://tigrblast.tigr.org/tgi/ |
| Ensembl | http://www.ensembl.org |
| PHD-htm | http://pbil.ibcp.fr/htm/index.php |
| JGI | http://genome.jgi-psf.org |
| Animal species-specific banks | |
| IMCB fugu genome project | http://www.fugu-sg.org/Blast2.htm |
| Takifugu rubripes | http://fugu.hgmp.mrc.ac.uk/blast/ |
| Tetraodon nigroviridis | http://www.genoscope.cns.fr/ |
| Oryzias latipes | http://dolphin.lab.nig.ac.jp/medaka/index.php |
| Ciona savignyi | http://www.broad.mit.edu/annotation/ciona/ |
| Ciona intestinalis | http://genome.jgi-psf.org/cgi-bin/runAlignment?db=ciona4 |
| Ciona intestinalis | http://ghost.zool.kyoto-u.ac.jp/indexr1.html |
| Drosophila melanogaster | http://www.fruitfly.org/seq_tools/splice.html |
| Anopheles gambiae | http://www.genoscope.cnrs.fr/ |
The initial searches were performed between 2000 and 2004, and they were all updated by a last search performed in January 2005.
Phylogeny
Protein sequences were aligned with ClustalW 1.8 (Thomson et al., 1994), the selection of informative sites was made by G‐BLOCKS (Castresana, 2000), and the output saved in PHYLIP format. Phylogeny analysis was carried out with PHYLOWIN (Galtier et al., 1996), using neighbor joining, observed distances, and 500 bootstrap replicates (Felsenstein, 1985).
Determination of subfamily-specific conserved positions
New sequences were a priori considered to belong to the subfamily of the sialyltransferase used for the search and giving the highest BLAST scores. However, the subfamily of each hypothetical sialyltransferase was further confirmed by the determination of the relative proportions of subfamily-specific conserved positions in ClustalW alignments. The alignments of all the subfamilies of each family (taken 2 × 2) were carried on with the region comprised between the first position before the first motif (L) and the first position after the last motif (VS). Shading of alignments was based on a chemical alphabet comprising five groups: acidic or amide (E, D, Q, N); hydrophobic (I, L, V, M); aromatic (F, Y, W); basic (R, H, K); and hydroxyl (S, T), and the remaining four amino acids, A, G, P, and C, were analyzed separately. This alphabet is based on frequencies of evolutionary replacement among amino acids, chemical characterizations, and minimal base differences between codons. Amino acids of the same group were considered equivalent for the definition of conserved positions. A threshold of >50% of conserved amino acids in each position was used for the definition of the subfamily-specific amino acids. The total number of shaded amino acids specific from either one or the other subfamily is reported at the end of each 2 × 2 clustal line, preceded by the relative proportion (%) of conserved amino acids specific from each of the two subfamilies. Finally, the different lines of each 2 × 2 clustal were ordered by decreasing proportion of specific positions for one subfamily (from 100 to 0%) and increasing proportion of specific positions for the second subfamily (from 0 to 100%). This simple test helps to find intermediate sialyltransferases that have equivalent proportions of specific positions for each of the two subfamilies and therefore may constitute a new intermediate group, which has similarities with both subfamilies, but cannot be considered to belong to one or the other of the two original subfamilies. One example of the 2 × 2 subfamily alignments from each sialyltransferase family is included in the text, and the other alignments are available as online supplement data.
Conclusion
The molecular phylogeny analysis of the animal sialyltransferase super family indicates that there are many sialyltransferase sequences containing the sialylmotifs (signatures of the sialyltransferases). In addition, we have defined five new distinct evolutionary groups (ST3Gal I/II, ST6Gal I/II, ST6GalNAc I/II, ST6GalNAc III/IV/V/VI, and ST8Sia I/II/III/IV/V/VI) that correspond to orthologs of the common ancestors of different sialyltransferase subfamilies that might provide a useful new foundation for understanding the structure–function relatedness of the sialyltransferase super family members.
Supplementary material
Supplementary files of Table II and Figures 11, 12, and 13 are available at Glycobiology online (http://glycob.oupjournals.org).
