-
PDF
- Split View
-
Views
-
Cite
Cite
Corinne E. Rouquette-Loughlin, Jacqueline T. Balthazar, William M. Shafer, Characterization of the MacA–MacB efflux system in Neisseria gonorrhoeae, Journal of Antimicrobial Chemotherapy, Volume 56, Issue 5, November 2005, Pages 856–860, https://doi.org/10.1093/jac/dki333
Close - Share Icon Share
Abstract
Objectives: A homologue of the MacA–MacB ABC transporter of Escherichia coli, which recognizes and exports macrolides, was identified in Neisseria gonorrhoeae. This study was undertaken to determine whether gonococci could use the MacA–MacB homologue to express decreased susceptibility to macrolides.
Methods: Techniques of DNA sequencing, gene cloning and expression of recombinant proteins in E. coli, gene mutation construction, transcriptional analysis and antimicrobial susceptibility testing were used in the study.
Results: Although the gonococcal MacA–MacB efflux pump enhanced bacterial resistance to macrolides when overexpressed in an E. coli background, its loss in a gonococcal clinical isolate only slightly decreased bacterial resistance to azithromycin and erythromycin. However, a mutation in the −10 sequence of the promoter used in macAB expression enhanced the macrolide resistance of gonococci that produced a defective MtrC–MtrD–MtrE pump, which also recognizes macrolides.
Conclusions: The results from this study indicate that gonococci can employ both the MacA–MacB and MtrC–MtrD–MtrE efflux pumps to develop resistance to macrolides, particularly if mutations develop in the promoter that drives transcription of macAB.
Introduction
Gonococcal resistance to host-derived antimicrobials and classical antibiotics can involve multidrug efflux pumps.1–11 Two major classes of drug efflux systems exist in bacteria.12,13 One class contains drug efflux pumps that use the proton motive force of the cytoplasmic membrane as their energy source [e.g. the resistance/nodulation/cell division (RND), major facilitator protein (MFP), small multidrug resistance (SMR) families14 and multidrug and toxic compound extrusion (MATE)15 families], whereas the other class consists of the ATP binding cassette (ABC) transporter family that uses ATP as their energy source.16
Given the continued problem of emerging antibiotic resistance in gonococci and the recognition that efflux pumps play an important role in bacterial resistance to antimicrobials in general, we are interested in determining the number and function of efflux pumps expressed by gonococci. We have studied the mtrCDE,2,3,6,7,9farAB5 and norM8 efflux pump protein systems and have reported on their capacity to export host-derived antimicrobial agents and antibiotics. The online availability of the Neisseria gonorrhoeae FA1090 genome sequence (www.genome.ou.edu, date last accessed 26 July 2005) allowed us to identify the farAB and norM efflux pumps. Unlike other bacteria (e.g. Pseudomonas aeruginosa17), genome sequence information predicts that gonococci encode a limited number of efflux pumps potentially involved in antibiotic resistance. In this respect, through an analysis of the FA1090 genome sequence, we detected two open reading frames (ORFs) that were predicted to encode homologues of the MacA and MacB proteins of Escherichia coli.18 These proteins, along with the TolC outer membrane protein, form an ABC transporter system that exports macrolides. MacA belongs to the membrane fusion protein (MFP) family, while MacB is an integral membrane protein with one ATP-binding domain. Because azithromycin has been used in some parts of the world to treat gonorrhoea and chlamydial infections,19 we have been interested in the mechanisms by which gonococci could develop decreased susceptibility to macrolides. We now report that the gonococcal MacA–MacB efflux pump can recognize macrolides and its action with the MtrC–MtrD–MtrE pump can contribute to the decreased susceptibility of gonococci to macrolides.
