-
PDF
- Split View
-
Views
-
Cite
Cite
C Costello, KE Nelson, V Suriyanon, S Sennun, S Tovanabutra, CM Heilig, S Shiboski, DJ Jamieson, V Robison, K Rungruenthanakit, A Duerr, HIV-1 subtype E progression among northern Thai couples: traditional and non-traditional predictors of survival, International Journal of Epidemiology, Volume 34, Issue 3, June 2005, Pages 577–584, https://doi.org/10.1093/ije/dyi023
Close - Share Icon Share
Abstract
Background In the continuing effort to introduce antiretroviral therapy in resource-limited settings, there is a need to understand differences between natural history of HIV in different populations and to identify feasible clinical measures predictive of survival.
Methods We examined predictors of survival among 836 heterosexuals who were infected with HIV subtype CRF01_AE in Thailand.
Results From 1993 to 1999, 269 (49.4%) men and 65 (25.7%) women died. The median time from the estimated seroconversion to death was 7.8 years (95% confidence interval 7.0–9.1). Men and women with enrolment CD4 counts <200 cells/μl had about 2 and 11 times greater risk of death than those with CD4 counts of 200–500 and >500, respectively. Measurements available in resource-limited settings, including total lymphocyte count (TLC), anaemia, and low body mass index (BMI), also predicted survival. Men with two or more of these predictors had a median survival of 0.8 (0.5–1.8) years, compared with 2.7 (1.9–3.3) years for one predictor and 4.9 (4.1–5.2) years for no predictors.
Conclusions The time from HIV infection to death appears shorter among this Thai population than among antiretroviral naïve Western populations. CD4 count and viral load (VL) were strong, independent predictors of survival. When CD4 count and VL are unavailable, individuals at high risk for shortened HIV survival may be identified by a combination of low TLC, anaemia, and low BMI. This combination of accessible clinical measures of the disease stage may be useful for medical management in resource-limited settings.
HIV-1 circulating recombinant form (CRF) 01_AE (subtype E) is the predominant HIV subtype in Thailand and in much of Southeast Asia.1,2 Although the HIV epidemic in Thailand began in 1988 with an explosive Thai-B (subtype B) epidemic among injection drug users (IDUs), a second and larger subtype E epidemic began in 1989 among female prostitutes and their male clientele.1,2 To date, researchers' and clinicians' understanding of subtype E disease progression has often been extrapolated from studies conducted primarily in North American and European individuals infected with subtype B. However, this extrapolation is less than ideal due to the potential differences not just between HIV subtypes, but also between ethnic and racial groups and their ability to respond to the HIV virus due to innate differences in factors that affect progression such as host genetics,3 levels of immune cell populations,4 nutrition,5 co-infections,6 and other factors. The many differences in host and viral factors between HIV-infected Thai and North American/European populations, along with diverse socioeconomic and geographic differences, demonstrate the need for independent studies of progression in Thai subjects with HIV subtype E infection.
To benefit from the enhanced provision of antiretroviral treatment to low-income and middle-income countries, these countries may need to rely on available and cheaper prognostic measures, than the standard CD4 count and viral load (VL) used in developed countries, to determine eligibility for therapy and monitoring of treatment response. This analysis estimates the survival time from subtype E infection until death and identifies significant traditional and non-traditional predictors of survival among men and women in northern Thailand.
Methods
In 1992, a study of heterosexual transmission of HIV was initiated in Chiang Mai, Thailand, where the HIV prevalence had been increasing rapidly among blood donors (0% in 1987, 0.7% in 1988, and 2.7% in 1989).2 The study was approved by ethical review boards at Chiang Mai University, the Thailand Ministry of Public Health, Johns Hopkins University, and the Centers for Disease Control and Prevention. In this study men who donated seropositive blood from 1989 to 1997 at the Chiang Mai Red Cross blood bank, the Chiang Mai University hospital, or the Lampang Provincial Hospital were contacted. Seropositive men who returned to the blood bank were counselled on their HIV status and offered a confirmatory HIV-1 antibody test. All men aged ≥18 years who agreed to bring their regular female sexual partners (i.e. wives) to the study site were invited to participate. Informed consent was obtained from all participants prior to enrolment. Couples in whom the woman had known risk factors for HIV infection other than intercourse with the index male [e.g. engaged in either sex with another HIV-positive male, injection drug use, or commercial sex work (CSW)] were excluded. Over 80% of the women enrolled had no indication from their sexual history or 5-year contraceptive calendar data of having any other sexual partner than the index male since the onset of the HIV epidemic in Thailand. By March 1997, 590 HIV-positive men and their female partners, 276 (46.8%) of whom were HIV-positive, had enrolled in the study.
The study's data collection procedures have been described in detail.7 The enrolment visit consisted of the drawing of blood, an interview, and a physical exam. Serum samples were tested by HIV-1 enzyme linked immunosorbent assay (ELISA) and, if positive, by Western blot. Numerous other laboratory tests were performed for a variety of markers, including immune parameters (immunophenotyping) and viral burden (HIV-1 RNA by Roche Amplicor 1.5). Retrospective assessment of clinical, historical, and laboratory data by a collaborating physician (co-author D.J.) was used to determine whether a participant met the WHO guidelines for onset of antiretroviral therapy in resource-limited settings where CD4 testing is unavailable. The December 2003 revised World Health Organization (WHO) guidelines for the onset of antiretroviral therapy (ART) in resource-limited settings where CD4 count is not available use one of two criteria: (i) WHO HIV-disease stage 3 or 4, or (ii) WHO HIV-disease stage 2 with TLC < 1200 cells/μl.8
An HIV-1 envelope peptide enzyme immunoassay9 was used to determine the subtype of the 866 HIV-positive subjects. For this analysis, those with non-reactive HIV-1 antibodies (3.7%) or missing subtype data (5.8%) were assigned the viral subtype of their spouses. Subtypes E and B accounted for 96.9% and 1.2%, respectively, whereas 1.3% were non-reactive and 0.1% had missing data. The main analysis was limited to participants with only subtype E virus (N = 839).