Acknowledgments
The work was partially supported by the “Centre National de la Recherche Scientifique” (CNRS), the “Institut National de la Santé et de la Recherche Médicale” (INSERM), and the “Association pour la Recherche sur le Cancer” (ARC) grant 3611.
References
Author notes
2Glycobiologie Structurale et Fonctionnelle, UMR CNRS/USTL 8576, Laboratoire de Chimie Biologique, Bâtiment C9, Université des Sciences et Technologies de Lille, 59655 Villeneuve d’Ascq cedex, France; 3Glycobiologie et signalisation cellulaire, INSERM U 504, Université de Paris Sud XI, 16 Avenue, P. Vaillant-Couturier, 94807 Villejuif cedex, France; and 4GDR CNRS 2590, Génomique et génie des glycosyltransférases, France



![ClustalW alignment of the peptide sequences of the sialylmotif regions of the ST3Gal I and II subfamilies. White letters with dark gray background represent conserved positions specific from ST3Gal I, and black letters on pale gray background represent conserved positions specific from ST3Gal II. The total number of subfamily-specific conserved positions is represented in the last column preceded by the relative proportions (%) of ST3Gal I and II specific positions, for each protein. The position of the sialylmotifs [L (large), S (small), motif III, and motif VS (very small)] is indicated in the first line of each subfamily. The two urochordate genes from Ciona savignyi (Csa AJ626814) and Ciona intestinalis (Cin AJ626815), flanked by horizontal thick lines, cannot be classified in either of the two subfamilies because they have similar proportions of ST3Gal I and II specific conserved positions (between 45 and 55%, bold characters).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/glycob/15/8/10.1093_glycob_cwi063/1/m_glycob063.f2.gif?Expires=1716357457&Signature=pmUCK7kXr1GDiB6L~o4-4qmZyl2BuSX-B1I7Cl9733Rl-WmWS8LztIpBNU0JdzSKE6cyUB22W0I1c49lj5HzVtPild5Fwr4HwQUs5UmZtROeNluEwPBxiH2534B~EsuTfNSre7smdaUo~4PFspYqAGoGnirptzKRe2GcjxqExOC3jhBaCIWpN9s4457lMArf29q5~P124xtKaLll80FXBWGX1v8o8hyGk-zDA1GWl8xEky23MovjfUttYlPX6evjhtE5QfLqWUEKAQ2Uy92y76gVG7efePZ19ukaDQaYzMbMVHSVseyPgcckMCtyxvuy2DWySHs8LU1ncfjqDjf7tA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![ClustalW alignments of the sialylmotif regions of the ST6Gal I and II subfamilies. White letters with dark gray background represent conserved positions specific from ST6Gal I, and black letters on pale gray background represent conserved positions specific from ST6Gal II. The total number of subfamily-specific conserved positions is represented in the last column preceded by the relative proportions (%) of ST6Gal I and II specific positions for each protein. The position of the sialylmotifs [L (large), S (small), motif III, and motif VS (very small)] is indicated in the first line of each subfamily. The four insect sialyltransferases from Drosophila pseudoobscura (Dps AJ821849), Drosophila melanogaster (Dme AF218237), Drosophila yakuba (Dya AJ821848), and Anopheles gambiae (Aga AJ821850), flanked by thick horizontal lines, cannot be classified in either of the two subfamilies, because they have similar proportions of ST6Gal I and II specific conserved positions (between 48 and 61%, bold characters).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/glycob/15/8/10.1093_glycob_cwi063/1/m_glycob063.f5.gif?Expires=1716357457&Signature=qRSVyA2znd3EqovbKeEVpMwObJeYCq6qxQdWZgqnwcR87FpIgZILY7ckU6r04h2SQBSDwkWaSgFB2wXw10sd9io~wAx7c41g6dMstVlTkH9gFSJtFHZV4McqAeLKizocC9Pv8oj9CAv-1yhPE0YdgjBHEYPGjaieoQ81XeyeipXBH9PkfBMH55Kvx6yQeCDgri~XOpcGXCBpJ-75Y-a6qUQyppGiRBp5SE6EQ5y5E46z1mEzeQyRxNoh8cFEzWIbNPp0gOYqwpGbvaFUoChfegnSO7C8HE2p7CtuULv8bfeXehQ-9Nsxt1qBZrXRoaoColHb4R8AgMpDV4wSjRaw7A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![ClustalW alignments of the sialylmotif regions of ST6GalNAc III and IV subfamilies. White letters with dark gray background represent conserved positions specific from ST6GalNAc III, and black letters on pale gray background represent conserved positions specific from ST6GalNAc IV. The total number of subfamily-specific conserved positions is represented in the last column, preceded by the relative proportions (%) of ST6GalNAc III and IV subfamily-specific positions, for each protein. The position of the sialylmotifs [L (large), S (small), motif III, and motif VS (very small)] is indicated in the first line of each subfamily. The gene from the sea urchin Strongylocentrotus purpuratus (Spu AJ699425) flanked by horizontal thick lines, could not be classified in either of the two subfamilies, because it has similar proportions of ST6Gal III and IV specific conserved positions (41 and 59%, bold characters). The three genes from bony fish Oryzias latipes (Ola AJ871604), Takifugu rubripes (Tru AJ646869), and Danio rerio (Dre AJ868430) also have intermediate values of subfamily-specific positions, but they probably belong to the ST6GalNAc IV (see Discussion and Figure 6).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/glycob/15/8/10.1093_glycob_cwi063/1/m_glycob063.f7.gif?Expires=1716357457&Signature=aaJoLPvKXW3YoHA23qG-t1nyqOwCBZdk1ZgtFlCkFSVg39kXXjgghZpheIDT-~qVzoUz6BQW0gA~EJxyktjurQzw72BPFgo6jzATMUjL6XYtoNJsrT-jajlhkL2pB52Ve5QBob5UWuwjLOYCkUDcgd-CKM8MinkNAxriVFTiCQJQXx1jflGwRPg17VIa9r3dmYTSNQc5XeR5sbvD8RFK3P-o-VL0917isHoo6lMEJuAB42Vvmw252sceLGpAmCzbADhnlMt-O-EkS-m5E~q5gkrMwm3FRUk-PQ~i1SafZhKLyq9PBYrMO2HaA1bJs~obZjFneiRhKFvcwpng8MZvzw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![ClustalW alignment of the sialylmotif regions of the ST8Sia V and VI subfamilies. White letters with dark gray background represent conserved positions specific from ST8Sia V, and black letters on pale gray background represent conserved positions specific from ST8Sia VI. The total number of subfamily-specific conserved positions is represented in the last column, preceded by the relative proportions (%) of ST8Sia V and ST8Sia VI specific positions, for each protein. The position of the sialylmotifs [L (large), S (small), motif III, and motif VS (very small)] is indicated in the first line of each subfamily. The cephalochordate gene from Branchiostoma floridae (Bfl AJ715545) cannot be classified in either of the two subfamilies, because it contains the same proportion of ST8Sia V and VI subfamily-specific positions (50%). Three genes from bony fish Takifugu rubripes (Tru AJ715549 and Tru AJ715550) and Danio rerio (Dre AJ715551) have also similar proportions of subfamily-specific positions (between 43 and 57%), but they probably belong to the ST8Sia VI subfamily (see Discussion and Figure 8).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/glycob/15/8/10.1093_glycob_cwi063/1/m_glycob063.f9.gif?Expires=1716357457&Signature=rB8DyHidBi~qZPJmVl6qyDdkQ~7Ib0hXYIpO4R8Mp6gLXVT71GNqvIAKxzUZ0IZ7tnSCqNWgh~3iGVTFhrM6AMGRQPpJIZ0D9QIbrsFyyLQ-3x8xbuvPwLRqH8lCzqfQ1poxcArnWKVgQ1aA2DqLyhH2lS7pVnT8btsMTWo01Mkg0koX9vaL5u1Ywo7KD6JaLy7MfiGCRyFiSbz78Ghvo4im33NI29Y9dUyMH~wV4mllY95bp71E0O2g9HOI5QSy8VeenN333yKHiAnSfE3WBLdCnVBR21JJuoeS-hxx1xUQhhwCs~B2tAzyp973JSSDf8orJbQ-3n00tJyvhr5Fnw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)