Materials and methods
Bacterial strains used and growth conditions
N. gonorrhoeae strain FA19 is a well-described laboratory-maintained strain2,20 that has been used extensively in studies dealing with the development and genetics of antibiotic resistance mechanisms in the gonococcus. It displays an antibiotic-susceptible phenotype. Strain BR54 is an antibiotic-hypersusceptible genetic derivative of strain FA140, which is in the FA19 genetic lineage, and contains a 10 bp deletion in the mtrD gene3 that results in the production of a truncated MtrD protein and as a consequence, an inactive MtrC–MtrD–MtrE efflux pump. Strain KH15 is a transformant of strain FA19 that has a single bp deletion in a 13 bp inverted repeat sequence within the mtrR promoter;2mtrR encodes a transcriptional repressor of mtrCDE. Owing to this mutation, the mtrCDE efflux pump-encoding operon is overexpressed resulting in an eightfold increase in gonococcal resistance to azithromycin and erythromycin. Clinical isolate 0722 [kindly provided by D. Trees, Centers for Disease Control and Prevention (CDC), Atlanta, Georgia, USA], which was isolated from a patient in Kansas City,4 contains the mtrR promoter mutation described above as well as a Correia Element in the DNA sequence that intervenes mtrR and mtrCDE. Strain 0722 expresses high-level resistance to macrolides. Gonococci were grown on GC agar base containing glucose and iron supplements at 37°C under 3.8% (v/v) CO2. E. coli strain JMZ120 (kindly provided by H. Nikaido), which has a deletion of the acrAB-encoded efflux operon, was used as a recipient strain to express the gonococcal macAB operon and was routinely grown on Luria-Bertani (LB) agar.
Antimicrobial susceptibility testing and isolation of spontaneous mutants
The susceptibility of N. gonorrhoeae and E. coli to macrolides and other antimicrobials was determined by the agar dilution method described previously.6,21 An efficiency of plating (EOP) assay, described previously,6,21 was also used to measure differences in susceptibility of gonococci to azithromycin and erythromycin. The EOP was calculated by dividing the cfu on antibiotic-containing GC agar base by the cfu growing on GC agar base without antibiotic. Human antimicrobial peptide LL-37, kindly provided by J. Pohl (Microchemical Facility of Emory University), was tested against gonococci as described previously.9
PCR and DNA sequencing
Chromosomal DNA from strain FA19 was prepared from gonococci6 and used in PCR to amplify the macA and macB genes as described previously.6 Oligonucleotide primers macA4 (5′-ATGGCAAAAATGATGAAATGGGC-3′) and macA5 (5′-TCAATCCTGCGCCAATGCATC-3′) were designed based on the FA1090 genome sequence (available at www.genome.ou.edu). Sequencing was performed through the Microchemical Facility of Emory University as described previously.3,8
Cloning and expression of the gonococcal macAB operon in E. coli
The macAB operon was amplified by PCR from a chromosomal DNA preparation of strain FA19 using oligonucleotide primers macA4 and macA5. The purified PCR product was cloned under the control of an arabinose-inducible promoter using the pBAD vector (Invitrogen). This construct and the vector control were individually transformed into competent E. coli strain JMZ12022 and transformants were selected on LB agar supplemented with ampicillin 100 mg/L.
Construction of an insertional mutation in macA and mtrD
The macA gene from strain FA19 was PCR-amplified from FA19 chromosomal DNA using primers macAF (5′-GGATGGTCTTATCTGAAGCC-3′) and macAR (5′-CATCAGCGTGGACTTGCCC-3′). The resulting PCR product was cloned into vector pCR2.1 (Invitrogen) as described by the manufacturer. The pBADΩmacA construct was then digested by the restriction enzyme HincII and a spectinomycin (SPT) resistance cassette was cloned in the HincII restriction site at nucleotide position 776 in the macA coding sequence. The resulting construct (pCR2.1ΩmacAΩSPT) was then transformed20,21 into gonococcal strain 0722 and transformants were selected on GCB agar containing spectinomycin 80 mg/L. The mtrD gene of strain 0722 was replaced by the mtrD::Km sequence by transformation using donor DNA from strain KH14 (as FA19 but mtrD::Km) as described previously.3
Construction of a mutation in the −10 sequence of the macAB promoter
A PCR-based mutagenic procedure was used to introduce a single nucleotide change in the −10 sequence of the macAB promoter from strain BR54. A mutagenic oligonucleotide primer (MacA6-10) that anneals 42 bp upstream of the translational start codon for macA and is within the promoter (5′-GGGACCTGCGGTATAATCCG–3′; the point mutation in the promoter is underlined) was used in a PCR along with oligonucleotide primer MacA5 (5′-TCAATCCTGCGCCAATGCATC-3′). The PCR product was purified and used to transform strain BR54 as described previously3 with transformants selected on GCB agar plates containing erythromycin 0.06 mg/L. Transformants were then screened for levels of susceptibility to macrolides. The promoter mutation in the BR54 transformants was identified by automated DNA sequencing on a PCR product that was amplified from chromosomal DNA using a forward oligonucleotide primer [MacA1 (5′-CGGCAATCATCAGCTCGAGC-3′)], which anneals 255 bp upstream of the macA translational start codon, and a reverse oligonucleotide primer [MacA3 (5′-GACGACATCGTGGTCAGCC-3′)], which anneals 792 bp downstream from the translational start codon.
