-
PDF
- Split View
-
Views
-
Cite
Cite
Marie-Odile Sadoulet, Cécile Franceschi, Muriel Aubert, Françoise Silvy, Jean-Paul Bernard, Dominique Lombardo, Eric Mas, Glycoengineering of αGal xenoantigen on recombinant peptide bearing the J28 pancreatic oncofetal glycotope, Glycobiology, Volume 17, Issue 6, June 2007, Pages 620–630, https://doi.org/10.1093/glycob/cwm028
Close - Share Icon Share
Abstract
In human pancreatic adenocarcinoma, alterations of glycosylation processes leads to the expression of tumor-associated carbohydrate antigens, representing potential targets for cancer immunotherapy. Among these pancreatic tumor-associated carbohydrate antigens, the J28 glycotope located within the O-glycosylated mucin-like C-terminal domain of the fetoacinar pancreatic protein (FAPP) and expressed at the surface of human tumoral tissues, can be a good target for anticancer therapeutic vaccines. However, the oncodevelopmental self character of the J28 glycotope associated with the low immunogenicity of tumor-associated carbohydrate antigens may be a major obstacle to effective anti-tumor vaccine therapy. In this study, we have investigated a method to increase the immunogenicity of the recombinant pancreatic oncofetal J28 glycotope by glycoengineering Galα1,3Galß1,4GlcNAc-R (αGal epitope) which may be recognized by natural anti-αGal antibody present in humans. For this purpose, we have developed a stable Chinese hamster ovary cell clone expressing the αGal epitope by transfecting the cDNA encoding the α1,3galactosyltransferase. These cells have been previously equipped to produce the recombinant O-glycosylated C-terminal domain of FAPP carrying the J28 glycotope. As a consequence, the C-terminal domain of FAPP produced by these cells carries the αGal epitope on oligosaccharide structures associated with the J28 glycotope. Furthermore, we show that this recombinant “α1,3galactosyl and J28 glycotope” may not only be targeted by human natural anti-αGal antibodies but also by the mAbJ28, suggesting that the J28 glycotope remains accessible to the immune system as vaccinating agent. This approach may be used for many identified tumor-associated carbohydrate antigens which can be glycoengineered to carry a αGal epitope to increase their immunogenicity and to develop therapeutic vaccines.
Introduction
Many modifications in glycosylation of cell surface glycoconjugates have been described in cancer (Hakomori 1996). The biological significance of these alterations still remains poorly understood, but several studies reported that aberrant glycosylation of glycosphingolipids and glycoproteins expressed in tumor cells has been implicated in aggressiveness, invasion, metastasis, and tumor progression of these cells (Hakomori 1996). In adenocarcinoma of the pancreas, which is highly aggressive and resistant to current treatments (Abbruzzese 2002), many alterations of carbohydrate antigens were observed. Among these tumor-associated antigens, the fetoacinar pancreatic protein (FAPP) was defined as a member of the oncodevelopment-associated pancreatic antigens. FAPP is an oncofetal glycovariant of the bile salt-dependent lipase (BSDL) (Escribano and Imperial 1989; Mas et al. 1993), a lipolytic enzyme present in the pancreatic secretion and involved in the duodenal hydrolysis of cholesteryl esters (Lombardo and Guy 1980). FAPP was first identified using a polyclonal antiserum in the Syrian golden hamster (Benedi et al. 1984) and was further characterized in human pancreas with a murine monoclonal antibody (mAb) referred to as mAbJ28 (Escribano et al. 1986). The reactivity of this mAb with serum is significantly increased in pancreatic adenocarcinoma, and mAbJ28 also recognized the antigen in human tumoral tissues (Escribano et al. 1986) and in human pancreatic tumor cell lines (Mazo et al. 1991; Miralles et al. 1993; Panicot-Dubois et al. 2004). It has been shown that the epitope recognized by mAbJ28, termed the J28 glycotope, is a carbohydrate-dependent antigenic structure (Escribano and Imperial 1989) located within the O-glycosylated mucin-like C-terminal domain of FAPP (Mas et al. 1997; Panicot, Mas, Pasqualini, et al. 1999). The formation of the J28 glycotope requires the core 2 ß1,6 N-acetylglucosaminyltransferase (C2 GnT1) and the α1,3/4 fucosyltransferase (FUT3) (Mas et al. 1997; Panicot, Mas, Pasqualini, et al. 1999), two glycosyltransferases the expression of which is activated during the pancreatic neoplastic processes (Mas et al. 1998). Many works suggested that the J28 glycotope is present at the surface of tumoral pancreatic cells. These studies included immunocytological localization of J28 glycotope within human pancreatic cell lines and human pancreatic tumoral tissue compared with normal tissue (Escribano et al. 1986; Panicot-Dubois et al. 2004), and the specific targeting of nitrosamine-induced pancreatic tumor in hamster (which expressed the J28 glycotope) with the radiolabelled mAbJ28 (Takeda et al. 1992).
Consequently, the J28 glycotope represents a potential target for the diagnosis and for the development of a new therapeutic strategy against pancreatic adenocarcinoma using the recombinant O-glycosylated C-terminal domain of FAPP carrying the J28 glycotope as preventive or therapeutic vaccines (Slovin et al. 2005). However, the oncodevelopmental self character of FAPP might have lead to immunological tolerance. In this context, the carbohydrate epitope Galα1,3Gal ß1,4GlcNAc-R, termed the αGal epitope, can be exploited to increase the immunogenicity of the recombinant J28 glycotope. Indeed, the αGal epitope is present at the cell surface of non-primate mammals and New World monkeys and is absent in humans and higher apes because the gene encoding the α1,3 galactosyltransferase (α1,3GT) is invalid in these species (Galili et al. 1987; Galili, Shohet, et al. 1988). As a consequence, natural anti-αGal antibodies against this epitope were found in humans (Galili et al. 1984, 1985). These antibodies contribute to the host defense (Galili, Mandrell, et al. 1988) and participate in the hyperacute rejection of xenografts (Cooper et al. 1993; Joziasse and Oriol 1999). The recognition of αGal epitopes present at the endothelial cell surface of the donor organ by natural anti-αGal antibodies leads to the activation of the complement cascade and to the xenograft hyperacute rejection (Good et al. 1992; Cooper et al. 1993; Oriol et al. 1993; Collins et al. 1995). In a therapeutic strategy of cancer treatment, it is possible to mimic the mechanisms of hyperacute rejection of a xenograft via expression of the αGal epitope by tumor cells and thus to use the immunological potential of naturally occurring human anti-αGal antibodies for the activation of an immune response eliminating human tumor cells (Jäger et al. 1999). It has also been shown that immunization of α1,3GT knockout mice with BL6 melanoma cells expressing the αGal epitope, elicits a protective immune response against the same tumors lacking the αGal epitope (LaTemple et al. 1999). These data corroborate results showing that the α1,3GT knockout mice, immunized wih rabbit red blood cells, produce high titer of anti-αGal antibodies providing immune protection against the development of colon carcinoma (Unfer et al. 2003). On the basis of these results, the immunological potential of the αGal epitope may be used to increase the immunogenicity of autologous tumor vaccines in humans by expressing the αGal epitope on tumoral cells (Galili 2004). The binding of anti-αGal antibodies to these tumor cells would increase their uptake by antigen-presenting cells, such as dendritic cells and macrophages, by the recognition of the Fc portion of anti-αGal antibodies by Fcγ receptors on antigen-presenting cells (LaTemple et al. 1999; Chen et al. 2001; Deriy et al. 2002). An alternative approach was to use an identified tumor-associated carbohydrate antigen, such as the J28 glycotope, as a source for vaccinating antigens which can be glycoengineered to carry the αGal epitope. Therefore, in this study we have investigated a method to synthesize the αGal epitope on the recombinant O-glycosylated C-terminal domain of FAPP carrying the J28 glycotope and we have shown that the recombinant material still bounds to mAbJ28 and may be targeted by human natural anti-αGal antibodies.
