-
PDF
- Split View
-
Views
-
Cite
Cite
E. Guzman-Novoa, G. J. Hunt, R. E. Page, J. L. Uribe-Rubio, D. Prieto-Merlos, F. Becerra-Guzman, Paternal Effects on the Defensive Behavior of Honeybees, Journal of Heredity, Volume 96, Issue 4, July/August 2005, Pages 376–380, https://doi.org/10.1093/jhered/esi038
Close - Share Icon Share
Abstract
The defensive behavior of 52 hybrid honeybee (Apis mellifera L.) colonies from four sets of crosses was studied and compared with that of European and Africanized bee colonies. Colonies containing F1 hybrid workers were obtained through reciprocal crosses between European and Africanized bees. The total number of stings deposited by workers in a moving leather patch in 1 min was recorded. In each of the four sets of crosses, bees from hybrid colonies of Africanized paternity left more stings in leather patches than bees from hybrid colonies of European paternity. Results strongly suggest paternal effects of African origin increasing the defensive behavior of hybrid colonies. Although some degree of dominance was observed for high-defensive behavior in one of the four sets of crosses involving European paternity, most of the dominance effects reported in the literature appear to be the result of paternal effects. Several hypotheses to explain this phenomenon, as well as the implications of these effects on the fitness and breeding of honeybees are discussed.
Africanized honeybees are bees of mixed descent from African (Apis mellifera scutellata) and European honeybee races. Africanized bees originated in Brazil in 1956 (Kerr 1967) and have since spread throughout most of the Americas. They were first seen in the United States in 1990 (Sugden and Williams 1991). Africanized bees appear to have retained their highly defensive behavior and predominantly African genotype in most areas where they are found (Collins et al. 1982; Hall 1990, 1992; Hall and Muralidharan 1989; Lobo et al. 1989; Muralidharan and Hall 1990; Smith et al. 1989). Africanized bees also outcompete European races in warmer climates. Feral colonies in areas with Africanized bees soon display the African characteristics, a process called “Africanization.” Associated with this, there is an increase in the prevalence of mitochondria of African origin, a consequence of the loss of feral European bee colonies and the occupation of nest sites by feral Africanized bees (Loper 1997; Rubink et al. 1996; Smith et al. 1989). In our study area in Mexico, the frequency of African mitochondrial type in feral swarms increased from 29% to 96% in 5 years (Guzmán-Novoa and Page 1999).
Studies suggest that the Africanization process of managed colonies occurs mainly through matings between European queens from the “newly invaded” areas and Africanized drones from the “invading” population of feral colonies. The number of drones of African origin in congregation areas (open areas in the air where honeybees mate) may exceed 90% of the total drone population in Africanized areas (reviewed by Rinderer and Hellmich [1991]). Thus, if there were strong paternal effects influencing the defensive behavior of honeybees, this could at least partially explain why after more than 45 years since they originated, Africanized bees have rapidly altered the behavior of commercial colonies soon after the invasion of feral Africanized bees into a region through gene introgression into these populations.
The high defensive behavior of Africanized bees is heritable (Collins et al. 1984; Hunt et al. 1998; Stort 1975) and is reported to be dominant (DeGrandi-Hoffman et al. 1998; Guzmán-Novoa et al. 2002; Guzmán-Novoa and Page 1994a; Stort 1975). In addition, quantitative trait loci (QTL) that influence honeybee stinging behavior have been identified based on colony phenotypes and were later confirmed to affect the tendencies of individual worker bees to either sting or guard the nest entrance (Arechavaleta and Hunt 2004; Arechavaleta et al. 2003; Hunt et al. 1998). Bees that guard the nest entrance exclude nonnestmate bees, but also appear to have a role in recruiting nestmates to sting other intruders, perhaps through the release of an alarm pheromone (Arechavaleta and Hunt 2003; Breed et al. 2004).