RNA preparation, RT–PCR, and primer extension analysis
Total RNA of strain FA19 was prepared according to the method of Baker and Yanofsky.23 RT–PCR was performed as described previously.5,6 For RT–PCR analysis, oligonucleotide primer macB3 (5′-CTCAATCAAAAACTGCTGC-3′), which anneals within the macB gene, was used for the reverse transcription reaction. The 5′-end of the resulting cDNA was then amplified by PCR using primers macAF (5′-GGATGGTCTTATCTGAAGCC-3′) and macA3 (5′-GACGACATCGTGGTCAGCC-3′), both of which anneal within macA. As a control for genomic DNA contamination, the same RT–PCR was performed in the absence of RT. The transcriptional start point (TSP) for the macAB operon was determined by primer extension (PE) analysis. PE was performed as described previously6 using primer macA2 (5′-CCGGCTGATGCCGCCGCGCCTGACCGTTTC-3′). The TSP was determined by electrophoresis of the extension product on a 6% (w/v) DNA sequencing acrylamide gel adjacent to reference sequencing reaction lanes.
Results
Discovery of the macAB operon in gonococci
Kobayashi et al.18 identified a novel macrolide-specific ABC-type efflux transporter in E. coli, which they termed MacA–MacB. By using the predicted amino acid sequence of macA and macB gene products to probe the FA1090 gonococcal genome available online (www.genome.ou.edu and www.stdgen.lanl.gov, date last accessed 26 July 2005), we found two open reading frames (ORF) (NG1440 and NG1439, respectively) that could encode MacA and MacB homologues in gonococci. The DNA sequence of these ORFs was determined and the predicted MacA (accession number AY768531) and MacB (accession number AY768532) proteins of strain FA19 shared 35.1% and 51.1% of identity with the MacA and MacB proteins of E. coli, respectively (data not presented). A Pfam search (http://pfam.wustl.edu, date last accessed 26 July 2005) showed that MacA belonged to the HlyD family of proteins (which are secretion proteins) that includes members from the ABC transporter family. A Pfam search also showed that residues 33 to 218 (E-value = 2.5 × 10−54) placed MacB in the ABC transporter family.
The putative ATP-binding domain of the gonococcal protein was aligned with the corresponding domain of the MacB protein of E. coli.18 Interestingly, the ATP binding sites in the MacB protein from these bacteria are positioned identically (data not presented). It is relevant to note, however, that while 12/14 residues of the gonococcal protein are identical to those of E. coli, there is one radical change (A41→Q41); whether this change impacts ATP binding is not yet known.
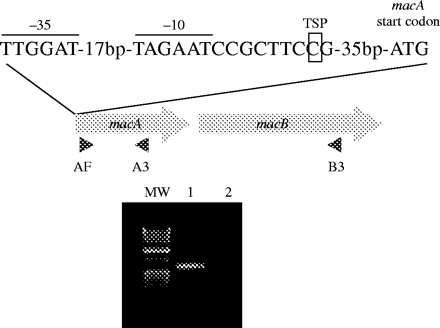
The macA and macB genes in gonococcal strains FA19 and FA1090 were separated by only 64 bp. Consequently, we hypothesized that they form a single transcriptional unit with a promoter element upstream of macA and used RT–PCR and PE analysis to test these ideas. For RT–PCR, an oligonucleotide primer that annealed at the 3′-end of the macB transcript was used to generate cDNA. The cDNA product was then subjected to PCR using primers within macA (Figure 1). Under these conditions, we obtained a nearly 750 bp (predicted size of 743 bp) RT–PCR product, which could only have been produced if macA and macB were co-transcribed (Figure 1). PE analysis revealed a TSP at a C nucleotide located 37 bp upstream of the ATG of the macA gene. Seven base pairs upstream of this TSP was a putative promoter with a near consensus −10 sequence (TAGAAT) and a −35 sequence (TTGGAT) spaced by an optimal5 17 bp (Figure 1). As described below, the G nucleotide in the −10 hexamer sequence is characteristic of the macAB promoter sequence in gonococci and meningococci and has a dampening effect on macAB transcription.