Results
Development of stable CHO cell clones expressing the αGal epitope and the recombinant O-glycosylated C-terminal domain of FAPP carrying the J28 glycotope
In a previous study, we have described the Chinese hamster ovary (CHO)-C2-F3-Ct6R cell line secreting the O-glycosylated C-terminal tandem repeated peptide of FAPP harboring the J28 epitope structure on this C-terminal domain (Panicot-Dubois et al. 2004). This cell line derived from CHO-K1 cell line does not express αGal epitope. Thus, the full-length cDNA, encoding the murine α1,3GT cloned into the pBK-CMV vector, was further transfected into CHO-C2-F3-Ct6R cells and several stable cell clones were selected. Control cell clones were concomitantly obtained by transfecting the empty pBK-CMV plasmid. Transfected and control cells were analyzed for the expression of the αGal epitope by flow cytometry and one representative clone of each cell line was selected for further studies.
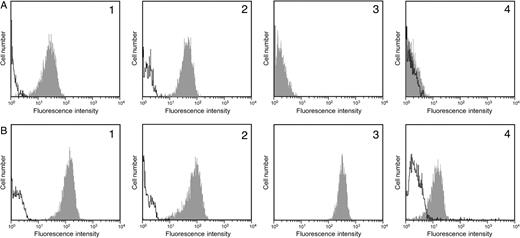
Flow cytometry analyses indicated that the recombinant O-glycosylated C-terminal domain of FAPP and the J28 glycotope were detected using pAbL64 and mAbJ28, respectively, in control cells (Figure 1A1 and A2) and in transfected cells (Figure 1B1 and B2). Using the Griffonia simplicifolia isolectin B4 (IB4 lectin) which recognizes the terminal galactose of Galα1,3Gal-R, a strong expression of the αGal epitope was observed on transfected cells (Figure 1B3), whereas the expression of the αGal epitope was undetectable in control cells (Figure 1A3). The expression of the αGal epitope in transfected and control cells was also investigated by flow cytometry using purified natural anti-αGal antibodies isolated from human AB blood group sera. As shown in Figure 1, the expression of the αGal epitope was not detected in control cells by natural anti-αGal antibodies (Figure 1A4). However, the expression of this epitope can be evidenced on transfected cells (Figure 1B4) albeit the fluorescence intensity was lower than that obtained by using IB4 lectin.
Flow cytometry analysis of the αGal epitope and J28 glycotope on control cells (A) and transfected cells (B). Expression of the O-glycosylated C-terminal domain of FAPP carrying the J28 glycotope was analyzed using pAbL64 (1) and mAbJ28 (2) antibodies. Expression of α1,3 galactosylated antigens was analyzed using FITC-labeled IB4 lectin (3) and human natural anti-αGal antibodies (4) followed by secondary FITC-labeled anti-human IgG or IgM. The shaded area represents the histogram of cell clones incubated with L64, J28, IB4 lectin, and natural anti-αGal antibodies. The empty area represents the histogram of the non-specific binding of secondary FITC-labeled antibodies. For each cell clone, the histograms were superimposed to compare the expression of α1,3 galactosylated antigens.
Secretion of the recombinant O-glycosylated C-terminal glycopeptide of FAPP
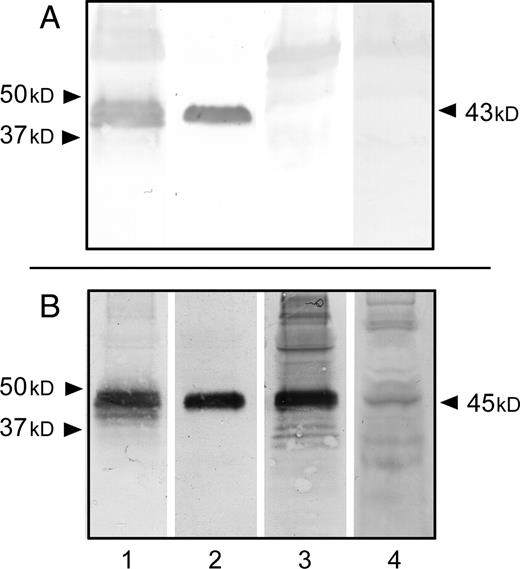
The cell-free medium of subconfluent transfected and control cells [16 h culture in fetal calf serum (FCS)-free OptiMEM] was lyophilized, desalted, and concentrated by lyophilization. Dry material was resuspended in water and analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis SDS–PAGE and western-blot using pAbL64. As previously described (Panicot-Dubois et al. 2004), a glycopeptide associated with a molecular mass of around 43–45 kDa corresponding to the O-glycosylated C-terminal domain of FAPP was present in the cell-free culture media of control (Figure 2A, lane 1) and transfected cells (Figure 2B, lane 1). These C-terminal glycopeptides produced by these two clones carry the J28 glycotope (Figure 2A, and 2B lane 2). Western-blot analyses of glycoproteins present in culture medium of transfected cells revealed one major glycopeptide stained with the IB4 lectin (Figure 2B, lane 3). This band exhibited an apparent molecular mass of 45 kDa likely corresponding to the O-glycosylated C-terminal domain of FAPP. Immunodetection with the human anti-αGal antibodies (Figure 2B, lane 4) revealed the presence of many faint bands including one band which corresponds to the band detected at 45 kDa with the IB4 lectin. No material produced by control cells can be detected by the lectin (Figure 2A, lane 3 and 4). These results corroborate the reactivities of anti-αGal antibodies and IB4 lectin of transfected cells as observed by cytofluorimetry (see Figure 1).