Between March 1998 and December 1999, vital status and, if applicable, date of death were collected on 96.7% (811/839) of subjects via survey letters, spouses or care providers, death certificates, or government records. The 28 subjects without vital status information from the follow-up survey were excluded from calculations on the proportion of deaths among seropositive subjects. However, if these subjects had at least two study visits while being seropositive (25/28), they were eligible for inclusion in the analysis of HIV survival time (N = 836: 569 men, 267 women), with their HIV survival censored at their last study visit. For 66.3% (236/356) of the deceased seropositive subjects, cause of death was obtained from death certificates. The 13 subjects who died from non-HIV-related causes were excluded from calculations on the proportion of deaths but included in the survival analysis, with their HIV survival censored at their date of death.
Two methods were used to accommodate uncertainty regarding the date of seroconversion. The first method selected subtype E infected subjects whose seroconversion occurred within a 2-year window of uncertainty; these participants are referred to here as the seroconverter analysis group. These subjects included the following: men with HIV-negative and HIV-positive blood bank donations less than 2 years apart (n = 21); wives with no other risk factors for HIV who were HIV-positive at the time of enrolment, which was within 2 years of their onset of sexual activity (n = 44) or their husbands' HIV-negative blood bank test for women who had no other sexual partners since the onset of epidemic (n = 2); women who became HIV-positive within 2 years after enrolling as HIV-negative (n = 12); and men without IDU histories who donated HIV-positive blood in 1989 or 1990 (n = 64) or who reported previous HIV-positive results in 1989 or 1990 during their enrolment interviews (n = 6) and, thus, were HIV-positive within 2 years of the onset of the non-IDU, male epidemic among blood donors in Chiang Mai (January 1, 1989).2
For the 91 men and 58 women classified as seroconverters, the seroconversion date was imputed as the midpoint between the dates last known as being HIV-negative and first known as being HIV-positive. The imputed seroconversion date was incorporated into a Kaplan–Meier survival model. The second method of accommodating the uncertainty of the seroconversion date used the monthly, male HIV prevalence at participating blood banks to calculate the male seroconversion probabilities for each month between the dates last known HIV-negative and first known HIV-positive.10,11 The date in which those subjects were last known as being HIV-negative was either the date of a negative blood bank donation or January 1, 1989. Sensitivity analyses tested the effect on the results if the assumptions used for modelling the survival based on seroconversion probabilities among blood donors varied. Two additional assumptions were tested: a uniform trend in HIV prevalence from 1989 to 1998 and a non-zero prevalence starting in 1988 (0.64% prevalence).
The analysis of potential predictors of survival focused on two models: one utilized the CD4 count and VL measurement; the other, for use in resource-limited settings, included total lymphocyte count (TLC), haemoglobin level, and body mass index (BMI). Receiver operating characteristics (ROC) curves were used to assess predictability of varying cut-offs of haemoglobin level and BMI by gender. Because these factors were measured when a subject enrolled in the study, ‘survival time’ began at enrolment. Statistically significant predictors of survival time were identified using the Kaplan–Meier and Cox proportional hazards methods. Analytical models that allowed adjustment for other factors included age at enrolment.
Results
At enrolment, most subjects were asymptomatic, and all were antiretroviral naïve. Within the previous 3 years, ∼12% reported a history of hospitalization, while very few reported a history of either IDU or opium use (Table 1). The median age was 29 (range: 18–56) for men and 26 (range:15–52) for women. Of the subjects 95.9% (802/836) had an enrolment CD4 count, and 99.6% (833/836) had an enrolment VL measurement.
Prevalence of baseline factors in men and women infected with HIV-1 subtype E in the Thai heterosexual couples study
| . | Seroconverters [% (n = 149)] . | Women [% (n = 267)] . | Men [% (n = 569)] . | |||
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | 61.1 (91) | 0 | 100 (569) | |||
| Female | 38.9 (58) | 100 (267) | 0 | |||
| Education | ||||||
| None | 3.3 (5) | 3.1 (8) | 1.2 (7) | |||
| Primary | 63.1 (94) | 76.8 (198) | 60.2 (342) | |||
| Secondary | 26.2 (39) | 14.7 (38) | 29.6 (168) | |||
| Post-secondary | 7.4 (11) | 5.4 (14) | 9.0 (51) | |||
| Hospitalized within past 3 years | 13.5 (20) | 12.5 (32) | 12.2 (69) | |||
| Injected drugs or smoked opium within past 3 years | 0 | 0 | 2.1 (12) | |||
| CD4 (cells/μl) | ||||||
| <200 | 23.2 (33) | 10.7 (27) | 30.3 (167) | |||
| 200–500 | 41.6 (59) | 55.0 (138) | 48.8 (269) | |||
| >500 | 35.2 (50) | 34.3 (86) | 20.9 (115) | |||
| VL (copies/ml) | ||||||
| <10 000 | 36.2 (54) | 56.1 (148) | 20.9 (119) | |||
| 10 000–100 000 | 47.7 (71) | 34.8 (92) | 58.9 (335) | |||
| >100 000 | 16.1 (24) | 901 (24) | 20.2 (115) | |||
| . | Seroconverters [% (n = 149)] . | Women [% (n = 267)] . | Men [% (n = 569)] . | |||
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | 61.1 (91) | 0 | 100 (569) | |||
| Female | 38.9 (58) | 100 (267) | 0 | |||
| Education | ||||||
| None | 3.3 (5) | 3.1 (8) | 1.2 (7) | |||
| Primary | 63.1 (94) | 76.8 (198) | 60.2 (342) | |||
| Secondary | 26.2 (39) | 14.7 (38) | 29.6 (168) | |||
| Post-secondary | 7.4 (11) | 5.4 (14) | 9.0 (51) | |||
| Hospitalized within past 3 years | 13.5 (20) | 12.5 (32) | 12.2 (69) | |||
| Injected drugs or smoked opium within past 3 years | 0 | 0 | 2.1 (12) | |||
| CD4 (cells/μl) | ||||||
| <200 | 23.2 (33) | 10.7 (27) | 30.3 (167) | |||
| 200–500 | 41.6 (59) | 55.0 (138) | 48.8 (269) | |||
| >500 | 35.2 (50) | 34.3 (86) | 20.9 (115) | |||
| VL (copies/ml) | ||||||
| <10 000 | 36.2 (54) | 56.1 (148) | 20.9 (119) | |||
| 10 000–100 000 | 47.7 (71) | 34.8 (92) | 58.9 (335) | |||
| >100 000 | 16.1 (24) | 901 (24) | 20.2 (115) | |||
Data were not available on all subjects; percentages reflect a changing denominator.
