-
PDF
- Split View
-
Views
-
Cite
Cite
Jay S. Charleston, Karl R. Hansen, Angela C. Thyer, Lynne B. Charleston, Alain Gougeon, Joseph R. Siebert, Michael R. Soules, Nancy A. Klein, Estimating human ovarian non-growing follicle number: the application of modern stereology techniques to an old problem, Human Reproduction, Volume 22, Issue 8, August 2007, Pages 2103–2110, https://doi.org/10.1093/humrep/dem137
Close - Share Icon Share
Abstract
Previous published reports on the number of non-growing follicles (NGFs) in the human ovary have employed model-based methods for number estimates. These methods are time-intensive, and require correction factors and assumptions that ultimately limit their accuracy. Here, we describe the modification, application and validation of a modern fractionator/optical disector technique for the estimation of human ovarian NGF number.
Forty-eight pairs of normal human ovaries were collected from women (age 8–51 years) undergoing elective bilateral oophorectomy, organ donation, or from autopsy. After gross pathologic examination, systematic random sampling was utilized to obtain tissue for analysis by the fractionator/optical disector method. The precision of individual NGF counts was determined by calculating the observed coefficient of error (OCE). Intra-observer variability and variation in NGF number between ovaries within a pair were also determined.
The mean OCE was 16.6% with larger variations observed at lower follicle counts. In recount experiments of the same ovary, NGF number estimates varied by 15–29%, except at very low follicle counts where variation was greater, but absolute differences were small. There was no significant difference in NGF number between ovaries within a pair (Wilcoxon signed rank test, P = 0.81).
Modern stereology methods provide an unbiased, efficient method for estimating NGF number in the human ovary. Both ovaries within a pair contain similar numbers of NGFs.
Introduction
At birth the human ovary is endowed with a finite number of non-growing (primordial, intermediate and primary) follicles representing the complete reproductive potential for the individual (Block, 1952; Richardson et al., 1987; Gougeon et al., 1994). The average human female is believed to possess approximately 500 000–1 000 000 follicles at birth (Forabosco et al., 1991; Gougeon et al., 1994). This pool of non-growing follicles (NGFs) is progressively reduced during the life of the individual, either through atresia or due to recruitment along a path of dominant follicle development and ovulation. Ultimately, the pool of NGFs becomes exhausted, representing the end of the reproductive lifespan. This loss is associated with the onset of menopause when the total number of available follicles reaches a threshold level below which ovulation ceases and estrogen secretion is diminished (Block, 1952; Richardson et al., 1987; Thomford et al., 1987; Faddy et al., 1992; Richardson, 1993; Gougeon et al., 1994). The age of menopause is highly variable (average age 51 ± 8years), yet the factors accounting for this variability are unknown. Given that reproductive lifespan is ultimately determined by ovarian NGF number, accurate and efficient methods for estimating NGF number are paramount for investigations into the ovarian aging process.
Previous estimates of human ovarian NGF number have utilized model (i.e. assumption)-based techniques. These techniques, as applied in previous investigations, involved embedding the ovaries in paraffin followed by serial sectioning and counting the first 10 out of 100 sections (Richardson et al. 1987), every 200th section (Block 1952, 1953), and ‘up to 1 in 200’ sections (Faddy et al., 1992). The number of follicles counted was then multiplied by the inverse of the sampling fraction to obtain a raw estimate of the total NGF number within a given ovary. Raw counts were then frequently multiplied by a correction factor to obtain a ‘corrected’ follicle count. These correction factors, as first described by Abercrombie (1946) and Floderus (1944) assume that the particle of interest (in this case, the follicle) has a particular size, shape and orientation. In essence, they are an attempt to correct for the observation that larger particles appear in more tissue sections, and tend to be overcounted, whereas smaller particles appear in fewer sections and tend to be undercounted. The problem with model-based strategies, in general, are that these assumptions may or may not be true, and as a result, how much the correction factor improves the accuracy of the final estimate is unknown (Abercrombie 1946). Furthermore, the application of correction factors has been inconsistent, with some investigations always utilizing them (Block 1952, 1953), and others incorporating them ‘as appropriate’ (Faddy et al., 1992). Beyond these considerable limitations of model-based counting techniques, their greatest limitation is their inefficiency, even if it was assumed they provided reasonable estimates of ovarian NGF number.