Transcription of the macAB operon. The −10 and −35 sequences of the macAB operon are represented by solid bars. The macA transcription start point (TSP) is boxed. Grey arrows represent the macA and macB genes, the filled triangles represent the primers used for the RT–PCR. An agarose gel showing the RT–PCR products is also presented. MW, molecular weights. Lane 1, the reverse transcription reaction was performed with primer B3 and the PCR was done with primers AF/A3. Lane 2, same as lane 1 but reverse transcriptase was omitted in the reverse transcription reaction.
In order to determine whether the neisserial macAB-encoded efflux pump could recognize macrolides, the entire coding sequence from strain FA19 was expressed in E. coli strain JMZ120, which lacks the AcrA and AcrB proteins of the AcrA–AcrB–TolC efflux pump that exports macrolides.22 We noted a twofold increase in erythromycin resistance and a fourfold increase in azithromycin resistance for strain JMZ120/pBADΩmacAB when it was grown in the presence of 0.2% of arabinose (Table 1). This result suggested that the MacA–MacB efflux pump of the gonococcus could functionally recognize and export macrolides.
MICs of N. gonorrhoeae strains with or without the MacAB efflux genes
| . | MIC (mg/L) . | . | |
|---|---|---|---|
| Antibiotic . | JMZ120/pBAD . | JMZ120/pBADΩmacAB . | |
| ERY | 4a | 4 | |
| ERY + 0.2% arabinose | 4 | 8 | |
| AZM | 1 | 1 | |
| AZM + 0.2% arabinose | 1 | 4 | |
| . | MIC (mg/L) . | . | |
|---|---|---|---|
| Antibiotic . | JMZ120/pBAD . | JMZ120/pBADΩmacAB . | |
| ERY | 4a | 4 | |
| ERY + 0.2% arabinose | 4 | 8 | |
| AZM | 1 | 1 | |
| AZM + 0.2% arabinose | 1 | 4 | |
ERY, erythromycin; AZM, azithromycin.
Representative values from three independent determinations.
MICs of N. gonorrhoeae strains with or without the MacAB efflux genes
| . | MIC (mg/L) . | . | |
|---|---|---|---|
| Antibiotic . | JMZ120/pBAD . | JMZ120/pBADΩmacAB . | |
| ERY | 4a | 4 | |
| ERY + 0.2% arabinose | 4 | 8 | |
| AZM | 1 | 1 | |
| AZM + 0.2% arabinose | 1 | 4 | |
| . | MIC (mg/L) . | . | |
|---|---|---|---|
| Antibiotic . | JMZ120/pBAD . | JMZ120/pBADΩmacAB . | |
| ERY | 4a | 4 | |
| ERY + 0.2% arabinose | 4 | 8 | |
| AZM | 1 | 1 | |
| AZM + 0.2% arabinose | 1 | 4 | |
ERY, erythromycin; AZM, azithromycin.
Representative values from three independent determinations.
Insertional inactivation of the macAB operon in gonococci
Since expression of the gonococcal macAB operon could decrease the susceptibility of E. coli to macrolides, we next asked if its loss in gonococci would result in increased macrolide susceptibility. However, our previous studies indicated that the mtrCDE-encoded efflux pump could also export macrolides1,10,11 and it may play a more predominant role in the development of gonococcal resistance to macrolides.4 In both strains FA19 and KH15, insertional-inactivation of macA (verified by PCR) did not change levels of gonococcal susceptibility to either azithromycin or erythromycin or hydrophobic antimicrobial agents (Triton X-100 and ethidium bromide) known to be exported by the MtrC–MtrD–MtrE efflux system (data not presented). Based on this observation, we hypothesized that the MacA–MacB efflux system might contribute to macrolide resistance in gonococcal strains expressing levels of macrolide resistance higher than that expressed by strains FA19 and KH15. Accordingly, we introduced the macA::SPT mutation into clinical isolate strain 0722, which was previously studied by Johnson et al.4 and reported to have an mtr-dependent mechanism of resistance to macrolides. Indeed, we have shown that insertional inactivation of the mtrD gene in this strain resulted in enhanced susceptibility (36- and 64-fold) to both azithromycin and erythromycin, respectively (data not presented). In contrast, the macA::SPT mutation resulted in a twofold decrease in the MIC of these macrolides (data not presented). In order to better assess the impact of the macA::SPT mutation in strain 0722, we performed an EOP using strains 0722 and 0722 macA::SPT on GC agar plates supplemented with erythromycin 4 mg/L or azithromycin 2 mg/L. The calculated EOP ratios (EOP of 0722/EOP of 0722 macA::SPT) indicated that parental strain 0722 was twofold more resistant to azithromycin and erythromycin than its isogenic transformant 0722 macA::SPT (data not presented).