In Mexico, Africanized bees have caused thousands of stinging incidents that have resulted in the deaths of more than 300 people, as well as several thousand animal fatalities (Cajero 1995; Guzmán-Novoa and Page 1994b). In the United States, fewer than 20 people have died as a result of stinging incidents since the arrival of Africanized bees (Erickson EH, personal communication), but the number of fatalities could increase if these bees continue to spread.
Whether the defensive behavior of honeybee colonies is influenced by paternal effects may have implications for the fitness of honeybee colonies, for human and animal health, and for honeybee breeding. If there are paternal effects on defensive behavior, hybrid colonies from Africanized paternity would be expected to increase their fitness in environments in which there are benefits associated with defensiveness for reducing predation, such as the tropics. Moreover, breeding gentler bees by mating European queens in Africanized areas would be more difficult to accomplish and more human and animal lives could be at risk.
This article describes results from defensive assays of F1 hybrid colonies. We made reciprocal crosses (from Africanized and European paternities) to test for paternal effects on the defensive behavior of honeybee colonies.
Materials and Methods
Experimental Colonies
The experiments were conducted in Ixtapan de la Sal, Mexico (19° N, 99° W). Defensive data were obtained from hybrid European-Africanized colonies that were produced with reciprocal crosses in four different years (1993, 2000, 2001, and 2003) and were part of a population of bees used for different studies of defensive behavior. The European colonies used for the experiments were derived from queens that had been imported from the United States or Canada and were maintained by instrumental insemination in Mexico. The Africanized sources were derived from colonies obtained locally. Morphometric and mitochondrial DNA (mtDNA) analyses (Nielsen et al. 1999) of the queens' progeny were used to confirm the strain origin of our source colonies.
We made reciprocal crosses to produce colonies containing F1 worker bees with either European or Africanized paternity and evaluated their behavior with a stinging assay. Fifty-two F1 colonies were produced in the 4-year study as follows: 6 in year 1 (3 each of European and Africanized paternity), 18 in year 2 (10 of European and 8 of Africanized paternity), 9 in year 3 (5 of European and 4 of Africanized paternity), and 19 in year 4 (10 of European and 9 of Africanized paternity). Colonies of years 1, 2, and 4 were derived from queens that were instrumentally inseminated with the mixed semen of six to eight drones each. Four source colonies per genotype (European and Africanized) were used to produce queens and drones for the crosses of year 1, whereas six source colonies per genotype were used in years 2, 3, and 4. For year 3, single-drone instrumental insemination (one drone for each queen) was used in all the crosses for reasons unrelated to this report. Inseminated queens were identified by gluing colored, numbered plastic tags (Graze KG, Weinstadt, Germany) to their thoraxes and by clipping their right wings. Experimental queens were introduced into Dadant jumbo-size hives initially containing approximately 5000 workers and three combs with brood, pollen, and honey. Hives were positioned at least 5 m apart to minimize interhive drifting of workers. In addition, 32 Africanized and 31 European bee colonies were established as above to be used as controls. In year 1 we used 6 colonies of each type, in year 2 we used 9 Africanized and 11 European bee colonies, in year 3 we used 12 Africanized and 9 European bee colonies, and in year 4 we used 5 colonies of each type.
Behavioral Assays
Nine weeks after being established, each experimental colony was tested three to eight times with a defensive behavior assay. Fourteen days before conducting the defensive trials, the populations of the colonies were equalized by removing bees and frames of brood from the most populous colonies. Each of the colonies contained about 4000 cm2 (four frames) with capped brood and five frames covered with adult bees after equalization. Colony equalization was necessary to control for differences in colony population that would affect the intensity of defensive responses (Breed et al. 2004).
We used a “traditional” behavioral assay to test the stinging behavior of the experimental colonies (see Guzmán-Novoa et al. [2003] for more details about methods for testing the defensiveness of honeybee colonies). The assay consisted of a 10 cm × 8 cm black suede leather patch (a “flag”) suspended on a piece of white wood (0.7 cm × 0.5 cm × 100 cm). In order to provide a stimulus to trigger the defensive response of colonies, a flag was rhythmically elevated (about 4 cm) and lowered (about 4 cm) at two swings per second approximately 5–10 cm in front of the entrance of each hive. Bees were permitted to sting for 60 s. After completing each test, the leather patch was placed inside a labeled plastic bag for subsequent sting counts. All colonies were tested simultaneously. Tests were conducted blindly; that is, the operators did not know the type of colony they were testing.