We previously described the use of the fractionator/physical disector technique to determine NGF number in the non-human primate ovary (Miller et al., 1997, 1999). These modern design-based stereology techniques avoid the introduction of correction factors necessary with older methods. The fractionator technique involves exhaustively counting follicles within a known fraction of the total ovary using either the physical or optical disector method. This raw count is then multiplied by the inverse of the sampling fractions to give an unbiased estimate of total follicle number. In the present paper, we describe the adaptation of the fractionator/optical disector technique (Gunderson et al., 1988; West, 1993) for the purpose of estimating NGF number in the human ovary. Like the fractionator/physical disector method, the fractionator/optical disector technique does not rely on any correction factor(s) of the raw data to improve the number estimate. Furthermore, NGF counts obtained by these stereologic methods are not impacted by any potential distortion of the ovary that may occur during tissue processing. The optical disector is more efficient than the physical disector in that the preparation and evaluation of adjacent ‘look-up’ and ‘reference’ sections are not needed, reducing the time and effort required to generate an estimate of NGF number (Myers et al., 2004). Modern stereology methods using a combination of the fractionator and the physical or optical disector are rapidly becoming the standard in structural analysis, and have been utilized to determine nephron and glomerular number in the kidney (Bertram 1995, 2001), germ cell number in the testis (Wreford 1995; Kumar et al., 2001), neuron number in the central nervous system (Braendgaard et al., 1990; West et al., 1991; Galvin and Oorschot 2003), and follicle number in the neonatal (Sonne-Hansen et al., 2003) and adult mouse ovary (Myers et al., 2004).
From a practical standpoint, another important consideration in determining ovarian NGF count for studies investigating reproductive aging is whether or not both ovaries within a pair contain similar number of follicles. If so, then a reasonable approximation of the total NGF endowment of an individual could be obtained by counting a single ovary. Thus the purpose of the current report is 2-fold: first, to present a detailed description and validation of the fractionator/optical disector technique for the estimation of NGF number in the human ovary, and second, to examine the relative equivalency in NGF number between the right and left ovaries.
Methods and Materials
Source of human ovaries
For procurement of normal human ovaries, approval was obtained from the human subjects committees of the University of Washington and six other local hospitals. Written informed consent was obtained from women scheduled for elective bilateral oophorectomy (n = 27) before collection of any specimens or medical history. Subjects were asked to complete an extensive medical history with attention to menstrual history, hormonal therapy, and exposure to potential gonadotoxic agents. In addition, medical records, surgical pathology reports, and operative reports were subsequently reviewed. Subjects previously exposed to chemotherapy, radiation, or with previous ovarian surgery were excluded from participation. In addition, specimens with gross or microscopic evidence of ovarian pathology or with ovarian cysts greater than 2 cm diameter were excluded from the analysis.
In addition to the surgical specimens, we collaborated with two local organ/tissue donation agencies and a national tissue bank (see acknowledgements) for procurement of ovarian pairs from recently deceased women (n = 21). After receiving family next-of-kin consent, donor network technicians collected whole ovaries at the time of other tissue and organ donation. From organ donors, the ovaries were perfused up to the time of collection. From tissue banks, we did not accept ovaries that were procured 72 h or more after the time of death. For this latter group of subjects, the only medical information available to us was cause of death, with or without a full autopsy report.
Tissue preparation
Ovaries were obtained as soon as practical following surgical removal or autopsy. In the case of elective surgical removal, the whole ovary was trimmed of non-ovarian tissue, large obvious follicles were lanced and drained (to prevent large volumes of follicular fluid from significantly contributing to the weight of the ovary), and then the entire ovary was weighed. A small sample was removed and prepared separately for review by a surgical pathologist. The remainder of the ovary was then re-weighed. The difference in weights recorded was used to determine the fraction of the whole ovary that was available for use in this study (see fractionator sampling below). Whole ovaries obtained from tissue banks or organ donors were received in fixative and non-ovarian tissue was removed prior to processing. The ovary was then immersed in Bouin's fixative (picric acid 0.9% w/v, formaldehyde 9% v/v, acetic acid 5% w/v) for at least 2 weeks prior to additional preparation for stereology examination. After receiving whole or biopsied ovaries from the procurement source, one of the investigators grossly examined the tissue before it was deemed suitable as a study specimen. Specimens were rejected if they contained cysts > 2 cm, areas of missing cortex, or any obvious gross pathology.