Immunodetection of the αGal epitope and J28 glycotope in extracellular media of control cells and transfected cells. Total (glyco)proteins (100 µg/lane) of concentrated and desalted cell culture media of control cells (A) and transfected cells (B) were separated by SDS–PAGE and electrotransferred onto a nitrocellulose membrane. The membranes were then probed with pAbL64 (lane 1), mAbJ28 (lane 2), biotin-labeled IB4 lectin (lane 3) and human natural anti-αGal antibodies (lane 4). Arrowheads (left) indicate the position of calibrated molecular mass standard proteins and arrows (right) indicate the apparent molecular size of recombinant O-glycosylated C-terminal glycopeptides of FAPP produced by cell clones and detected in extracellular media.
Ex vivo synthesis of the αGal epitope on oligosaccharide structures associated with the J28 glycotope by α1,3GT
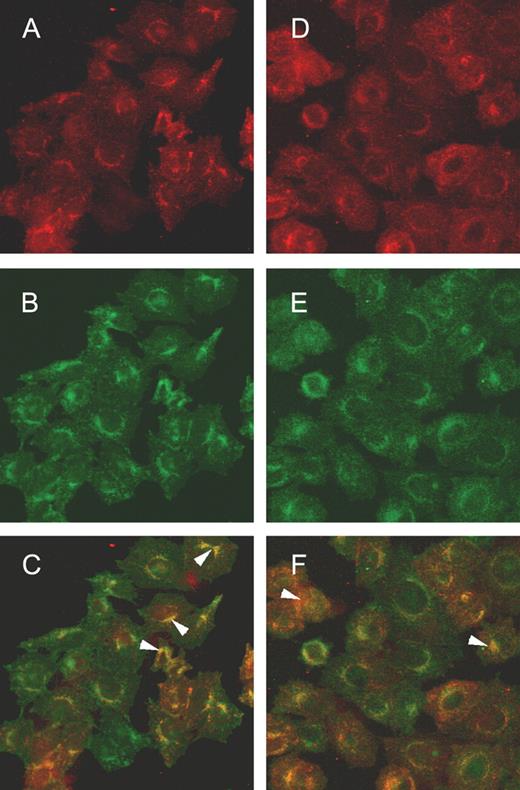
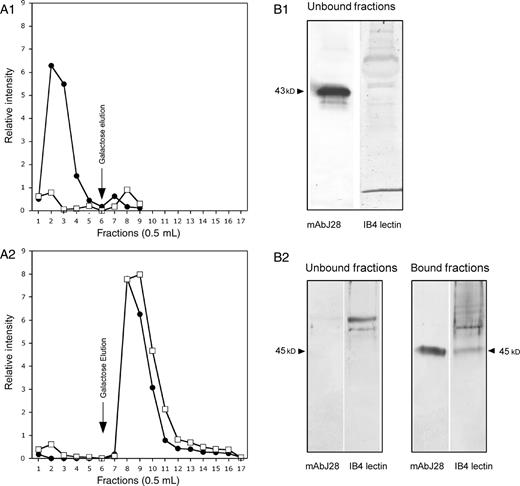
Results obtained in the Secretion of the recombinant O-glycosylated C-terminal glycopeptide of FAPP section suggested that the αGal epitope and J28 glycotope are carried by the O-glycosylated C-terminal domain of FAPP. Thus, confocal microscopy and affinity binding to an agarose-immobilized IB4 lectin column were used as a direct approach to confirm the coexistence of each antigenic structure on the C-terminal domain of FAPP. For this purpose, transfected cells were cultured on slides and stained with mAbJ28 and anti-mouse-Alexa 594 antibodies (red fluorescence, Figure 3A and D) and fluorescein isothiocyanate (FITC)-labeled IB4 (Figure 3B) or human natural anti-αGal antibodies and anti-human-Alexa 488 (green fluorescence, Figure 3E). Although the labeling of the αGal epitope appeared dispersed in the whole cell, on merged images the J28 glycotope was found to be colocalized with the αGal epitope mainly in the Golgi (Figure 3C and F, white arrowheads). To confirm that the antigenic structures recognized by mAbJ28 and IB4 lectin were carried by the same glycopeptide, recombinant C-terminal glycopeptides produced by control cells and transfected cells were separated by affinity chromatography on an agarose-immobilized IB4 lectin column (Figure 4). The results indicated that control recombinant C-terminal glycopeptide bearing the J28 glycotope was recovered in unbound fraction (Figure 4A1), whereas recombinant C-terminal glycopeptide bearing the J28 glycotope and produced by transfected cells was recovered in the bound fractions eluted with galactose (Figure 4A2). These data suggested that αGal epitope may be associated with the J28 glycotope. The eluted material was further separated by SDS–PAGE, and electrotransferred onto nitrocellulose membranes. As expected, the J28 glycotope carried by recombinant material produced by control cells was detected in unbound fractions (Figure 4B1). No reactivity of IB4 lectin with this glycopeptide was observed (Figure 4 B1). No reactivity of mAbJ28 and IB4 lectin was observed in bound fractions (data not shown). The 45 kDa glycopeptide produced by transfected cells and retained on the agarose-immobilized IB4 lectin column was reactive with the mAbJ28 and IB4 lectin (Figure 4B2), suggesting that the α1,3GT can synthesize the αGal epitope on oligosaccharide structures of the recombinant C-terminal domain of FAPP on which the J28 glycotope is also present.
Colocalization of the αGal epitope and J28 glycotope in transfected cells by confocal microscopy. Transfected cells were incubated with mAbJ28 followed by anti-mouse-Alexa 594 antibodies (A) and FITC-labeled IB4 (B). Panel C shows the merged images. The cells were also incubated with mAbJ28 followed by anti-mouse-Alexa 594 (D) and human natural anti-αGal antibodies followed with anti-human-Alexa 488 antibodies (E). Panel F shows the merged images. The yellow color represents the coincidence of red and green pixels, indicating the colocalization of the J28 glycotope and the αGal epitope. The arrowheads in C and F indicate some specific areas of the J28 glycotope and the αGal epitope colocalization. The magnification is ×40.