Prevalence of baseline factors in men and women infected with HIV-1 subtype E in the Thai heterosexual couples study
| . | Seroconverters [% (n = 149)] . | Women [% (n = 267)] . | Men [% (n = 569)] . | |||
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | 61.1 (91) | 0 | 100 (569) | |||
| Female | 38.9 (58) | 100 (267) | 0 | |||
| Education | ||||||
| None | 3.3 (5) | 3.1 (8) | 1.2 (7) | |||
| Primary | 63.1 (94) | 76.8 (198) | 60.2 (342) | |||
| Secondary | 26.2 (39) | 14.7 (38) | 29.6 (168) | |||
| Post-secondary | 7.4 (11) | 5.4 (14) | 9.0 (51) | |||
| Hospitalized within past 3 years | 13.5 (20) | 12.5 (32) | 12.2 (69) | |||
| Injected drugs or smoked opium within past 3 years | 0 | 0 | 2.1 (12) | |||
| CD4 (cells/μl) | ||||||
| <200 | 23.2 (33) | 10.7 (27) | 30.3 (167) | |||
| 200–500 | 41.6 (59) | 55.0 (138) | 48.8 (269) | |||
| >500 | 35.2 (50) | 34.3 (86) | 20.9 (115) | |||
| VL (copies/ml) | ||||||
| <10 000 | 36.2 (54) | 56.1 (148) | 20.9 (119) | |||
| 10 000–100 000 | 47.7 (71) | 34.8 (92) | 58.9 (335) | |||
| >100 000 | 16.1 (24) | 901 (24) | 20.2 (115) | |||
| . | Seroconverters [% (n = 149)] . | Women [% (n = 267)] . | Men [% (n = 569)] . | |||
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | 61.1 (91) | 0 | 100 (569) | |||
| Female | 38.9 (58) | 100 (267) | 0 | |||
| Education | ||||||
| None | 3.3 (5) | 3.1 (8) | 1.2 (7) | |||
| Primary | 63.1 (94) | 76.8 (198) | 60.2 (342) | |||
| Secondary | 26.2 (39) | 14.7 (38) | 29.6 (168) | |||
| Post-secondary | 7.4 (11) | 5.4 (14) | 9.0 (51) | |||
| Hospitalized within past 3 years | 13.5 (20) | 12.5 (32) | 12.2 (69) | |||
| Injected drugs or smoked opium within past 3 years | 0 | 0 | 2.1 (12) | |||
| CD4 (cells/μl) | ||||||
| <200 | 23.2 (33) | 10.7 (27) | 30.3 (167) | |||
| 200–500 | 41.6 (59) | 55.0 (138) | 48.8 (269) | |||
| >500 | 35.2 (50) | 34.3 (86) | 20.9 (115) | |||
| VL (copies/ml) | ||||||
| <10 000 | 36.2 (54) | 56.1 (148) | 20.9 (119) | |||
| 10 000–100 000 | 47.7 (71) | 34.8 (92) | 58.9 (335) | |||
| >100 000 | 16.1 (24) | 901 (24) | 20.2 (115) | |||
Data were not available on all subjects; percentages reflect a changing denominator.
During follow-up, 269 (49.4%) of the men and 65 (25.7%) of the women died. The estimated median time to death for seroconverters in this cohort was 7.8 (7.0–9.1) years (Table 2). A comparison between the first-quartile survival for seroconverter men and women revealed no difference: 5.8 (5.3–6.5) years and 6.3 (5.0–unbounded) years, respectively. When the male blood bank HIV-prevalence data were used in conjunction with the first documented HIV-positive date, the estimated male median survival was 8.3 (7.5–9.1) years (n = 560). Sensitivity analyses based on varying the assumptions used for modelling the survival based on seroconversion probabilities among blood donors resulted in very similar survival times.
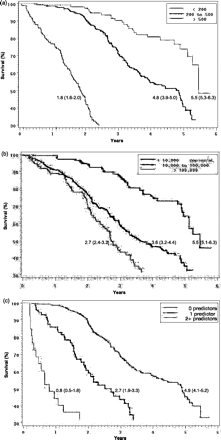
Only 2.2 (2.0–2.5) years after enrolment, 25% of the men were deceased, and by 3.9 (3.6–4.8) years, 50% had died. The 25% mortality among women, who, by study design, were infected after their husbands, was reached at 4.4 (3.7–5.6) years after enrolment. The median time from enrolment to death was analysable for men and varied significantly across categories of enrolment CD4 count (Figure 1a). Men with enrolment CD4 counts >500 cells/μl lived on average 4.9 years longer after enrolment than those with CD4 counts <200 cells/μl. Men and women with CD4 counts <200 cells/μl had hazards of death ∼10 times greater than that for men and women with CD4 counts >500 cells/μl (Tables 3 and 4). Participants with VLs > 100 000 had hazards of death about three times greater than that of subjects with VLs < 10 000 copies/ml (Tables 3 and 4). Men with VLs > 100 000 copies/ml had significantly shorter median survival beyond enrolment, averaging ∼3 years less, than men with VLs < 10 000 copies/ml (Figure 1b). The survival time beyond enrolment varied markedly by age, with a 30–60% increased risk of death for each 10-year increment. When combined with age at enrolment in a multivariate model satisfying the proportional hazard assumption, the CD4 count and VL provided significant, independent predictability of HIV survival.