Analyses
Data from the individual trials of each colony were summed and averaged, and were square-root transformed to normalize their distribution before being analyzed. Data were subjected to analysis of variance (ANOVA) and Scheffe tests for each of the four years. Moreover, we used t-tests to compare data from hybrid colonies with the mid Africanized-European value to test for possible dominance effects. The mid Africanized-European value was calculated by adding the average responses of the Africanized and European colonies. The resulting figure was then divided by two.
Results
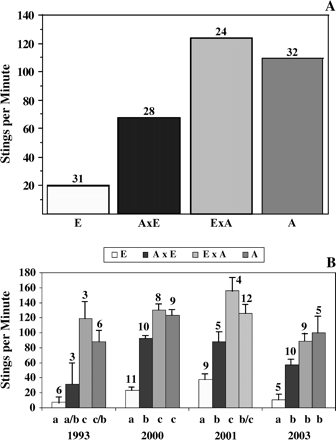
Overall we observed a pattern suggesting a paternal effect on stinging behavior (Figure 1A). In each of the four years, a significant difference between the genotypes was found for the number of stings (F = 13.2, P < .001, df = 3.14; F = 104.3, P < .0001, df = 3.34; F = 22.4, df = 3.26; F = 17, P < .0001, df = 3.25). In three of the four years, hybrid colonies of Africanized paternity were more defensive (left more stings in leather patches; P < .01) than hybrid colonies of European paternity. Hybrids did not give stings that were significantly different from the Africanized type, except for hybrids with European paternity in year 2 (Figure 1B). Colonies of hybrid workers with European paternity were more defensive than the expected midparent value in year 2, suggesting genetic dominance (t = 5.6, df = 9, P < .001). However, no difference between hybrids of European paternity and the expected midparent value was found for the other three years, even though colonies were significantly more defensive than the European type (Figure 1B).
Results of tests for paternal effects on honeybee stinging behavior. (A) Summary of the number of stings for four genotypes from 4 years of testing. (B) Number of stings + standard error (SE) for four genotypes each year. Statistical tests were performed on square-root transformed data. For each year, values are significantly different (P < .01, Scheffe test) if they do not share the same lowercase letter beneath the bar. The number above each bar indicates how many colonies were used to determine the average. “E” refers to a colony of European origin; “AxE” refers to a hybrid colony of European paternity; “ExA” refers to a hybrid colony of Africanized paternity; “A” refers to an Africanized colony.
Discussion
Our results suggest that paternal effects of African origin increase the defensive behavior of hybrid colonies, and they are consistent with those of previous studies. Guzmán-Novoa and Page (1993) had colonies with F1 workers of Africanized paternity that gave as many stings as the Africanized control colonies. Sister F1 queens were backcrossed to European drones for two generations. In each backcross, the colonies were not different from European controls. Stort and Gonçalves (1991) also reported dominance for high defensiveness. They used Africanized drones for the F1 cross and drones from F1 queens for their backcrosses. Their backcross colonies fell mostly into the highly defensive category. DeGrandi-Hoffman et al. (1998) reported that the most defensive colonies in their study were those with European or Africanized queens mated to Africanized drones. Collins and Rinderer (1991) refer to some matings in which the F1 progeny had intermediate defensiveness, but do not state which parent was used as the drone source.
Results from our current study strongly suggest that the dominance reported for defensive behavior in the literature can be attributed to paternal effects. Paternal effects on the defensive behavior of honeybees may explain in part the ecological success of Africanized honeybees. Defensive traits are beneficial to honeybee colonies in tropical environments because they help them reduce predation (Rinderer and Hellmich 1991). If a simple dominance mechanism were involved, then colonies with queens that were homozygous for defensive behavior alleles, or heterozygous, may have an unacceptably high number of defensive individuals that may be prone to disrupt routine colony functions, such as foraging, or may sting any animal near the colony, resulting in the death of the bees that sting. On the other hand, a paternal effect would allow for more specialization in defensive behavior within the colony.