Promoter mutation can enhance macAB expression and macrolide resistance
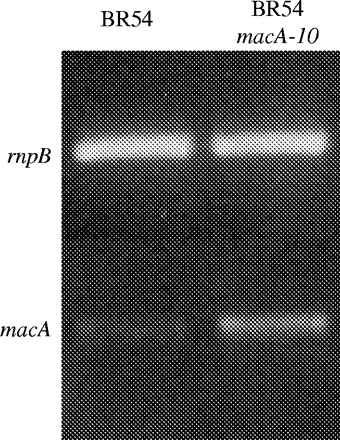
Based on the above-described results, we concluded that the MtrC–MtrD–MtrE efflux pump performs a significant role in mediating gonococcal resistance to macrolides and that its presence overshadows any contribution of the MacA–MacB efflux pump. Accordingly, we hypothesized that mutations that elevate expression of macAB would increase macrolide resistance in gonococci. As described above, we noted that the −10 hexamer (5′-TAGAAT-3′)of the macAB promoter in strain FA19 had a G nucleotide instead of the consensus T and hypothesized that this would negatively impact transcription. DNA sequencing of the macAB promoter from eight gonococcal strains (strains 0722, FA19, BR54, F62, UUI, EU75, FA889 and FA1090) by us or others (FA1090, www.genome.ou.edu) revealed that all contained a G residue at this position (data not presented). In order to test if the presence of the G nucleotide has a dampening effect on macAB expression, we employed a mutagenic PCR strategy to replace it with the consensus T nucleotide in the −10 sequence. The purified PCR product was then used to transform BR54, which produces a non-functional MtrC–MtrD–MtrE efflux pump3 and is hypersusceptible to macrolides.3,20 (For ethical reasons, we purposely used this strain as a recipient for transformation because we could not predict the extent of macrolide resistance that could be endowed by the mutation. We were concerned that if the mutation was placed in a wild-type or mtrR mutant, the level of resistance endowed by it may be higher than what has been previously observed in clinical isolates.) Transformants were selected on GCB agar plates containing erythromycin 0.12 mg/L and were then analysed for differences in susceptibility to azithromycin and erythromycin, compared with parental strain BR54. DNA from a representative transformant (BR54 macA-10) was prepared and the macAB promoter region was sequenced and the results confirmed that the G nucleotide in the −10 promoter had been replaced by a T nucleotide (data not presented). As shown in Table 2, BR54 macA-10 was 33.3- and 8.3-fold more resistant to azithromycin and erythromycin, respectively, compared with parental strain BR54; both strains were equally sensitive to antimicrobial peptide LL-37 and the non-ionic detergent Triton X-100, both of which are substrates for the MtrC–MtrD–MtrE efflux pump.3,9 In order to verify that this difference in macrolide susceptibility was due to changes in expression levels of macAB, RT–PCR was performed on total RNA extracted from the strains BR54 and BR54 macA-10. The results (Figure 2) confirmed that BR54 macA-10 expressed macAB at a level higher than BR54.