Stereology
The fractionator method is based on directly counting the particles of interest (in this case, the oocyte nucleoli of NGFs) in a known fraction of the original structure. The total number of particles encountered in this fraction is then multiplied by the inverse of a hierarchy of systematic random sampling fractions in order to generate an estimate of the total number in the original specimen (Gunderson et al., 1988; West, 1993; Charleston, 2000).
The sampling design is outlined in Fig. 1. For surgical specimens, the first sample fraction (F1) consisted of the original ovary minus the small portion previously removed for pathological examination. For both whole ovaries and post-surgical specimens, each ovary was cut into approximately 1 mm slabs perpendicular to the long axis of the ovary using a device designed after Michel and Cruz-Orive (1988). Approximately eight slabs were selected out of the total generated (yielding a second fraction, F2) using systematic random sampling rules. Systematic random sampling rules require that an interval for sampling be set, a random position within that interval be determined for the initial sample, and then sequential samples be selected at that fixed interval. From initial pilot observations, we determined that eight slabs, systematically spaced across the ovary, would provide sufficient samples at this level of the sampling hierarchy. In this scheme, the sampling interval changed for different sized ovaries so that eight or nine slabs were collected per ovary. For example, from an ovary with 32 slabs, every 4th slab is collected, starting with a random position. In this example, if the position in the first interval was randomly chosen as 3, then the 3rd, 7th, 11th, etc. slabs were selected for further processing and sampling.
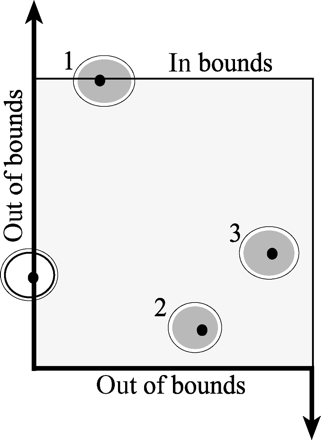
Ovarian sampling method. F1 represents the fraction of the whole ovary remaining after a sample was removed for pathology. F2 represents the slab fraction (in this example, eight slabs [shaded] out of 34 total). F3 represents the set of sections collected from the entire set of sections generated (1/10). F4 represents the area of the sections selected for counting (one section with the largest cross-sectional area is selected to represent each of the original slabs) relative to the total area of all of the sections collected. F5 represents the ratio of the total sum of the area of the disector frames relative to the entire area of the section. F6 represents the ratio of the height of the disector relative to the total height of the tissue section. The area of the upper and lower ‘guard zones’ (the unshaded region above and below the shaded optical disector volume), represents the area in which counting was not performed in order to avoid potential artefacts associated with the cut surface of the tissue section
The selected slabs were dehydrated in a graded ethanol series, passed through a transition solvent of 100% acetone, and embedded as a group in one or two large (2″ × 3″) blocks of glycol methacrylate (GMA, Technovit 8100, Energy Beam Sciences, Inc., Agawam, MA) to maximize sectioning efficiency (Charleston et al., 2003). With this embedding material the slabs are effectively suspended in a plastic block. The blocks were exhaustively sectioned at a thickness of 25 µm using a rotary microtome (Leica 2535, Leica Instruments, Bannockburn, FL). Every 10th section (the third fraction, F3) was collected in the order generated on glass slides for staining (Fig. 1). Sections were stained with Richardson's stain as previously described (Miller et al., 1999), and then mounted with coverslips using Cytoseal 280 (Stephens Scientific, Kalamazoo, MI).