Cofractionation of αGal epitope and J28 glycotope on a IB4 agarose column. (A) The lyophilized (glyco)proteins (approximately 1 mg) obtained from cell culture media of control cells (A1) and transfected cells (A2) and prepared as described in the Materials and methods section were loaded onto a IB4 agarose column and incubated overnight. The gel was washed with the loading buffer, then the bound material was eluted with 100 mM galactose in PBS, 1 mM CaCl2 and 100 µM MgCl2. Fractions were collected and analyzed by dot blot using mAbJ28 (closed circles •) and IB4 lectin (open squares □) and the quantification of this dot blot is performed by the NIH image Program. The elution profiles were superimposed to compare the elution of the αGal epitope and the J28 glycotope from each cell clone production. (B) Affinity blotting of recombinant O-glycosylated C-terminal glycopeptides eluted from each IB4 agarose column was performed after SDS–PAGE and electrotransfer to nitrocellulose membranes. Membranes were incubated with mAbJ28 and IB4 lectin. Arrows indicate the apparent molecular size of the recombinant O-glycosylated C-terminal domain of FAPP produced by each cell clone.
Selective recognition of the α1,3 galactosylated J28 glycotope by human natural anti-αGal antibodies and mAbJ28
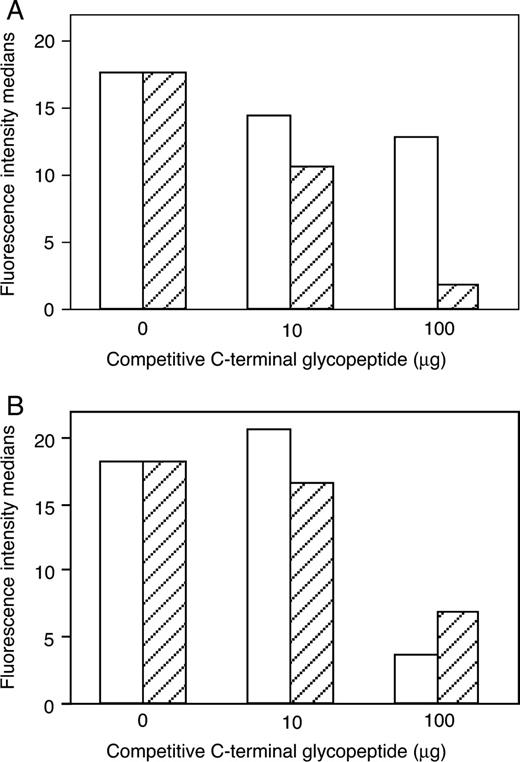
Up to now, data suggest that the α1,3 galactosylated C-terminal domain of FAPP can be detected by human natural anti-αGal antibodies and by mAbJ28. To demonstrate that the αGal epitope does not mask the J28 glycotope, we have constructed a competition assay between mAbJ28 or human natural anti-αGal antibodies and recombinant O-glycosylated C-terminal domains of FAPP using Bx-αGT.9 cells. These cells constitutively express the J28 glycotope and the αGal epitope at their surface (Aubert et al. 2003). The reactivity of mAbJ28 or human natural anti-αGal antibodies with Bx-αGT.9 cells was tested in the presence of increasing amounts of recombinant C-terminal domains of FAPP produced by control cells or transfected cells as soluble competitive material. As expected, binding of human anti-αGal antibodies to the Bx-αGT.9 cells was significantly decreased as the concentration of competitive peptide bearing both the αGal epitope and the J28 glycotope increases, whereas a poor effect on anti-αGal antibodies binding was obtained with the glycopeptide bearing the J28 glycotope only (Figure 5A). On the other hand, binding of mAbJ28 to these cells decreases with an increasing amount of each competitive glycopeptide (Figure 5B). These results indicate that the J28 glycotope and the αGal epitope do not compete and are recognized by their respective antibodies independently of each other.
Inhibition of mAbJ28 or human natural anti-αGal antibodies binding to Bx-αGT.9 cells by recombinant C-terminal glycopeptides. (A) Bx-αGT.9 cells were exposed for 2 h to human anti-αGal antibodies in the presence of increasing amounts (0, 10, and 100 µg) of recombinant C-terminal glycopeptides harboring the J28 glycotope and produced by control cells (empty column) or both the αGal epitope and J28 glycotope produced by transfected cells (dashed column). Binding of human anti-αGal antibodies to the Bx-αGT.9 cells was detected with FITC-labeled anti-human IgG. (B) Bx-αGT.9 cells were exposed for 2 h to mAbJ28 in the presence of increasing amounts (0, 10 and 100 µg) of recombinant C-terminal glycopeptides produced by control cells (empty column) or transfected cells (dashed column). Binding of mAbJ28 to the Bx-αGT.9 cells was detected with FITC-labeled anti-mouse IgG. The fluorescence intensity medians of cells stained with the respective antibodies has determined by fluorescence-activated cell sorter analysis.
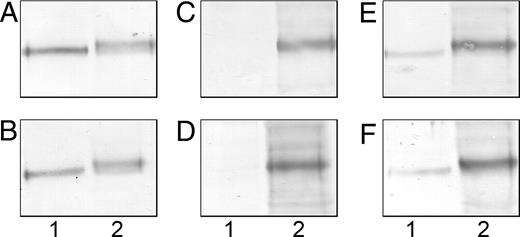
To confirm these data, we have developed a masking assay between mAbJ28 and IB4 lectin on recombinant O-glycosylated C-terminal glycopeptides. The reactivity of mAbJ28 on recombinant C-terminal domains of FAPP produced by control or transfected cells was tested by western-blot after a preincubation of membranes with IB4 lectin. As shown in Figure 6A and B, the binding of mAbJ28 was not modified after the preincubation with this lectin. Identical results were obtained when the reactivity of IB4 lectin was tested after a preincubation with mAbJ28 (Figure 6C and D) or when the reactivity of IB4 lectin associated with mAbJ28 was tested at the same time (Figure 6E and F). These results have been confirmed in dot blot dilution assays, where no differences were observed in the number of positive dilution of mAbJ28 reactivity after a preincubation with IB4 lectin or IB4 lectin reactivity after a preincubation with mAbJ28 (data not shown). These data demonstrate that the αGal epitope and the J28 glycotope are separate structures harbored by the same glycopeptide.