MICs (mg/L) of N. gonorrhoeae wild-type and macA strains
| . | N. gonorrhoeae strain . | . | |
|---|---|---|---|
| Antibiotic . | BR54 . | BR54 macA-10 . | |
| Azithromycin | 0.015 | 0.5 | |
| Erythromycin | 0.06 | 0.5 | |
| Triton X-100 | 12.5 | 12.5 | |
| LL-37 | 3.12 | 3.12 | |
| . | N. gonorrhoeae strain . | . | |
|---|---|---|---|
| Antibiotic . | BR54 . | BR54 macA-10 . | |
| Azithromycin | 0.015 | 0.5 | |
| Erythromycin | 0.06 | 0.5 | |
| Triton X-100 | 12.5 | 12.5 | |
| LL-37 | 3.12 | 3.12 | |
MICs (mg/L) of N. gonorrhoeae wild-type and macA strains
| . | N. gonorrhoeae strain . | . | |
|---|---|---|---|
| Antibiotic . | BR54 . | BR54 macA-10 . | |
| Azithromycin | 0.015 | 0.5 | |
| Erythromycin | 0.06 | 0.5 | |
| Triton X-100 | 12.5 | 12.5 | |
| LL-37 | 3.12 | 3.12 | |
| . | N. gonorrhoeae strain . | . | |
|---|---|---|---|
| Antibiotic . | BR54 . | BR54 macA-10 . | |
| Azithromycin | 0.015 | 0.5 | |
| Erythromycin | 0.06 | 0.5 | |
| Triton X-100 | 12.5 | 12.5 | |
| LL-37 | 3.12 | 3.12 | |
Levels of macAB transcript in strains BR54 and BR54 macA-10. Shown are the results from RT–PCR of total RNA extracted from these isogenic strains. The rnpB RT–PCR product is a control to show equivalent amounts of RNA in the reaction.
In order to verify that the increased resistance of BR54 macA-10 was due to overexpression of the macAB operon, the macA gene was insertionally inactivated by transformation using macA::SPT DNA. Analysis of the erythromycin susceptibility of these transformants revealed that they expressed a level of susceptibility similar to strain BR54 (data not presented). In addition to the mtrCDE- and macAB-encoded efflux pumps, gonococci also possess and express two additional loci (farAB and norM) that encode efflux pumps. Although neither the farAB nor norM efflux systems were found to modulate levels of macrolide susceptibility in wild-type strain FA19,5,8 we did not know whether overexpression of macAB in strain BR54, which produces an inactive MtrC–MtrD–MtrE efflux pump,3 in conjunction with these pumps was required for the increased macrolide resistance property of strain BR54macA-10. Accordingly, we inactivated the norM and farAB efflux pump loci in this strain using donor chromosomal DNA from strain EL15 (as FA19 but farB::Km) and strain CR288 (as strain FA19 but norM::Km). Both classes of transformants expressed levels of macrolide resistance identical to that of BR54macA-10 (data not presented), a result consistent with the ability of the MacA–MacB efflux system to modulate levels of gonococcal susceptibility to macrolides.
Discussion
Because of the emergence in some parts of the world4,10,11,24,25 of gonococci expressing decreased susceptibility to azithromycin, it is important to identify the mechanisms that could confer macrolide resistance in gonococci. Macrolide resistance in gonococci has been previously studied and mechanisms of efflux26 and ribosome protection27 have been shown to be important. Evidence has been presented that the MtrC–MtrD–MtrE efflux system in gonococci can recognize macrolides and that overexpression of the mtrCDE operon can enhance gonococcal resistance to this class of antibiotics.
It is our hypothesis that the gonococcal MacA–MacB efflux pump, which belongs to the ABC transporter family, also has the ability to recognize macrolides. However, its contribution to gonococcal resistance to macrolides is overshadowed by the presence of the MtrC–MtrD–MtrE efflux pump. This is also likely to be true for those strains containing the conjugative mef gene26 or rRNA methylase genes,27 which appear to provide levels of macrolide resistance greater than that of the mtrCDE efflux system. Our results indicate that this is due, in part, to an imperfect −10 sequence of the macAB promoter, which seems to be a common feature in this promoter among gonococci. We have shown that when the G nucleotide in the −10 hexamer was changed to the consensus T nucleotide, transcription of macAB is increased, resulting in enhanced macrolide resistance in gonococci (Figure 2). Although we have performed only limited sequence analysis of the macAB promoter region in gonococci (eight strains), the presence of this G nucleotide appears to be a common feature and its presence has a dampening impact on macAB transcription and levels of macrolide resistance mediated by the MacA–MacB efflux pump system.