A single section, representing the largest 2D profile of each slab was then selected for counting with the optical disector. The fraction that this section represented from the entire collected stack of sections from each slab (F4 in Fig. 1.) was determined by placing a point grid over the section and summing the points that fell over the section. This value was then divided by the total number of points landing over all collected sections (including the initial and trailing partial slab fragments encountered at the beginning and end of the sectioning run across each slab).
Optical disector counting frames (3D cubes) were placed over the selected stained sections using systematic random sampling rules (Gunderson et al., 1988). Placement of optical disectors and delineation of the areas of interest was accomplished by use of StereoInvestigator software (Version 4.31, MicroBright Field, Colchester, MA) operating on a PC style computer coupled to a Zeiss Photomicroscope II. Real time digital video images were obtained with an Optronics DEI-750 camera (Goleta, CA) and displayed as a window within the StereoInvestigator software screen image. Sequential placement of optical disector frames was performed by a Ludl stepper motor-driven microscope stage directed by the StereoInvestigator software.
The entire cortex of each section in the counting sample was outlined under low magnification for placement of the disector frames. The area of the disector frame divided by the area of the steps between placements (representing a grid) represented a fifth sampling fraction (F5). The frequency of grid placement was determined as that frequency required for identification of at least 100 NGFs within the entire tissue section. When the NGF number fell below this threshold, dissector frames were placed consecutively to cover the entire cortex. The next sampling fraction (F6) consisted of the height of the optical disector counting volume (20 µm) divided by the height of the tissue section (25 µm). This fraction accounts for the portion of the tissue section represented by the guard area, in which no counting was performed (Fig. 1).
Follicle identification and counting
Demonstration of the counting rule, determined when the oocyte nucleolus is present within the disector counting volume. If an oocyte nucleolus is within the disector frame or touches the upper or right boundary, it is counted (represented by the shaded follicles). If it is not within the disector frame, or touches the left or lower boundary, it is not counted (represented by the unshaded follicle)
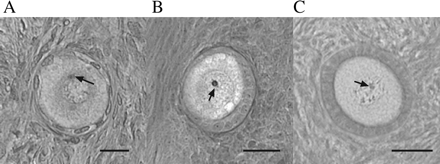
Follicle identification. (A) Primordial, bar = 20 µm; (B) intermediate, bar = 20 µm; (C) primary, bar = 40 µm. Tissue section thickness is 25 µm. The arrow in each photomicrograph identifies the nucleolus, which must be in sharp focus in the optical disector counting volume for a follicle to be counted
Estimation of NGF count by a model-based method (adapted ‘old’ technique)
Statistical analysis
Analysis of the precision of NGF counts was performed at the level of the individual ovary by calculating the observed coefficient of error (OCE) as described by West and Gundersen (1990) to ascertain that the sampling scheme did not introduce undue variability into the individual observations. Estimates of variation between ovaries within a given pair and variation between estimated NGF count in recount experiments were determined by calculating the coefficient of variation (CV). Comparisons between NGF count estimates obtained by the fractionator/optical disector method and model-based techniques were made by calculating the CV and Spearman rank correlation. Estimates of the total number of NGFs in the left and right ovaries of individuals were compared by Spearman rank correlation and Wilcoxon signed rank tests.
Results
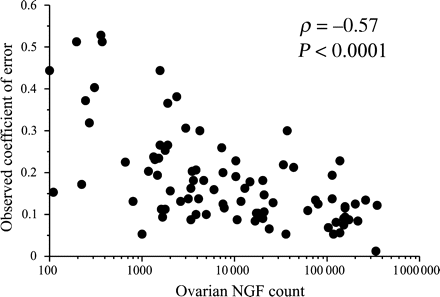
The average number of NGFs per ovary was 46 213 (range 0–348 124). To determine the precision of individual number estimates, the OCE was calculated for each of the ovaries counted. The mean OCE was 16.6% (range 0–52.8%). There was an inverse relationship between the OCE and the number of NGFs (Spearman rank correlation ρ = − 0.57, P < 0.0001, Fig. 4), suggesting that the fractionator/optical disector technique has greater precision at higher NGF number.