Masking assay of the αGal epitope and J28 glycotope. Affinity blotting of recombinant O-glycosylated C-terminal glycopeptides (10 µg/lane) produced by control cells (lane 1) or transfected cells (lane 2) was performed after SDS–PAGE and electrotransfer to nitrocellulose membranes. The membranes were incubated with either mAbJ28 (A) or biotin-labeled IB4 lectin followed by mAbJ28 (B). Detection of mAbJ28 was performed with alkaline phosphatase-conjugated anti-mouse IgG. The membranes were incubated with either biotin-labeled IB4 lectin (C) or mAbJ28 followed with biotin-labeled IB4 lectin (D). Detection of IB4 lectin was performed with alkaline phosphatase-conjugated anti-biotin IgG. The membranes were incubated with either biotin-labeled IB4 lectin followed by mAbJ28 (E) or biotin-labeled IB4 lectin and mAbJ28 (F). Detection of IB4 lectin and mAbJ28 was performed at the same time with alkaline phosphatase-conjugated anti-biotin IgG and alkaline phosphatase-conjugated anti-mouse IgG.
In vitro galactosylation of the J28 glycotope
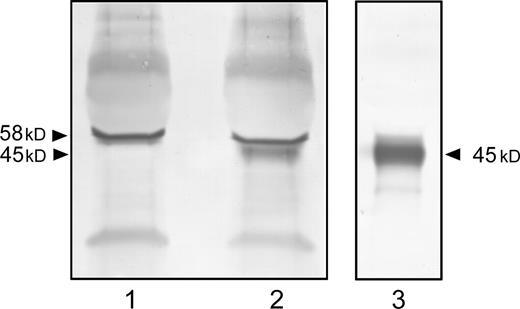
To determine whether another way can be used to synthesize the αGal epitope onto the peptide bearing the J28 glycotope, the J28 glycotope carried by the O-glycosylated C-terminal domain of FAPP produced by control cells was in vitro α1,3 galactosylated by recombinant soluble α1,3GT. The galactose transfer was then analyzed by western-blot using IB4 lectin. Analyses of α1,3 galactosylated glycopeptides revealed one major glycopeptide stained with the IB4 lectin (Figure 7, lane 2) associated with an apparent molecular mass of 45 kDa. In the absence of UDP-galactose as substrate donor, no reactivity of this lectin with this glycopeptide was observed (Figure 7, lane 1), while immunodetection with mAbJ28 revealed the presence of the J28 glycotope (Figure 7, lane 3). These data suggested that terminal galactose residues present on the glycopeptide bearing the J28 oligosaccharide structure remain accessible to α1,3GT to transfer a galactose in α1,3 linkage and generate the αGal epitope. Note that the band exhibiting an apparent molecular size of 58 kDa and detected with IB4 lectin corresponds to the recombinant soluble form of α1,3GT which seems to be α1,3 galactosylated.
In vitro α1,3 galactosylation of the recombinant J28 glycotope by recombinant soluble α1,3GT. The recombinant O-glycosylated C-terminal domain of FAPP carrying the J28 glycotope produced by control cells was galactosylated in vitro with the recombinant soluble form of α1,3GT. The reaction was performed in absence (lane 1) or presence (lane 2) of UDP-galactose and analyzed by western-blot using IB4 lectin. The presence of the J28 glycotope was determined by mAbJ28 immunostaining (lane 3). The apparent molecular size of recombinant O-glycosylated C-terminal domain is 45 kDa.
Discussion
The expression of carbohydrate antigens in normal pancreas and their alteration in malignant neoplasms have been reported in several studies. These altered carbohydrate patterns at the surface of cancer cells have been implicated in aggressiveness, invasion, metastasis, and tumor progression of these cells (Hakomori 1996). Among these antigens, an increase of Lewis x, sialyl-Lewis x, sialyl-Lewis a, and sialyl-Tn antigens was detected in pancreatic cancer tissues (Kim et al. 1988; Itzkowitz et al. 1991). These tumor-associated carbohydrate antigens expressed at the surface of neoplastic cells represent a potential target in immune therapies after tumor resection and chemotherapy treatments to avoid cancer recurrence.
Some of the tumor-associated carbohydrate antigens such as globo H (Gilewski et al. 2001) and GM2 glycolipid-derived antigens (Chapman et al. 2000) or sialyl-Tn antigens (Sandmaier et al. 1999) have been included in clinical trials for the treatment of breast or melanoma cancers. In the case of identified tumor-associated carbohydrate antigens, various approaches have been adopted to induce and enhance immune responses against these carbohydrate antigens. One of these approaches is to link the carbohydrate antigens to an immunogenic carrier protein. Many carbohydrate antigens linked to keyhole limpet hemocyanin (KLH) such as GM1-KLH, GM2-KLH, LewisY-KLH, or sialyl-Tn-KLH, have been developed and investigated as vaccine agents in cancer patients (Slovin et al. 2005). Vaccination of patients suffering from melanoma with GM2-KLH (Chapman et al. 2000) or GD3-KLH (Ragupathi et al. 2000) or patients with small-cell lung cancer with fucosyl GM1-KLH (Krug et al. 2004) induced the highest titer of immunoglobulin-M and -G (IgM and IgG) antibodies against their respective immunogens as well as against tumor cells expressing these antigens. These responses were found to be associated with an increase in survival of patients with breast cancers undergoing active specific immunotherapy with synthetic sialyl-Tn-KLH vaccine (MacLean et al. 1996). However, the possibility of an immune response against KLH carrier protein associated with a poor carbohydrate:KLH carrier protein ratio limits the efficacy of these antigens (Slovin et al. 2005). Therefore, an alternative strategy has been developed to design a synthetic glycopeptide vaccine associating a high density of Tn carbohydrate antigen, which is over-expressed on the membrane of breast, lung, colon, and pancreatic cancer cells, with a non immunogenic lysine core constituting a multiple antigenic glycopeptide (MAG) (Lo-Man et al. 2001). The efficiency of this Tn-MAG was tested in mouse and non-primate and was found to induce strong production of anti-Tn IgG antibodies and increased the survival of mammary tumor-bearing mice upto 80% in either therapeutic or prophylatic protocols (Lo-Man et al. 2001, 2004). However, specific tumor-associated antigens have not been yet identified for the majority of cancers. Consequently, another approach was developed which increased the immunogenicity of autologous tumor vaccines in humans by expressing the αGal epitope on glycoconjugates of tumoral membranes (Galili 2004) with a recombinant α1,3GT (Chen et al. 2001; Deriy et al. 2002). The binding of natural anti-αGal antibody that represents approximately 1% of circulating IgG in humans to these tumor cells would increase the uptake by antigen-presentating cells (LaTemple et al. 1999) to activate the immune system against cancer cells.