Survival probability for men by years since entrance into follow-up stratified by (a) CD4 count, (b) viral load, and (c) number of resource-limited predictors (TLC < 1200, anaemia, and low BMI)
Survival time from HIV infection of men and women in Thailand
| . | . | . | . | . | . | Survival (%) at . | . | |
|---|---|---|---|---|---|---|---|---|
| Subtype . | Cohort . | N . | Age . | 25% mortality . | 50% mortality . | 5 years . | 7 years . | |
| Ea | Chiang Mai (Heterosexual couples) | 149 SC | 27 (15–50) | 5.8 (5.2–6.3) | 7.8 (7.0–9.1) | 87.3 | 57.1 | |
| 58 SC women | 22 (15–50) | 6.3 (5.0–d) | N/A | 86.9 | N/A | |||
| 91 SC men | 30 (21–48) | 5.8 (5.3–6.5) | 7.8 (7.0–9.1) | 88.3 | 58.3 | |||
| 569 SC + SP men | 29 (18–56) | 8.3 (7.5–9.1)e | 55.9e | |||||
| Eb | Chiang Rai (Female sex workers) | 34 SC | – | 5.8 years | – | 77.8 | – | |
| 194 SC + SP | 20.9 (mean) | 6.0 years | – | 85.9 | 68.7 | |||
| Ec | Bangkok (Royal Thai Army—men) | 235 SC | (21–23) | – | 7.0 (6.8–7.2) | 82.2 | ||
| . | . | . | . | . | . | Survival (%) at . | . | |
|---|---|---|---|---|---|---|---|---|
| Subtype . | Cohort . | N . | Age . | 25% mortality . | 50% mortality . | 5 years . | 7 years . | |
| Ea | Chiang Mai (Heterosexual couples) | 149 SC | 27 (15–50) | 5.8 (5.2–6.3) | 7.8 (7.0–9.1) | 87.3 | 57.1 | |
| 58 SC women | 22 (15–50) | 6.3 (5.0–d) | N/A | 86.9 | N/A | |||
| 91 SC men | 30 (21–48) | 5.8 (5.3–6.5) | 7.8 (7.0–9.1) | 88.3 | 58.3 | |||
| 569 SC + SP men | 29 (18–56) | 8.3 (7.5–9.1)e | 55.9e | |||||
| Eb | Chiang Rai (Female sex workers) | 34 SC | – | 5.8 years | – | 77.8 | – | |
| 194 SC + SP | 20.9 (mean) | 6.0 years | – | 85.9 | 68.7 | |||
| Ec | Bangkok (Royal Thai Army—men) | 235 SC | (21–23) | – | 7.0 (6.8–7.2) | 82.2 | ||
SC, seroconverters; SP, seroprevalent subjects.
Current study population.
Reference 12.
Reference 13.
Upper limit of 95% CI is unbounded.
Results calculated using model that incorporated date last known HIV2; date first documented HIV+; and monthly, male HIV-prevalence at participating blood banks.
Survival time from HIV infection of men and women in Thailand
| . | . | . | . | . | . | Survival (%) at . | . | |
|---|---|---|---|---|---|---|---|---|
| Subtype . | Cohort . | N . | Age . | 25% mortality . | 50% mortality . | 5 years . | 7 years . | |
| Ea | Chiang Mai (Heterosexual couples) | 149 SC | 27 (15–50) | 5.8 (5.2–6.3) | 7.8 (7.0–9.1) | 87.3 | 57.1 | |
| 58 SC women | 22 (15–50) | 6.3 (5.0–d) | N/A | 86.9 | N/A | |||
| 91 SC men | 30 (21–48) | 5.8 (5.3–6.5) | 7.8 (7.0–9.1) | 88.3 | 58.3 | |||
| 569 SC + SP men | 29 (18–56) | 8.3 (7.5–9.1)e | 55.9e | |||||
| Eb | Chiang Rai (Female sex workers) | 34 SC | – | 5.8 years | – | 77.8 | – | |
| 194 SC + SP | 20.9 (mean) | 6.0 years | – | 85.9 | 68.7 | |||
| Ec | Bangkok (Royal Thai Army—men) | 235 SC | (21–23) | – | 7.0 (6.8–7.2) | 82.2 | ||
| . | . | . | . | . | . | Survival (%) at . | . | |
|---|---|---|---|---|---|---|---|---|
| Subtype . | Cohort . | N . | Age . | 25% mortality . | 50% mortality . | 5 years . | 7 years . | |
| Ea | Chiang Mai (Heterosexual couples) | 149 SC | 27 (15–50) | 5.8 (5.2–6.3) | 7.8 (7.0–9.1) | 87.3 | 57.1 | |
| 58 SC women | 22 (15–50) | 6.3 (5.0–d) | N/A | 86.9 | N/A | |||
| 91 SC men | 30 (21–48) | 5.8 (5.3–6.5) | 7.8 (7.0–9.1) | 88.3 | 58.3 | |||
| 569 SC + SP men | 29 (18–56) | 8.3 (7.5–9.1)e | 55.9e | |||||
| Eb | Chiang Rai (Female sex workers) | 34 SC | – | 5.8 years | – | 77.8 | – | |
| 194 SC + SP | 20.9 (mean) | 6.0 years | – | 85.9 | 68.7 | |||
| Ec | Bangkok (Royal Thai Army—men) | 235 SC | (21–23) | – | 7.0 (6.8–7.2) | 82.2 | ||
SC, seroconverters; SP, seroprevalent subjects.
Current study population.
Reference 12.
Reference 13.