Correlation between NGF counts for each ovary and the OCE (Spearman rank correlation, n = 96)
To assess intra-observer variability, five ovaries were counted a second time by the same examiner using the same slabs as those used in the initial count. The CV for follicle count estimates ranged from 2% to 21%, mean 14 ± 4% (Table 1).
Intra-observer recounts
| Ovary . | First count . | Second count . | CV (%) . |
|---|---|---|---|
| 1 | 334 098 | 261 800 | 17 |
| 2 | 31 396 | 32 246 | 2 |
| 3 | 11 500 | 15 250 | 20 |
| 4 | 5944 | 7988 | 21 |
| 5 | 2651 | 3092 | 11 |
| Average | 14 |
| Ovary . | First count . | Second count . | CV (%) . |
|---|---|---|---|
| 1 | 334 098 | 261 800 | 17 |
| 2 | 31 396 | 32 246 | 2 |
| 3 | 11 500 | 15 250 | 20 |
| 4 | 5944 | 7988 | 21 |
| 5 | 2651 | 3092 | 11 |
| Average | 14 |
Intra-observer recounts
| Ovary . | First count . | Second count . | CV (%) . |
|---|---|---|---|
| 1 | 334 098 | 261 800 | 17 |
| 2 | 31 396 | 32 246 | 2 |
| 3 | 11 500 | 15 250 | 20 |
| 4 | 5944 | 7988 | 21 |
| 5 | 2651 | 3092 | 11 |
| Average | 14 |
| Ovary . | First count . | Second count . | CV (%) . |
|---|---|---|---|
| 1 | 334 098 | 261 800 | 17 |
| 2 | 31 396 | 32 246 | 2 |
| 3 | 11 500 | 15 250 | 20 |
| 4 | 5944 | 7988 | 21 |
| 5 | 2651 | 3092 | 11 |
| Average | 14 |
To assess the variability in estimated NGF count derived from different sets of ovarian slabs, three ovaries had NGF counts estimated from adjacent slab sets prospectively prepared using the same methods. Distance between slab sets was 1 mm. The CV for ovary A is 15%, while the CV for ovary B is 29% (Table 2). In ovary C, the CV between counts derived from adjacent slabs is considerably larger (87%), but the difference in absolute terms is small (<200 NGFs, Table 2).
Estimated ovarian NGF counts derived from adjacent slab sets
| Ovary . | First slab set . | Second slab set . | Third slab set . | Mean . | Standard deviation . | CV (%) . |
|---|---|---|---|---|---|---|
| A | 81 607 | 66 240 | NA | 73 924 | 10 866 | 15 |
| B | 41 375 | 63 065 | NA | 52 220 | 15 337 | 29 |
| C | 173 | 0 | 163 | 112 | 97.1 | 87 |
| Ovary . | First slab set . | Second slab set . | Third slab set . | Mean . | Standard deviation . | CV (%) . |
|---|---|---|---|---|---|---|
| A | 81 607 | 66 240 | NA | 73 924 | 10 866 | 15 |
| B | 41 375 | 63 065 | NA | 52 220 | 15 337 | 29 |
| C | 173 | 0 | 163 | 112 | 97.1 | 87 |
Estimated ovarian NGF counts derived from adjacent slab sets
| Ovary . | First slab set . | Second slab set . | Third slab set . | Mean . | Standard deviation . | CV (%) . |
|---|---|---|---|---|---|---|
| A | 81 607 | 66 240 | NA | 73 924 | 10 866 | 15 |
| B | 41 375 | 63 065 | NA | 52 220 | 15 337 | 29 |
| C | 173 | 0 | 163 | 112 | 97.1 | 87 |
| Ovary . | First slab set . | Second slab set . | Third slab set . | Mean . | Standard deviation . | CV (%) . |
|---|---|---|---|---|---|---|
| A | 81 607 | 66 240 | NA | 73 924 | 10 866 | 15 |
| B | 41 375 | 63 065 | NA | 52 220 | 15 337 | 29 |
| C | 173 | 0 | 163 | 112 | 97.1 | 87 |
To determine if increased sampling improved the precision of NGF count estimates, we doubled the counting in two ovaries, counting two parallel sets of eight slabs each, and tripled it in a third ovary, counting three parallel sets of eight slabs each. Counting additional slabs did not improve the precision of the counts. The CV remained within the range of 14–29%, therefore, this additional work in sampling does not improve the estimate.