We can consider two potential mechanisms that would explain a paternal effect for defensive behavior. First, interactions between European mitochondrial genes and African nuclear alleles could result in a more active, defensive bee. The vast majority of proteins functioning in the mitochondria are nuclear encoded, so there is a potential for nuclear-mitochondrial interactions, and mitochondrial dysfunction has been implicated in a number of neurodegenerative diseases and ageing (Betts et al. 2004; Poyton and McEwen 1996). Africanized bees have higher mass-specific metabolic rates than European bees, and Harrison and Hall (1993) reported that hybrids had low metabolic rates similar to European bees, probably as a result of interaction between mitochondrial and nuclear genomes. Although we do not know what specific mitochondrial/nuclear interactions explain our results, this remains a possibility.
Another explanation for the observed paternal effects, and one that we favor, is that imprinting mechanisms selectively reduce expression of maternal alleles or increase expression of paternal alleles. Imprinting usually is caused by methylation and chromatin modifications that silence particular alleles depending on the sex of the parent from which the alleles were inherited (Constancia et al. 1998). Thus imprinting can cause specific sets of genes to show allele-specific expression depending on the sex of the parent that transmitted the alleles. If the maternal alleles are silenced, the effect would be asymmetries in the phenotypes from reciprocal crosses, as we observed in our results. Such epigenetic effects are usually observed early in development in most species, but there are a few examples in which imprinting has influenced adult behaviors, such as maternal care in mice, or resulted in neuropathologies in adults (Jaenisch and Bird 2003; Murphy and Jirtle 2003; Tremolizzo et al. 2002). DNA methylation has been studied in a small number of insect species and methylation levels were found to be generally low, except for one report of high methylation levels in the cabbage moth (Mandrioli and Volpi 2003; Marhold et al. 2004). Interestingly, a positive association was reported between methylation and gene expression of insecticide-resistance genes in the aphid (Myzus persicae) (Field 2000). Despite the complete lack of information on epigenetic phenomena in honeybees, there are theoretical reasons that might lead us to expect imprinting in this species.
Hamilton (1964a,b) stated that haplodiploidy should favor the evolution of cooperative behavior among full sisters because they share 75% of their alleles by descent. But the kinship theory of imprinting predicts that alleles of paternal origin in polyandrous social insects could favor the father's progeny at the expense of other nestmates (Haig 2000). The queen bee mates with multiple drones and most workers are only half sisters. So one possible interpretation of our results is that major defensive behavior alleles in bees are methylated when inherited from the mother to reduce the costs associated with a high degree of defensive behavior in the colony, but the expression of the paternal alleles results in progeny workers and queens that exhibit behavior that promotes the spread of these alleles. For example, when multiple queens are simultaneously produced in a honeybee colony, a fight ensues and one queen will sting her rivals to death. Genes that make worker bees more likely to sting also may make a queen more likely to sting her rivals. Then as the sole queen of the colony, her drone progeny would transmit the paternally expressed alleles to other colonies.
In addition to these theoretical considerations, we are aware of one study involving crosses between two closely related species of parasitic wasps that showed differences in behavior between reciprocal crosses. In these experiments, sons of F1 females resulting from crosses between Nasonia longicornis and Nasonia vitripennis had mating behaviors that were significantly skewed toward the paternal phenotype. This effect was termed “the grandfather effect” because hymenopteran males are haploid and result from parthenogenetic development of unfertilized eggs (Beukeboom and van den Assem 2001). Even after introgression crosses that were designed to eliminate possible cytoplasmic-nuclear interactions, the grandfather effect persisted (Beukeboom and van den Assem 2002). The authors suggested that an epigenetic signal or imprint is passed to the haploid males from their F1 mothers. This would be an unusual case of imprinting because the epigenetic signal would have to survive gametogenesis.
From the perspective of breeding, paternal effects on genes influencing defensive behavior can have important consequences. A queen may have high defensive alleles yet produce a fairly gentle colony. The alleles may show reduced expression in the workers because they were maternally inherited; but if she is used as a drone source, the mating could result in a defensive colony. The traditional way to deal with defensive Africanized stocks is simply to introduce European queens, if available, into all the colonies. However, a European queen will most likely be superseded by a daughter after about 7 months, and the daughter queen will fly out and mate with primarily Africanized drones (Guzmán-Novoa et al. 1998). Since high defensive behavior apparently shows paternal effects, this would not be the best strategy. It would be better to saturate the mating congregation areas with drones of European origin. This could be accomplished by replacing the queens of colonies located near mating yards with mothers of European origin and by providing those colonies with comb containing drone-size cells to foster the production of many European drones. High mating control and successful breeding using “drone flooding” techniques have been demonstrated in the past (Guzmán-Novoa and Page 1999; Hellmich and Waller 1990; Loper and Fierro 1990).
Corresponding Editor: Ron C. Woodruff
We thank Guillermo García of Miel Vita Real for providing the labor force during the test periods. We are also grateful to M. Kim Fondrk and Enrique Estrada for their assistance with instrumental insemination and maintenance of the experimental queens. This study was funded in part by grants from the National Science Foundation (IBN 0110842) and the U.S. Department of Agriculture (NRI 35302-10137).
References
Arechavaleta-Velasco ME and Hunt GJ,
Arechavaleta-Velasco ME and Hunt GJ,
Arechavaleta-Velasco ME, Hunt GJ, and Emore C,
Beukeboom LW and van den Assem J,
Beukeboom LW and van den Assem J,
Betts J, Lightowlers RN, and Turnbull DM,
Breed MD, Guzmán-Novoa E, and Hunt GJ,
Cajero AS,
Collins AM and Rinderer TE,
Collins AM, Rinderer TE, Harbo JR, and Bolten AB,
Collins AM, Rinderer TE, Harbo JR, and Brown MA,
Constancia M, Pickard B, Kelsey G, and Reik W,
DeGrandi-Hoffman G, Collins AM, Martin JH, Schmidt JO, and Spangler HG,
Field LM,
Guzmán-Novoa E, Hunt GJ, Uribe JL, Smith C, and Arechavaleta-Velasco ME,
Guzmán-Novoa E and Page RE,
Guzmán-Novoa E and Page RE,
Guzmán-Novoa E and Page RE,
Guzmán-Novoa E and Page RE,
Guzmán-Novoa E, Page RE, and Prieto-Merlos D,
Guzmán-Novoa E, Prieto-Merlos D, Uribe-Rubio JL, and Hunt GJ,
Haig D,
Hall HG,
Hall HG,
Hall HG and Muralidharan K,
Hamilton WD,
Hamilton WD,
Harrison JF and Hall HG,
Hellmich RL and Waller GD,
Hunt GJ, Guzmán-Novoa E, Fondrk MK, and Page RE Jr,,
Jaenisch R and Bird A,
Kerr WE,
Lobo JA, Lama MAD, and Mestriner MA,
Loper GM,
Loper GM and Fierro MM,
Mandrioli M and Volpi N,
Marhold J, Rothe N, Pauli A, Mund C, Kuehle K, Brueckner B, and Lyko F,
Murphy SJ and Jirtle RL,
Muralidharan K and Hall HG,
Nielsen DI, Ebert PR, Hunt GJ, Guzmán-Novoa E, Kinee SA, and Page RE,
Poyton RO and McEwen JE,
Rinderer TE and Hellmich RL,
Rubink WL, Luévano-Martínez P, Sugden EA, Wilson WT, and Collins AM,
Smith DR, Taylor OR, and Brown WM,
Stort AC,
Stort AC and Gonçalves LS,
Sugden EA and Williams KR,
Tremolizzo L, Carboni G, Ruzicka WB, Mitchell CP, Sugaya I, Tueting P, Sharma R, Grayson DR, Costa E, and Guidotti A,