We thank D. Trees for gonococcal strain 0722, H. Nikaido for E. coli strain JMZ120 and the Gonococcal Genome Sequencing Project (supported by NIH grant AI-3899) of the University of Oklahoma (B.A. Roe, S.P. Lin, L. Song, X. Yuan, S. Clifton, T. Ducey, L. Lewis and D. Dyer) for providing the sequence of the FA1090 genome online. This work was supported by PHS grants AI021150 and AI062755 (both to W. M. S.) from the NIH. W. M. S. is the recipient of a Senior Research Career Scientist Award from the VA Medical Research Service.
References
Shafer WM, Veal WL, Lee EH et al. Genetic organization and regulation of antimicrobial efflux systems possessed by Neisseria gonorrhoeae and Neisseria meningitidis.
Hagman KE, Shafer WM. Transcriptional control of the mtr efflux system of Neisseria gonorrhoeae.
Veal W, Yellen A, Balthazar JT et al. Loss of function mutations in the mtr efflux system of Neisseria gonorrhoeae.
Johnson SR, Sandul AL, Parekh M et al. Mutations causing in vitro resistance to azithromycin in Neisseria gonorrhoeae.
Lee E-H, Shafer WM. The farAB-encoded efflux pump mediates resistance of gonococci to long-chained antibacterial fatty acids.
Rouquette C, Harmon J, Shafer WM. Induction of the mtrCDE-encoded efflux pump system of Neisseria gonorrhoeae requires MtrA, an AraC-like protein.
Rouquette-Loughlin C, Stojiljkovic I, Hrobowski T et al. Inducible, but not constitutive, resistance of gonococci to hydrophobic agents due to the MtrC–MtrD–MtrE efflux pump requires TonB–ExbB–ExbD proteins.
Rouquette-Loughlin C, Dunham SA, Khun M et al. The NorM efflux pump of Neisseria gonorrhoeae and Neisseria meningitidis recognizes antimicrobial cationic compounds.
Shafer WM, Qu XD, Waring AJ et al. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family.
Zarantonelli L, Borthagaray G, Lee E-H et al. Decreased azithromycin susceptibility of Neisseria gonorrhoeae due to mtrR mutations.
Zarantonelli L, Borthagaray G, Lee E-H et al. Decreased susceptibility to azithromycin and erythromycin mediated by a novel mtrR promoter mutation in Neisseria gonorrhoeae.
Paulsen IT, Brown MH, Skurray RA. Proton-dependent multidrug efflux systems.
Brown MH, Paulsen IT, Skurray RA. The multidrug efflux protein NorM is a prototype of a new family of transporters.
Putman M, van Veen HW, Konings WN. Molecular properties of bacterial multidrug transporters.
Stover CK, Pham XQ, Erwin AL et al. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen.
Kobayashi N, Nishino K, Yamaguchi A. Novel macrolide-specific ABC-type efflux transporter in Escherichia coli.
Robinson AJ, Ridgway GL. Concurrent gonococcal and chlamydial infection: how best to treat.
Sarubbi FA, Sparling PF, Blackman E et al. Loss of low-level antibiotic resistance in Neisseria gonorrhoeae due to env mutations.
Shafer WM, Guymon LF, Lind I et al. Identification of an envelope mutation (env-10) resulting in increased antibiotic susceptibility and pyocin resistance in a clinical isolate of Neisseria gonorrhoeae.
Ma D, Alberti M, Lynch C et al. The local repressor AcrR plays a modulating role in the regulation of acrAB genes of Escherichia coli by global stress signals.
Baker RF, Yanofsky CF. The periodicity of RNA polymerase initiations: a new regulatory feature of transcription.
Arreaza L, Vasquez F, Alcala B et al. Emergence of gonococcal strains with resistance to azithromycin in Spain.
Dillon JA, Rubabaza JP, Benzaken AS et al. Reduced susceptibility to azithromycin and high percentages of penicillin and tetracycline resistance in Neisseria gonorrhoeae isolates from Manaus, Brazil, 1998.
Luna VA, Cousin S, Whittington WLH et al. Identification of the conjugative mef gene in clinical Acinetobacter jejunii and Neisseria gonorrhoeae isolates.
Author notes
1Department of Microbiology and Immunology, Emory University School of Medicine, Atlanta, GA 30322, USA; 2Laboratories of Microbial Pathogenesis, VA Medical Center, Decatur, GA 30033, USA