Five pairs of ovaries had zero NGFs counted in one or both ovaries. We were concerned that the sampling had ‘missed’ some follicles and examined the zero counts more closely. While three pairs had zero follicles counted from both the right and left sides, two pairs had zero NGFs counted on one side, and corresponding very low counts on the other side (0 and 223; 0 and 335). In recounts of these ovaries using adjacent sections, estimated total NGF counts remained < 1000.
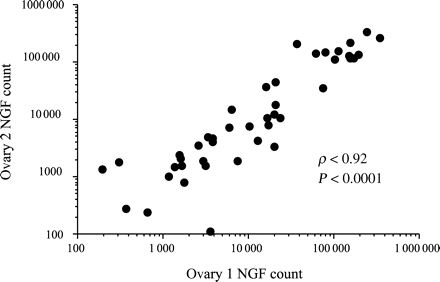
There was a direct correlation in the number of NGFs between the two ovaries from an individual (Spearman rank correlation, ρ = 0.92, P < 0.0001, Fig. 5). For all 96 ovaries, the CV was 168%. For the 48 pairs, the mean CV between ovaries in a given pair was 44%, and the median CV was 32%. Twenty-nine ovarian pairs had a known site of origin, right or left. In comparing the right versus the left side, there was no significant difference in the NGF counts (Wilcoxon signed rank test, P = 0.81).
Correlation between NGF count for both ovaries from the same individual (Spearman rank correlation, n = 48)
To compare the stereologic method described in this investigation with the model-based methods previously utilized by Gougeon (1994), Block (1952), and Richardson et al. (1987), we counted four ovaries using the fractionator/optical disector method and Alain Gougeon counted the same four ovaries using the previously established method. When plotted on a logarithmic scale, the correlation coefficient between the two independent counts was 0.92 (P = 0.08, Spearman rank correlation). In absolute terms, however, the differences in counts between the methods was considerable (CV 25–91%, Table 3), with greater discrepancies at higher NGF number.
Comparison of the optical fractionator technique versus the model-based technique
| Ovary . | Optical fractionator . | Model-based . | CV (%) . |
|---|---|---|---|
| 1 | 117 204 | 48 131 | 59 |
| 2 | 260 615 | 136 991 | 44 |
| 3 | 1869 | 2680 | 25 |
| 4 | 108 495 | 23 549 | 91 |
| Ovary . | Optical fractionator . | Model-based . | CV (%) . |
|---|---|---|---|
| 1 | 117 204 | 48 131 | 59 |
| 2 | 260 615 | 136 991 | 44 |
| 3 | 1869 | 2680 | 25 |
| 4 | 108 495 | 23 549 | 91 |
Comparison of the optical fractionator technique versus the model-based technique
| Ovary . | Optical fractionator . | Model-based . | CV (%) . |
|---|---|---|---|
| 1 | 117 204 | 48 131 | 59 |
| 2 | 260 615 | 136 991 | 44 |
| 3 | 1869 | 2680 | 25 |
| 4 | 108 495 | 23 549 | 91 |
| Ovary . | Optical fractionator . | Model-based . | CV (%) . |
|---|---|---|---|
| 1 | 117 204 | 48 131 | 59 |
| 2 | 260 615 | 136 991 | 44 |
| 3 | 1869 | 2680 | 25 |
| 4 | 108 495 | 23 549 | 91 |
Discussion
One of the greatest challenges facing investigations into the reproductive aging process has been the lack of an efficient method for accurately counting ovarian NGFs, the ultimate determinate of reproductive age. In addition to the inefficiency of older model-based techniques, they also depend upon correction factor(s) and assumptions to derive an estimate of NGF number. These assumptions may be inaccurate, and as a result, how much the application of correction factor(s) improves the estimate is unknown. As such, the goal of this investigation was to adapt modern unbiased stereology techniques using a combination of the fractionator and optical disector for the efficient estimation of human ovarian NGF number.