In this study, we have put together these two strategies and investigated a method to synthesize the αGal epitope on an identified pancreatic tumor-associated carbohydrate antigen to increase its immunogenicity. FAPP, defined as a member of the oncodevelopment-associated pancreatic antigens (Escribano and Imperial 1989), was characterized in human pancreas with mAbJ28 (Escribano et al. 1986) which recognized the J28 glycotope, a carbohydrate-dependent antigenic structure located within the O-glycosylated mucin-like C-terminal domain of FAPP (Mas et al. 1997; Panicot, Mas, Pasqualini, et al. 1999). Generally, the carbohydrate-based vaccine development was performed to mimic the tumor cell surface which is often characterized by mucins. Consequently, the J28 glycotope, which is present at the surface of tumoral pancreatic cells (Escribano et al. 1986; Mazo et al. 1991; Panicot-Dubois et al. 2004), represents an excellent target for development of an immune therapy strategy for pancreatic adenocarcinoma. For this purpose, we have developed stable CHO cell clones equipped with the cDNA encoding the mucin-like C-terminal domain of FAPP, the C2 GnT1, the FUT3, and the α1,3GT to produce the recombinant O-glycosylated C-terminal domain of FAPP carrying the αGal epitope and the J28 glycotope. Although the number of transfected cDNA copies is large enough, the obtained cell clones are stable, confirming the potential of the CHO cells, like COS cells, to express glycosyltransferases for glycoengineered recombinant glycoproteins the post-translational modifications of which are essential to optimize their therapeutic properties (Elliott et al. 2003; Wurm 2004). An alternative approach to α1,3 galactosylate, the oligosaccharide structure associated with the J28 glycotope is to use a recombinant soluble α1,3GT in vitro. This strategy has been employed successfully to synthesize the αGal epitope on membrane-bound carbohydrate from human myeloid leukemia cells and the tumor of a patient with ovarian cancer, using a recombinant soluble α1,3GT produced in the yeast Pischia pastoris (Chen et al. 2001). Transduction of α1,3GT gene into human HeLa cells by an adenovirus vector has also been used to express the αGal epitope at the surface of the cell to increase the immunogenicity of tumor-associated antigens (Deriy et al. 2002). Thus, it is possible to use these two strategies to synthesize the αGal epitope onto many recombinant tumor-associated carbohydrate antigens which are identified as being specific for a cancer.
The synthesis of the αGal epitope by recombinant soluble α1,3GT suggested that the terminal galactose residue of oligosaccharide structures on the glycopeptide also harboring the J28 glycotope remains accessible to α1,3GT to transfer a galactose in α1,3 linkage. Furthermore, we show that the recombinant “α1,3 galactosyl and J28 glycotope” may be recognized by IB4 lectin or human natural anti-αGal antibodies but also by mAbJ28, suggesting that the αGal epitope does not mask the J28 glycotope. Indeed, the formation of the J28 glycotope requires the expression of two glycosyltransferases, C2 GnT1 and FUT3 (Panicot-Dubois et al. 2004). C2 GnT1 is required to branch the GlcNAc residue on the inner GalNAc residue of the Gal ß1-3GalNAcα1-O linked structure (Bierhuizen and Fukuda 1992) and allows the possible elongation of the chain to form poly-N-acetyllactosamine (Gal ß1-4GlcNAc)n structures on which GlcNAc and terminal galactose residues can be α1,3 fucosylated and α1,3 galactosylated, respectively. Although the nature of different monosaccharides involved in the J28 glycotope structure has not been yet determined, one may strongly suspect that the first GlcNAc residue associated with a fucose residue is crucial for the expression of the J28 glycotope. In addition, it has been shown that CHO cells transfected with C2 GnT1 can synthesize glycoproteins branched O-linked glycans with poly-N-acetyllactosamines (Kim et al. 2001). In particular, the presence of C2 GnT1 is essential for the formation of poly-N-acetyllactosaminyl O-glycans in CHO cells transfected with cDNA encoding leukosialin and consequently, for the expression of T305 O-glycosylated antigen (Bierhuizen et al. 1994). It is therefore possible that the length of poly-N-acetyllactosamine extension on O-linked glycans generated by C2 GnT1 allows the synthesis of the αGal epitope at a distance from the J28 glycotope. Effectively, we show in our masking assays that the reactivity of mAbJ28 on recombinant C-terminal glycopeptide bearing the αGal epitope and the J28 glycotope was not modified after the preincubation with IB4 lectin. Identical results were obtained when the reactivity of IB4 lectin was tested after a preincubation with mAbJ28, suggesting that the αGal epitope and the J28 glycotope are distant structures. Recently, it has also been shown that the coexpression of a P-selectin glycoprotein ligand-1-mouse immunoglobulin Fc fusion protein with C2 GnT1 and α1,3GT in CHO-K1 cells led to increased αGal epitope density on the fusion protein while no O-linked glycan structures with the αGal epitope were identified on this fusion protein when expressed alone or in combination with the α1,3GT in CHO-K1 cells (Liu et al. 2005). These authors suggest that the density of the αGal epitopes on the fusion proteins which is used as an efficient adsorber of xenoreactive anti-αGal antibodies was dependent on the expression of O-linked glycans with core 2 structures and lactosamines extensions (Liu et al. 2005). Therefore, the inner core structure of the J28 glycotope, which will be able to influence the conformation of the lactosamine extension of O-linked glycans to allow the biosynthesis of the αGal epitope (Corzana et al. 2002), will be distant from the αGal epitope and will remain accessible to the immune system. The efficiency of this recombinant “α1,3galactosyl and J28 glycotope” to produce immunogenic responses will be further tested by immunization of α1,3GT knockout mice that lack the αGal epitope and can produce anti-αGal antibodies. However, some patho-physiological observations suggest that the O-glycosylated structures borne by the C-terminal domain can induce an immune response. Specific antibodies to the O-glycosylated C-terminal domain of BSDL were found in the circulating blood in 75% of patients suffering from type 1 diabetes and in 11% of patients suffering from pancreatic adenocarcinoma (Panicot, Mas, et al. 1999). Recently, we also have described two Norwegian kindreds autosomal dominantly inherited diabetes syndrome and exocrine pancreas dysfunction. This syndrome was associated with a single base deletion within the C-terminal domain-containing exon 11 of the BSDL gene resulting in modification of the sequence of this domain affecting several glycosylation sites (Ræder et al. 2006). This disruption may be involved in the molecular mechanisms responsible for exocrine pancreas alteration characterized by a pronounced fibrosis and mucinous metaplasia in these diabetic patients. In these kindreds, antibodies to the C-terminal domain of BSDL were also detected (unpublished observation). These data suggest that the alteration of the O-glycosylated C-terminal domain of BSDL could activate the immune system under inflammatory or pathological conditions.