Upper limit of 95% CI is unbounded.
Results calculated using model that incorporated date last known HIV2; date first documented HIV+; and monthly, male HIV-prevalence at participating blood banks.
Univariate and multivariate HRs for HIV-related death by CD4 and VL
| . | Men . | . | Women . | . | ||||
|---|---|---|---|---|---|---|---|---|
| Variable . | Unadjusted HRa . | Adjusted HRb . | Unadjusted HRa . | Adjusted HRb . | ||||
| CD4 (cells/μl) | ||||||||
| >500 | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| 200–500 | 2.6 (1.7–4.0) | 2.2 (1.5–3.5) | 2.4 (1.2–4.8) | 2.4 (1.2–4.7) | ||||
| <200 | 12.9 (8.3–20.1) | 10.9 (7.0–17.1) | 12.4 (5.8–26.5) | 11.4 (5.2–24.7) | ||||
| VL (copies/ml) | ||||||||
| <10 000 | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| 10 000–100 000 | 2.6 (1.8–3.8) | 2.0 (1.3–2.9) | 2.0 (1.1–3.5) | 1.7 (0.9–3.0) | ||||
| >100 000 | 3.9 (2.6–6.0) | 3.0 (2.0–4.7) | 3.5 (1.8–7.1) | 3.4 (1.7–7.0) | ||||
| . | Men . | . | Women . | . | ||||
|---|---|---|---|---|---|---|---|---|
| Variable . | Unadjusted HRa . | Adjusted HRb . | Unadjusted HRa . | Adjusted HRb . | ||||
| CD4 (cells/μl) | ||||||||
| >500 | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| 200–500 | 2.6 (1.7–4.0) | 2.2 (1.5–3.5) | 2.4 (1.2–4.8) | 2.4 (1.2–4.7) | ||||
| <200 | 12.9 (8.3–20.1) | 10.9 (7.0–17.1) | 12.4 (5.8–26.5) | 11.4 (5.2–24.7) | ||||
| VL (copies/ml) | ||||||||
| <10 000 | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| 10 000–100 000 | 2.6 (1.8–3.8) | 2.0 (1.3–2.9) | 2.0 (1.1–3.5) | 1.7 (0.9–3.0) | ||||
| >100 000 | 3.9 (2.6–6.0) | 3.0 (2.0–4.7) | 3.5 (1.8–7.1) | 3.4 (1.7–7.0) | ||||
The unadjusted analysis is limited to subjects who have data on all factors in the adjusted analysis.
Multivariate model adjusted for CD4 count, VL, and age at enrolment.
Univariate and multivariate HRs for HIV-related death by CD4 and VL
| . | Men . | . | Women . | . | ||||
|---|---|---|---|---|---|---|---|---|
| Variable . | Unadjusted HRa . | Adjusted HRb . | Unadjusted HRa . | Adjusted HRb . | ||||
| CD4 (cells/μl) | ||||||||
| >500 | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| 200–500 | 2.6 (1.7–4.0) | 2.2 (1.5–3.5) | 2.4 (1.2–4.8) | 2.4 (1.2–4.7) | ||||
| <200 | 12.9 (8.3–20.1) | 10.9 (7.0–17.1) | 12.4 (5.8–26.5) | 11.4 (5.2–24.7) | ||||
| VL (copies/ml) | ||||||||
| <10 000 | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| 10 000–100 000 | 2.6 (1.8–3.8) | 2.0 (1.3–2.9) | 2.0 (1.1–3.5) | 1.7 (0.9–3.0) | ||||
| >100 000 | 3.9 (2.6–6.0) | 3.0 (2.0–4.7) | 3.5 (1.8–7.1) | 3.4 (1.7–7.0) | ||||
| . | Men . | . | Women . | . | ||||
|---|---|---|---|---|---|---|---|---|
| Variable . | Unadjusted HRa . | Adjusted HRb . | Unadjusted HRa . | Adjusted HRb . | ||||
| CD4 (cells/μl) | ||||||||
| >500 | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| 200–500 | 2.6 (1.7–4.0) | 2.2 (1.5–3.5) | 2.4 (1.2–4.8) | 2.4 (1.2–4.7) | ||||
| <200 | 12.9 (8.3–20.1) | 10.9 (7.0–17.1) | 12.4 (5.8–26.5) | 11.4 (5.2–24.7) | ||||
| VL (copies/ml) | ||||||||
| <10 000 | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| 10 000–100 000 | 2.6 (1.8–3.8) | 2.0 (1.3–2.9) | 2.0 (1.1–3.5) | 1.7 (0.9–3.0) | ||||
| >100 000 | 3.9 (2.6–6.0) | 3.0 (2.0–4.7) | 3.5 (1.8–7.1) | 3.4 (1.7–7.0) | ||||
The unadjusted analysis is limited to subjects who have data on all factors in the adjusted analysis.
Multivariate model adjusted for CD4 count, VL, and age at enrolment.