The precision of individual ovarian NGF counts derived using the fractionator/optical disector technique was determined by calculating the OCE. The goal of the sampling design was to sample as small a fraction of the total ovary as possible while producing an OCE that was less than half the observed group CV. In other words, the variation in NGF count between individuals, as determined with any technique, consists of two components. First, the true biological variation between individuals, and second, the variation introduced by the technique itself. Ideally, the variation introduced by the technique itself would be relatively small compared to the variation between individuals. The CV for our sample group of 98 ovaries was 168.8%, and the mean OCE was 16.6%, demonstrating that variations in NGF estimates due to the technique itself contribute minimally to the observed differences between individuals. Given that most counting techniques based on sampling fractions tend to produce greater precision at higher counts, we expected that at the lower counts the OCE would be higher, which is proved to be correct. These findings suggest that with our current sampling scheme, the precision of estimated counts is improved at higher NGF number.
We also evaluated the variation in estimated NGF counts in recount experiments of the same ovary using both the same sections as those initially counted, and using sections obtained from an adjacent set of ovarian slabs. NGF number estimates varied by 15–29%, except at very low NGF counts, where variation was greater, but absolute differences were small. Although this degree of variation (15–29%) likely contributes minimally to the observed differences in NGF counts between individuals, it was greater than anticipated, and underscores the challenges of estimating NGF number with any technique. Indeed, the heterogeneous distribution of NGFs in the ovarian cortex likely also contributes to the greater OCEs observed at lower NGF counts. Increased sampling did not significantly improve the precision of the estimates. There was no significant difference in the number of NGFs in each ovary from a pair, suggesting that reasonable estimates of total NGF endowment for an individual can be obtained by counting a single ovary.
From an efficiency standpoint, the fractionator/optical disector technique has considerable advantages over older, model-based strategies. Relatively small counts are required to estimate the total number of NGFs in the entire ovary. In practice, we are able to estimate the total number in a human ovary with between 6 and 8 h of counting effort. Although not disclosed in previous publications, much less time is required to implement the fractionator/optical disector method compared to prior methods. For example, Richardson et al. (1987) would count stacks of 10 sections out of every 100 sections. It would not be unexpected to obtain at least 3000 total sections (at 10 µm per section) per ovary from a large ovary, therefore this method would require scanning a minimum of 300 sections. For a young woman with an estimated count of 300 000, about 30 000 NGFs would need to be counted in order to arrive at an estimate, a feat that likely took days to weeks. Furthermore, the fractionator method does not require exhaustive sectioning of the entire ovary in order to arrive at its estimate, rather only the eight slabs. All of our slabs were sectioned in one or two blocks, representing a further potential reduction in workload. Finally, our method utilized systematic random scanning (counting) of the section, versus counting all follicles on the entire section (see Charleston et al., 2003). Systematic sampling designs are much more efficient by representing the larger portion of the section not being counted.
In addition to the greater efficiency of the fractionator/optical disector technique, the resulting number estimate does not require nor utilize any correction factors to push the result closer to the ‘correct’ result. Previously reported NGF counts in the human and non-human primate ovary (Green and Zuckerman, 1951; Block, 1952; Green and Zuckerman, 1954; Baker et al., 1963; Richardson et al., 1987; Thomford et al., 1987; Faddy et al., 1992; Gougeon et al., 1994) have employed model-based stereology methods that, by definition, require assumptions to be made regarding follicular size, shape and orientation. These model-based methods are inherently biased, and hence, require adoption of model-based correction factors to compensate for systematic errors produced by attempting to generate non-dimensional values (number, N) from 2D samples (West et al., 1991). Commonly employed correction factors were first described by Abercrombie (1946) and Floderus (1944) as models to correct for these biases (see comments in Miller et al., 1997). Some of these biases include the systematic overestimation of follicle number due to ‘split cells’ (follicles cut in half during sectioning, thus appearing in two adjacent sections and counted twice), and systematic underestimations due to ‘lost-caps’ (tangentially sectioned follicles that are overlooked or physically lost from the section during tissue processing). Thus, model-based counting strategies can produce systematic overestimations or underestimations of follicular counts, depending on which factors come more into play for a particular ovary. The introduction of correction factors attempt to compensate for these biases, however, the level of improvement in the final estimate remains unknown (Abercrombie, 1946). Furthermore, the accuracy of any number estimate generated from a model-based method cannot be tested by use of the model itself (i.e. confirming a biased estimate with another biased estimate generated by use of the same model is useless). An important point to be made is that model-based strategies may provide reasonable estimates of ovarian NGF number, but there is no clear way to estimate the bias and error associated with each investigation.