Materials and methods
Chemicals and reagents
RPMI 1640, HamF12 and OptiMEM media, glutamine, penicillin, streptomycin, trypsin-ethylenediaminetetraacetic acid (EDTA), fungizone, neomycin, hygromycin, and fetal calf serum were purchased from InVitrogen (Cergy-Pontoise, France). Zeocin was from Invivogen (Toulouse, France). Paraformaldehyde was from Fluka (Buchs, Switzerland). Biotin- and FITC-labeled isolectin B4, bovine serum albumin (BSA) and cell dissociation solution were obtained from Sigma (St Louis, MO). Complete™ EDTA-free (a mix of protease inhibitors) was purchased from Roche Diagnostic (Meylan, France). Isoflow buffer was purchased from Coulter (Hialeah, FL).
Antibodies
Human AB blood group sera, obtained from Sigma, were used as a source of natural anti-αGal antibodies. Anti-αGal antibodies were purified from these sera by affinity chromatography on Galα1,3Gal-agarose (Calbiochem, La Jolla, CA). pAbL64 antibodies raised against purified human BSDL were obtained in our laboratory and isolated by affinity chromatography on protein A–Sepharose (Abouakil et al. 1988). The mAb mAbJ28 specific for the fucosylated J28 glycotope carried by repeated C-terminal sequences of the oncofetal glycoisoform of BSDL i.e. FAPP, was a generous gift from Dr M-J. Escribano. FITC-labeled anti-human IgG or IgM, FITC-labeled anti-mouse IgG, FITC-labeled anti-rabbit IgG, alkaline phosphatase-conjugated anti-biotin IgG, alkaline phosphatase-conjugated anti-mouse IgG, and alkaline phosphatase-conjugated anti-rabbit IgG were from Sigma. Anti-human-Alexa 488 and anti-mouse-Alexa 594 antibodies were from Molecular Probes (InVitrogen).
Plasmids, transfection, and cell culture
The construction of α1,3GT plasmid was carried out as previously described (Aubert et al. 2003). Briefly, the cDNA coding for the full-length α1,3GT sequence obtained by reverse transcription-polymerase chain reaction using RNA isolated from mouse heart as template was cloned into the pBK-CMV vector from which the lac promoter was removed, to obtain pBK-α1,3GT plasmid. The latter plasmid confers neomycin resistance to transfected cells.
The human pancreatic adenocarcinoma BxPC-3 cell line that constitutively expresses FAPP and the J28 glycotope (Mazo et al. 1991) was obtained from the American Type Culture Collection (Rockville, MD) (Tan et al. 1986). The Bx-αGT.9 cell line derived from the BxPC-3 cell line was obtained after transfection with cDNA encoding α1,3GT to express the αGal epitope constitutively (Aubert et al. 2003). Bx-αGT.9 cells were grown in RPMI 1640 medium supplemented with 10% FCS, 2 mM glutamine, penicillin (100 U/mL), streptomycin (100 µg/mL), fungizone (0.1%), and geneticin (500 µg/mL). The CHO-C2-F3-Ct6R cell line derived from the CHO-K1 cell line (ATCC, Rockville, MD) was transfected with the cDNA encoding the C-terminal domain of FAPP (Ct6R) (Panicot-Dubois et al. 2004) and further equipped with cDNA encoding C2 GnT1 (C2) and FUT3 (F3) (Panicot, Mas, Pasqualini, et al. 1999). These two glycosyltransferases are required for the formation of the J28 glycotope on the O-glycosylated C-terminal domain of FAPP. CHO-C2-F3-Ct6R cells were grown in Ham F12 medium supplemented with 10% FCS, 2 mM glutamine, penicillin (100 U/mL), streptomycin (100 µg/mL), fungizone (0.1%), geneticin (500 µg/mL), hygromycin (200 µg/mL), and zeocin (500 µg/mL). This recombinant cell line secretes the recombinant C-terminal domain of FAPP constituted of six tandemly repeated identical sequences (Pasqualini et al. 1998) carrying the J28 glycotope in the cell culture medium. Cells were kept at 37°C in a humidified atmosphere of 95% air and 5% CO2. CHO-C2-F3-Ct6R cells were transfected with the pBK-α1,3GT or with the pBK empty vector using lipofectamine plus (InVitrogen) according to the manufacturer's instructions. After 6 h of incubation, the transfection medium was replaced by Ham-F12 medium containing geneticin, hygromycin, and zeocin. After 4–5 weeks, individual colonies were isolated using cloning cylinders and selected for further studies.
Production of recombinant glycopeptides
Subconfluent transfected and control cells were cultured in OptiMEM medium for 16 h. Then the cell culture media were withdrawn and concentrated by lyophilization. Dry material was resuspended in water and desalted on a Sephadex G-25 column (10 cm × 1.5 cm). (Glyco)protein fractions were pooled and lyophilized before analyses. The concentration of proteins was determined using the bicinchoninic acid assay (Perbiosciences, Brebières, France).
Western-blotting
Proteins in reducing SDS buffer were separated on 10% polyacrylamide gels and transferred onto a nitrocellulose membrane. The membranes were probed with the pAb to pancreatic BSDL (pAbL64), mAb specific for the J28 glycotope (mAbJ28) or IB4 lectin or purified human antibodies specific for αGal structures. Detection and development were as previously described (Aubert et al. 2003).
Fractionation on a IB4 agarose column
The lyophilized material obtained from cell culture media was resuspended in 150 µL of phosphate-buffered saline (PBS), 1 mM CaCl2, and 100 µM MgCl2, loaded onto a IB4-agarose column (0.5 mL) (Calbiochem) and incubated overnight under agitation. Thereafter, the gel was washed with the loading buffer, then the bound material was eluted with 100 mM galactose in PBS, 1 mM CaCl2, and 100 µM MgCl2. The entire procedure was performed at 4 °C. Fractions (1 mL) were collected and analyzed for reactivity with mAbJ28 and IB4 lectin. For this purpose 1 µL of each fraction was dotted on a nitrocellulose membrane and detected as described above. Dot quantitations were performed using the NIH image program (http://rsb.info.nih.gov/nih-image/). Nonreactive and reactive fractions were pooled, dialyzed against water, and further subjected to western-blot analysis.