Univariate and multivariate RRs for HIV-related deaths by predictors available in resource-limited settings
| . | Men . | . | Women . | . | ||||
|---|---|---|---|---|---|---|---|---|
| Variable . | Unadjusted RRa . | Adjusted RRb . | Unadjusted RRa . | Adjusted RRc . | ||||
| Total lymphocyte count (cells/μl) | ||||||||
| <1200 | 4.6 (3.3–6.4) | 3.6 (2.5–5.1) | 4.4 (2.0–9.9) | 3.9 (1.7–8.8) | ||||
| ≥1200 | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Anaemicd | ||||||||
| Yes | 4.0 (2.7–5.9) | 2.6 (1.7–4.0) | 1.3 (0.7–2.4) | N/A | ||||
| No | 1.0 | 1.0 | 1.0 | |||||
| BMI (kg/m2) | ||||||||
| <18.5 | 2.7 (1.7–4.2) | 2.0 (1.2–3.3) | 1.4 (0.7–2.8) | N/A | ||||
| ≥18.5 | 1.0 | 1.0 | 1.0 | |||||
| . | Men . | . | Women . | . | ||||
|---|---|---|---|---|---|---|---|---|
| Variable . | Unadjusted RRa . | Adjusted RRb . | Unadjusted RRa . | Adjusted RRc . | ||||
| Total lymphocyte count (cells/μl) | ||||||||
| <1200 | 4.6 (3.3–6.4) | 3.6 (2.5–5.1) | 4.4 (2.0–9.9) | 3.9 (1.7–8.8) | ||||
| ≥1200 | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Anaemicd | ||||||||
| Yes | 4.0 (2.7–5.9) | 2.6 (1.7–4.0) | 1.3 (0.7–2.4) | N/A | ||||
| No | 1.0 | 1.0 | 1.0 | |||||
| BMI (kg/m2) | ||||||||
| <18.5 | 2.7 (1.7–4.2) | 2.0 (1.2–3.3) | 1.4 (0.7–2.8) | N/A | ||||
| ≥18.5 | 1.0 | 1.0 | 1.0 | |||||
Unadjusted analysis of factors included in adjusted analysis is limited to subjects who have data on all factors in the adjusted analysis.
Multivariate model adjusted for low TLC, anaemia, low BMI, and age at enrolment.
Multivariate model adjusted for low TLC and age at enrolment.
Anaemia was defined as a haemoglobin level <11.0 gm/dl for women and <12.0 gm/dl for men.
Univariate and multivariate RRs for HIV-related deaths by predictors available in resource-limited settings
| . | Men . | . | Women . | . | ||||
|---|---|---|---|---|---|---|---|---|
| Variable . | Unadjusted RRa . | Adjusted RRb . | Unadjusted RRa . | Adjusted RRc . | ||||
| Total lymphocyte count (cells/μl) | ||||||||
| <1200 | 4.6 (3.3–6.4) | 3.6 (2.5–5.1) | 4.4 (2.0–9.9) | 3.9 (1.7–8.8) | ||||
| ≥1200 | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Anaemicd | ||||||||
| Yes | 4.0 (2.7–5.9) | 2.6 (1.7–4.0) | 1.3 (0.7–2.4) | N/A | ||||
| No | 1.0 | 1.0 | 1.0 | |||||
| BMI (kg/m2) | ||||||||
| <18.5 | 2.7 (1.7–4.2) | 2.0 (1.2–3.3) | 1.4 (0.7–2.8) | N/A | ||||
| ≥18.5 | 1.0 | 1.0 | 1.0 | |||||
| . | Men . | . | Women . | . | ||||
|---|---|---|---|---|---|---|---|---|
| Variable . | Unadjusted RRa . | Adjusted RRb . | Unadjusted RRa . | Adjusted RRc . | ||||
| Total lymphocyte count (cells/μl) | ||||||||
| <1200 | 4.6 (3.3–6.4) | 3.6 (2.5–5.1) | 4.4 (2.0–9.9) | 3.9 (1.7–8.8) | ||||
| ≥1200 | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Anaemicd | ||||||||
| Yes | 4.0 (2.7–5.9) | 2.6 (1.7–4.0) | 1.3 (0.7–2.4) | N/A | ||||
| No | 1.0 | 1.0 | 1.0 | |||||
| BMI (kg/m2) | ||||||||
| <18.5 | 2.7 (1.7–4.2) | 2.0 (1.2–3.3) | 1.4 (0.7–2.8) | N/A | ||||
| ≥18.5 | 1.0 | 1.0 | 1.0 | |||||
Unadjusted analysis of factors included in adjusted analysis is limited to subjects who have data on all factors in the adjusted analysis.
Multivariate model adjusted for low TLC, anaemia, low BMI, and age at enrolment.
Multivariate model adjusted for low TLC and age at enrolment.
Anaemia was defined as a haemoglobin level <11.0 gm/dl for women and <12.0 gm/dl for men.
In univariate analysis, the CD8 level had a significant (P < 0.001), direct relationship with survival; however, when adjusted for CD4 count, CD8 was no longer significant. Natural killer cell levels, while associated with survival in univariate analysis among women, became insignificant when adjusted for CD4 count and VL. In univariate analysis, each 10-unit increase in neopterin level increased the risk of death almost 2-fold for the men [Hazard ratio (HR)(CI) = 2.0 (1.8–2.2) P < 0.001] and by 24% for the women [1.2 (1.1–1.4) P < 0.001]. Each 1-unit increase in beta-2 microglobulin level increased the risk of death ∼20% for the men [1.2 (1.2–1.2) P < 0.001] and 10% for women [1.1, (1.0–1.2) P = 0.009]. For men only, neopterin and beta-2 microglobulin remained independent predictors of survival when adjusted for CD4 count and VL; the adjusted HRs are [1.6 (1.5–1.8) P < 0.001] and [1.1 (1.1–1.2) P < 0.001], respectively.
Men and women who met the December 2003 revised WHO guidelines for onset of ART therapy in resource-limited settings where CD4 testing is unavailable (i.e. WHO HIV disease stage 3 or 4 or the combination of WHO disease stage 2 and TLC < 1200) had a greater hazard of death during follow-up (HR (CI): men = 2.5 (1.8–3.5), women = 1.6 (0.8–3.0)), although the increase for women did not reach statistical significance. The previous WHO guidelines for onset of ART therapy when CD4 count is unavailable published in April 2002 (i.e. WHO HIV disease stage 4 or the combination of WHO disease stage 2 or 3 and TLC < 1200) had higher hazards for death (men: 8.6 (5.1–14.5), women: 33.7 (6.9–164.2)). TLC < 1200 cells/μl was significantly associated with survival among men and women (P < 0.001) (Table 5). Anaemia—defined as a haemoglobin level <12 g/dl in men and haemoglobin level <11 g/dl in women—and low BMI (< 18.5 kg/m2) were significantly associated with decreased survival for men (P < 0.001) but not for women. For women, severe anaemia (<9 g/dl) was significantly associated with decreased survival [2.8 (1.1–7.0)]. Among men, all factors—including age, TLC, anaemia, and low BMI—remained significant predictors of survival when included in a multivariate model satisfying the proportional hazard assumption (Table 5). Survival from the time of enrolment was progressively shorter for men who had more, as opposed to fewer, of these survival predictors [TLC < 1200, low BMI, and anaemia] (Figure 1C); men with two or more predictors had a median survival of 0.8 (0.5–1.8) years, compared with 2.7 (1.9–3.3) years for one predictor, and 4.9 (4.1–5.2) years for no predictors. A similar, although statistically insignificant, trend was seen for number of predictors among HIV-infected women and the time until one-quarter were deceased. Having one or more of these three predictors had a sensitivity of 49.3% and specificity of 90.2% for CD4 < 200 among men.
The alternative markers of HIV disease stage (TLC, anaemia, and BMI) along with alternative markers associated with immune activation (beta-2 microglobulin and neopterin) were included as continuous, equally scaled variables in a hazards regression model adjusted for age at enrolment. All variables remained significant with TLC and neopterin having the strongest hazards for death among men (TLC = 0.7 (0.7–0.8), neopterin = 1.5 (1.3–1.7)). CD4 count and VL were included in the model to compare the relative strengths of the standard and proposed resource-limited predictors. With CD4 and VL included, TLC (after adjustment for the significant TLC–CD4 correlation), neopterin, and BMI remained significant among the resource-limited predictors (TLC = 0.8 (0.7–1.0), neopterin = 1.3 (1.2–1.5), BMI = 0.8 (0.6–1.0)).
A subanalysis was performed on deceased, male subjects (n = 269) of which 173 (66%) had follow-up data provided by either a spouse or caregiver on the use of antiretrovirals or medicines by the deceased for opportunistic infections (OI). Among the 173 men, 23 (13.3%) had reported antiretroviral use, which was always monotherapy, and 71 (41.0%) had reported use of medicines for opportunistic infections. There was no difference in median survival between subjects with reported use of ART or OI medications and those with reported lack of use, whether stratified by CD4 or VL or modelled in aggregate.
Conclusions
From 1993 to 1999, almost half the men and one-quarter of the HIV-positive women who enrolled in the Thai heterosexual couples study died. The median time from seroconversion to death was 7.8 years. This median survival time is similar to that reported in subtype E studies among commercial sexual workers12 and young Thai men,13 but appears more rapid than that of the analogous 24–35 year age group from the CASCADE database of 13 030 HIV-infected, antiretroviral naive individuals from cohorts in developed countries where subtype B predominates [10.9 (10.6–11.3) years].14 The difference in survival time between Thai HIV-positive subtype E populations and the Western HIV-positive populations studied in the CASCASE meta-analysis is not likely due to the overall lower life expectancy in Thailand,15 since AIDS mortality primarily affects young or middle-aged populations. Furthermore, non-AIDS mortality was not included in our mortality rate calculations. The apparent survival difference between Thai subtype E cohorts and the CASCADE combined population may depend on access to adequate medicine and prophylactic regimens as well as population prevalence of opportunistic infections and other infectious diseases, nutrition, host immunogenetics, and other differences in these populations.
In Thailand, comparisons of subtype E to subtype B HIV progression are confounded by the almost exclusive different modes of transmission (heterosexual vs IDU, respectively) between current cohorts with sufficient follow-up. However, some Thai studies have been reported: a study among in-patients found only an increased prevalence of extrapulmonary cryptococcosis among subtype E, compared with subtype B, infected Thai patients16 another study found shortened survival after AIDS diagnosis among those who acquired HIV through injection drug use (primary mode of transmission for subtype B) compared with heterosexual contact (primary mode of transmission for subtype E), however, they reported that both groups had shorter survival times after AIDS diagnosis than patients in developed countries.17 In order to answer the question of HIV-subtype effect on HIV progression in Thailand, it will be necessary for a cohort study to obtain sufficient follow-up on HIV-subtype B and HIV-subtype E positive Thais in whom the date of infection is known (seroconverters), who are infected by the same route of transmission.
Similar to subtype B, enrolment CD4 count and VL were powerful, independent predictors of time until death from subtype E infection. Other immune covariates were either not predictive after correction for CD4 count (e.g. CD8 count), not easily obtained by routine testing (e.g. B-2 microglobulin and neopterin), or both (e.g. natural killer cell count). The WHO criteria for the onset of antiretroviral therapy in resource-limited settings where CD4 testing is unavailable, although having a low sensitivity in this cohort, identified subjects with a high risk of death. A combination of TLC < 1200, low BMI, and anaemia could easily be assessed in resource-limited settings and was highly predictive for men of their survival time until death, with the survival time decreasing significantly with each additional predictor. Having one or more of these predictors identified half of the men with CD4 count <200, while maintaining a high specificity. The inability of anaemia and low BMI to predict disease progression among women in the study was probably complicated by the Thai female prevalence of anaemia and low BMI due to non-HIV related causes. The largest analysis of anaemia in HIV infection to date found the prevalence of anaemia to be 46–59% higher among HIV-infected patients with AIDS than in those without AIDS.18 Furthermore, anaemia predicts clinical prognosis independent of CD4 count and VL.19 Spacek et al. suggested the combination of anaemia with a TLC from 1200 to 2000 cells/mm3 in addition to TLC < 1200 cells/mm3 for increased sensitivity and specificity above TLC < 1200 alone for CD4 < 200 cells/mm3.20 In the current study, TLC < 1200 or the combination of TLC from 1200 to 2000 and anaemia led to a higher HR and lower survival among women compared with anaemia or TLC <2000 alone. The same was true when anaemia was replaced with low BMI in the above algorithm. Reports have suggested the utility of TLC, anaemia, and low BMI as predictors of either CD4 count <200 or HIV survival.18–22 This report indicates that combining these factors can be highly predictive for decreased survival among Thai persons infected with subtype E.
This analysis had some important limitations. First, the exact seroconversion date was unknown for most of the seroprevalent subjects. Although we used methods to accommodate the uncertainty in the seroconversion date, the window of uncertainty could not be <2 years on any analysis set with a sufficient sample size. Incorporating individuals with prevalent HIV infection in analyses of AIDS-related event times has been a common practice since the beginning of the epidemic. Because such analyses raise the problem of prevalent cohort bias, analytical techniques have been developed to deal with the unobserved time of seroconversion for prevalent subjects.10,11 The method we use in this paper is an example of this type of approach, and represents a refinement of previously published methods that incorporates both regression effects and smoothing. In our opinion, the assumption that the distribution of seroconversion dates follows the observed prevalence of infection from the blood bank data is very reasonable and makes optimal use of the available information. The weaker assumption that this distribution was uniform did not affect the results markedly. The use of the local blood banks for identification of HIV-positive men should not have biased the survival estimates to be shorter than in Western populations. In general, blood donors tend to be healthy, and although some blood donors early in the epidemic may have been IDUs receiving payment, few subjects in our study had a history of injection drug use. With regard to generalizability, this cohort may be better than currently available cohorts for estimating survival among the general, non-IDU and non-CSW, Thai population. Other strengths of this analysis are its relatively large subtype E population, the study's exclusive focus on heterosexual couples, and the availability of extensive clinical and laboratory prognostic markers.
The time period from infection with HIV subtype E to death appears to be shorter among this heterosexual Thai population than among subtype B infected populations in the pre-HAART era. However, similar to subtype B, both CD4 count and VL are independent, strong predictors of the time until death from HIV subtype E. When CD4 count and VL measurements are unavailable, patients at high risk for shortened HIV survival may be identified by the presence of a combination of low TLC, low BMI, and anaemia. The use of these accessible markers for decision-making purposes with regard to initiating antiretroviral treatment in resource-limited settings merits consideration.
The authors would like to thank Sonya Bowens and Antika Wongthanee for their data management contributions to the Thai heterosexual couples study. Support for the baseline (CSA-99-259) and follow-up (CSA-00-278) studies was provided by CONRAD (Contraceptive Research and Development) Program, Eastern Virginia Medical School, under a Cooperative Agreement with the United States Agency for International Development (USAID) (HRN-A-00-98-00020-00) which in turn receives funds for AIDS research from an interagency agreement with the Division of Reproductive Health, Centers for Disease Control and Prevention (CDC). The views expressed by the authors do not necessarily reflect the views of USAID, CDC or CONRAD.
References
Ruxrungtham K, Phanuphak P. Update on HIV/AIDS in Thailand.
Weniger BG, Limpakarnjanarat K, Ungchusak K et al. The epidemiology of HIV infection and AIDS in Thailand
Kaslow RA, McNicholl JM. Genetic determinants of HIV-1 infection and its manifestations.
Webster HK, Pattanapanyasat K, Phanupak P et al. Lymphocyte immunophenotype reference ranges in health Thai adults: Implications for management of HIV/AIDS in Thailand.
Piwoz EG, Preble EA. HIV/AIDS and Nutrition: A review of the literature and recommendations for nutritional care and support in sub-Saharan Africa. Washington DC: Academy for Educational Development,
Sheih B, Chang MJ, Ko WC et al. Effects of multiple virus coinfections on disease progression in HIV-positive patients.
Nagachinta T, Duerr A, Suriyanon V et al. Risk factors for HIV-1 transmission from HIV-seropositive male blood donors to their regular female partners in northern Thailand.
Scaling up antiretroviral therapy in resource-limited settings: Treatment guidelines for a public health approach.
Pau CP, Lee-Thomas S, Auwanit W et al. Highly specific V3 peptide enzyme immunoassay for serotyping HIV-1 specimens from Thailand.
Geskus RB. Methods for estimating the AIDS incubation time distribution when date of seroconversion is censored.
Law CG, Brookmeyer R. Effects of midpoint imputation on the analysis of doubly censored-data.
Kilmarx PH, Limpakarnjanarat K, Kaewkungwal J et al. Disease progression and survival with human immunodeficiency virus type 1 subtype E infection among female sex workers in Thailand.
Rangsin R, Chiu J, Khamboonruang C et al. The natural history of HIV-1 Infection in Young Thai men after seroconversion.
CASCADE Collaboration. Time from HIV-1 seroconversion to AIDS and death before widespread use of highly-active antiretroviral therapy: a collaborative re-analysis. Collaborative Group on AIDS Incubation and HIV Survival including the CASCADE EU Concerted Action on SeroConversion to AIDS and Death in Europe.
The World Health Report
Amornkul PN, Tansuphasawadikul S, Limpakarnjanarat K et al. Clinical disease associated with HIV-1 subtype B, and E infection among 2104 patients in Thailand.
Kitayaporn D, Tansuphaswadikul S, Lohsomboon P et al. Survival of AIDS Patients in the emerging epidemic in Bangkok, Thailand.
Sullivan PS, Hanson DL, Chu SY et al. Adult/Adolescent Spectrum of Disease Group. Epidemiology of anemia in human immunodeficiency virus (HIV)-infected persons: results from the multistate Adult and Adolescent Spectrum of HIV Disease Surveillance Project.
Mocroft A, Kirk O, Barton SE et al. Anemia is an independent predictive marker for clinical prognosisi in HIV-infected patients from across Europe.
Spacek LA, Griswold M, Quinn TC et al. Total lymphocyte count and hemoglobin combined in an algorithm to initiate the use of highly active antiretroviral therapy in resource-limited settings.
Beck EJ, Kupek CJ, Gompels MM et al. Correlation between total and CD4 lymphocyte counts in HIV infection: not making the good an enemy of the not so perfect.