Although based only on a small sample, estimates of NGF count from the same ovary derived with the different techniques suggest that the fractionator/optical disector method yields higher NGF counts compared to the model-based technique, except at very low follicle number. What can be made of this comparison? Do older model-based techniques underestimate follicle count, or alternatively, do modern stereology techniques overestimate them? First, from a practical standpoint, there is no sure way to demonstrate which estimate is more ‘correct’, and both must truly be considered estimates. Nevertheless, we at least have an understanding of the precision of the estimate derived with the fractionator/optical disector technique. The precision of NGF count estimates derived with older methods is only rarely reported, most likely because the process for counting a single ovary is so laborious. In this way, the fractionator/optical disector technique has a clear advantage, in that the precision of an individual estimate is readily determined for each ovary by calculating the OCE. Furthermore, no correction factor was introduced in the estimates derived with the fractionator/optical disector technique.
In summary, modern stereology techniques using a combination of the fractionator and optical disector provide for an efficient and reproducible method for determining human ovarian NGF count. While we consider the fractionator/optical disector method to be more accurate than older techniques, there is no clear way to prove this contention. Even if it was no more accurate than the older model-based methods, it clearly is more efficient (results available in 6-8 h compared to days/weeks of effort). In that regard this new method makes ovarian morphometric studies practical to address relevant research questions in the future.
Acknowledgements
This work was supported by NIA Grant R29-HD37360-04. The authors would like to thank Theresa Naluai-Cecchini for research coordination efforts among sites and for counting ovaries. They would also like to thank the pathology department at the University of Washington for assistance with collection of ovaries, and the Northwest Tissue Center, Life Center Northwest, and the National Disease Research Interchange for procurement of ovaries. Additionally, the authors would like to thank Don Clifton, Ph.D. for assistance with statistical analysis.
References
Author notes
Presented in part at the 2003 Society for Gynecological Investigation annual meeting.



![Ovarian sampling method. F1 represents the fraction of the whole ovary remaining after a sample was removed for pathology. F2 represents the slab fraction (in this example, eight slabs [shaded] out of 34 total). F3 represents the set of sections collected from the entire set of sections generated (1/10). F4 represents the area of the sections selected for counting (one section with the largest cross-sectional area is selected to represent each of the original slabs) relative to the total area of all of the sections collected. F5 represents the ratio of the total sum of the area of the disector frames relative to the entire area of the section. F6 represents the ratio of the height of the disector relative to the total height of the tissue section. The area of the upper and lower ‘guard zones’ (the unshaded region above and below the shaded optical disector volume), represents the area in which counting was not performed in order to avoid potential artefacts associated with the cut surface of the tissue section](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/humrep/22/8/10.1093/humrep/dem137/2/m_dem13701.gif?Expires=1716442024&Signature=SXWR~8PMR9v1ZDJDbB6AWGeGQ7GulYxadvEQwA4tTQNIQchu~slMR-MorNTQcIvjnO0ec04fIMHhmM5oEGoUw~54UxFtkFoDagE-CHtOdZiRcDDjzBMu8lDlrXfanG3G3~OJb3D3PVhAdLwHiFJGkFp8cf2iGpHh5H8azYvIk-EBzy9uu5Ofj15rtgx8FZewYPjQjq6WoVrOXw0D-FURK-W-a5g8UDJZ4m~vXTFHw1xE-Cbwm4~HQvv1NiZbyL~SSf6rLlQcJMpyHM9o~tH9WITdYNfaw5U7o9IB8TmRQUyR5R9IBupzqUMMuuX43LAPpamXt5FVZAxRJhbtOlmQ3Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)