Flow cytometry and confocal microscopy
Detection of the αGal epitope and J28 glycotope in transfected and control cell clones was carried out by indirect fluorescence under the following conditions: cells were released from culture plates by treatment with non-enzymatic cell-dissociation solution (Sigma) for 15 min at 37 °C. The cells were washed three times in PBS, fixed with 2% paraformaldehyde in PBS for 30 min and extensively washed with 1 M glycine buffer (pH 8.5). The cells were permeabilized with 0.05% saponin in PBS buffer and non-specific sites were saturated by incubation for 15 min in the same buffer with 1% BSA. The cells were then incubated for 1 h with pAbL64, mAbJ28, or human natural anti-αGal antibodies, washed three times with 0.05% saponin in PBS buffer, and finally incubated for 30 min with adequate fluorochrome-labeled secondary antibodies. Alternatively, cells were incubated with FITC-labeled IB4 lectin for 1 h. Cells were then washed, suspended in Isoflow buffer and analyzed using an EPICS Profile II flow cytometer (Coulter). For confocal microscopy assays, cells were grown to 50% confluence on sterile Falcon® culture slides (BD Falcon, Le Pont-De-Claix, France) and treated as above. The cells were then incubated for 1 h with mAbJ28 and FITC-labeled IB4, washed with 0.05% saponin in PBS buffer, and finally incubated for 30 min with anti-mouse-Alexa 594 antibodies directed against mAbJ28. Alternatively, cells were incubated with mAbJ28 and human natural anti-αGal antibodies, washed and incubated with anti-mouse-Alexa 594 and anti-human-Alexa 488 antibodies. After staining, slides were washed and mounted in Dako® fluorescent mounting medium (Dako, Trappes, France). Cells were examined by confocal microscopy using a Leica PCS SP2 microscope (Rueil-Malmaison, France).
Inhibition of mAbJ28 or human natural anti-αGal antibodies binding to Bx-αGT.9 cells by recombinant C-terminal glycopeptides
Bx-αGT.9 cells were released from culture plates, washed in PBS, fixed with 2% paraformaldehyde in PBS and extensively washed with 1% BSA in PBS. All subsequent steps were carried out at 4 °C. Then, Bx-αGT.9 cells were exposed for 2 h to human anti-αGal antibodies or mAbJ28 as primary antibodies in the presence of increasing amounts (0, 10, and 100 µg) of recombinant C-terminal domains of FAPP derived from transfected and control cell culture media. Under these conditions, the soluble material could compete with the primary antibodies for recognition of the αGal epitope or J28 glycotope present at the Bx-αGT.9 cell surface. Consequently, the fluorescence intensity medians could decrease with an increasing amount of competitive material. Binding of primary antibodies to the Bx-αGT.9 cells was detected with adequate FITC-labeled secondary antibodies. Cells were then washed, suspended in Isoflow buffer, and analyzed by flow cytometry.
Masking assay of αGal epitope and J28 glycotope
To confirm that the αGal epitope cannot mask the recognition of the J28 glycotope by mAbJ28, we have developped a masking assay between mAbJ28 and IB4 lectin on recombinant O-glycosylated C-terminal domains. For this purpose, affinity blotting of recombinant O-glycosylated C-terminal glycopeptides (10 µg/lane) produced by control or transfected cells was performed after SDS–PAGE and electrotransfer onto nitrocellulose membranes. The membranes were incubated with mAbJ28 (1 h) or biotin-labeled IB4 lectin (1 h), followed by mAbJ28 (1 h), and finally incubated for 1 h with alkaline phosphatase-conjugated anti-mouse IgG directed against mAbJ28. The membranes were incubated with biotin-labeled IB4 lectin (1 h) or mAbJ28 (1 h) followed by biotin-labeled IB4 lectin (1 h), and finally incubated for 1 h with alkaline phosphatase-conjugated anti-biotin IgG directed against IB4 lectin. The membranes were also incubated with biotin-labeled IB4 lectin (1 h), followed by mAbJ28 (1 h) or biotin-labeled IB4 lectin and mAbJ28 (1 h), and finally incubated for 1 h with alkaline phosphatase-conjugated anti-biotin IgG and alkaline phosphatase-conjugated anti-mouse IgG directed against IB4 lectin and mAbJ28, respectively.
In vitro α1,3 galactosylation using recombinant soluble α1,3GT
In vitro α1,3 galactosylation was performed in 50 µL final volume of 100 mM cacodylate pH 6.0 buffer (1X Complete™ EDTA-free, 10 mM AMP, 20 mM MnCl2), containing 100 µM UDP-galactose and 5 mU of porcine recombinant α1,3GT (Calbiochem). The reaction was initiated by the addition of 250 µg of glycopeptide as acceptor and terminated for 6 h at 37 °C. Control was performed in the absence of UDP-galactose. The final product was subjected to western-blot analysis.
Acknowledgments
Dr M.-J. Escribano is greatly acknowledged for her kind release of mAbJ28. This work was supported by grants 4473 and 3816 from the Association pour la Recherche sur le Cancer (Villejuif, France) (to E.M.), and by institutional funding from INSERM (Paris, France) and the Université de la Méditerranée (Marseille, France).
Conflict of interest statement
None declared.
Abbreviations
- α1,3GT
α1,3 galactosyltransferase
- αGal epitope
Galα1,3Galß1,4GlcNAc-R
- BSA
bovine serum albumin
- BSDL
bile salt-dependent lipase
- C2 GnT1
core 2 ß1,6N-acetylglucosaminyltransferase
- CHO
Chinese hamster ovary
- EDTA
ethylenediaminetetracetic acid
- FAPP
fetoacinar pancreatic protein
- FCS
fetal calf serum
- FITC
fluorescein isothiocyanate
- FUT3
α1,3/4fucosyltransferase
- IB4
Griffonia simplicifolia isolectin B4
- IgM and IgG
immunoglobulin-M and -G
- KLH
keyhole limpet hemocyanin
- mAb
monoclonal antibody
- MAG
multiple antigenic glycopeptide
- pAb
polyclonal antibody
- PBS
phosphate-buffered saline
- SDS–PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis.