-
PDF
- Split View
-
Views
-
Cite
Cite
Christopher D. Todd, Peter A. Tipton, Dale G. Blevins, Pedro Piedras, Manuel Pineda, Joe C. Polacco, Update on ureide degradation in legumes, Journal of Experimental Botany, Volume 57, Issue 1, January 2006, Pages 5–12, https://doi.org/10.1093/jxb/erj013
Close - Share Icon Share
Abstract
Warm season N2-fixing legumes move fixed N from the nodules to the aerial portions of the plant primarily in the form of ureides, allantoin and allantoate, oxidation products of purines synthesized de novo in the nodule. Ureides are also products of purine turnover in senescing tissues, such as seedling cotyledons. A combination of biochemical and molecular approaches in both crop and model species has shed new light on the metabolic pathways involved in both the synthesis and degradation of allantoin. Improved understanding of ureide biochemistry includes two ‘additional’ enzymatic steps in the conversion of uric acid to allantoin in the nodule and the mechanism of allantoin and allantoate breakdown in leaf tissue. Ureide accumulation and metabolism in leaves have also been implicated in the feedback inhibition of N2-fixation under water limitation. Sensitivity to water deficit differs among soybean cultivars. Manganese supplementation has been shown to modify relative susceptibility or tolerance to this process in a cultivar-dependent manner. A discussion of the potential roles for ureides and manganese in the feedback inhibition of N2-fixation under water limitation is presented. The existing data are examined in relation to potential changes in both aerial carbon and nitrogen supply under water deficit.
Introduction
Leguminous plants establish a symbiotic association with certain root nodule-forming soil bacteria such as Rhizobium spp., which are capable of fixing N2. Organic nitrogenous compounds formed from N2 fixation can be transported to the upper parts of the plant either as amides [mainly asparagine (Asn), but also glutamine (Gln)] or as ureides (allantoin and allantoate), so that legumes are classified as amide or ureide exporters according to the compounds used for the mobilization of fixed N (Schubert, 1986). Ureides dominate xylem N composition in warm weather legumes, while the plant is actively fixing atmospheric N. Otherwise, amides are exported. In amide-producing species, Asn is always the main form of xylem-transported N despite changes in N nutritional status.
Although some authors have proposed that ureides could be formed by the condensation of urea with a two-carbon compound (glyoxylate being the best candidate), there is no doubt that ureides are products from purine oxidative catabolism. Purines for ureide biogenesis may arise by turnover of nucleic acids or by de novo synthesis. The salvage pathway may be important in certain tissues such as developing seedlings whereas de novo synthesis of purines is the main route for ureide formation in nodules (Schubert, 1986; Atkins and Smith, 2000).
Interest in the route(s) of ureide degradation has been aroused by reports that the nature of the degradative pathway in aerial tissues may influence N2-fixation activity under water-deficit in soybean. Therefore, it is necessary to define the steps in the conversion of the transport ureides, allantoin and allantoic acid, to utilizable ammonia in green tissues of N2-fixing warm-season legumes. Fixed N requires carbon (C) skeletons for assimilation. From the standpoint of combined N and C assimilation, the ‘ureide strategy’ may be more conserving of photosynthate than the ‘amide strategy’ by which temperate climate legumes transport fixed N as Asn and Gln. Experimental determinations of C and N budgets of ureide-forming (Vigna unguiculata, V. radiata) and amide-forming (Lupinus albus, Pisum sativum) symbioses indicated that those based on ureides are generally more economical of C, requiring as little as 1.4 g C g−1 fixed N in cowpea compared with a minimum of 3.9 g C g−1 fixed N in lupin (Atkins, 1991).
Ureides do not accumulate to high levels in seeds. Streeter (2005) measured the ureide concentration in seeds derived from nodulated soybean plants as 1.9 nmol mg−1 dry wt. However, purine degradation leads to ureide accumulation in senescing cotyledons of both legumes and non-legume seedlings. Ureides were observed in seedling cotyledons of soybean (Stebbins and Polacco, 1995). In Arabidopsis seedlings, the accumulation of urea was partially inhibited by allopurinol (Zonia et al., 1995), an inhibitor of xanthine dehydrogenase (Triplett et al., 1980; Leydecker et al., 1995), the enzyme that catalyses the oxidation of xanthine to uric acid. Clearly, the efficient conversion of the C and N of ureides to metabolically active intermediates is important in seedling health.
Ureide degradation is equally important for the eukaryotic micro-organisms, fungi, yeasts, and algae. Advantage has been taken of yeast (Saccharomyces cerevisiae) mutants in ureide degradation in order to demonstrate the function of plant genes encoding ureide-degrading enzymes (CD Todd and JC Polacco, unpublished results). The green alga Chlamydomonas reinhardtii has been used as a plant model for more than 40 years (Harris, 2001). C. reinhardtii can grow with purines and ureides as the sole N source to a yield comparable with that of the inorganic N sources, ammonium or nitrate (Pineda et al., 1984). Therefore, C. reinhardtii must have all the uptake systems and enzymes for the efficient release of N from ureides (Pineda and Cárdenas, 1996; Piedras et al., 1998).
Current understanding of ureide degradation in legumes, focusing on soybean (Glycine max), French bean (Phaseolus vulgaris), and chickpea (Cicer arietinem) is reviewed here. First, two aspects of the ureide degradation pathway are addressed: the nature of recently identified enzymes intervening between uric acid and allantoin, and the apparent identity of allantoate degradation steps in two soybean varieties reported to have distinct allantoate degradation routes. Secondly, the interplay of Mn and ureides in N2-fixation in drought-stressed soybean is addressed.
The overall pathway of uric acid degradation
Urate to allantoin: recently characterized intervening enzymes
Urate is derived from xanthine dehydrogenase action in infected cells of the nodule. Urate is then moved to the uninfected cells of the nodule where it is oxidized in microbodies, eventually forming allantoin (Smith and Atkins, 2002). The formation of allantoin from urate begins with the reaction catalysed by urate oxidase (or uricase). The presence of uricase in legumes was established by Tracey (1955) and the enzyme was subsequently purified from different sources (for pertinent references, see Montalbini et al., 1997). Although the gene encoding uricase has been studied at the molecular level in several legumes, very little is known about its regulatory mechanisms (Nguyen et al., 1985; Sánchez et al., 1987), and only the genomic structure and the promoter region of the gene from soybean (Nguyen et al., 1985; Takane et al., 1997) and chickpea (Redondo-Nevado et al., 2004) have been described. As expected from purification data, this gene is also expressed in organs other than nodules (Capote-Maínez and Sánchez, 1997; Redondo-Nevado et al., 2004).
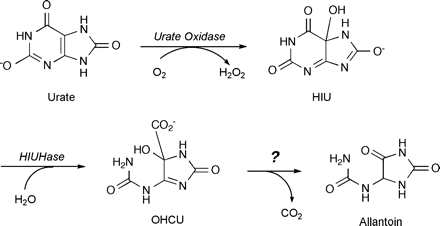
In spite of extensive study of legume uricases, it has been widely reported that allantoin is formed directly from urate by the action of uricase. However, when the reaction was monitored directly by 13C-NMR, the true product of the urate oxidase reaction was determined to be 5-hydroxyisourate (HIU) (Modric et al., 1992; Kahn et al., 1997) (Fig. 1). HIU is unstable in aqueous solution and, at neutral pH and room temperature, it decomposes non-enzymatically to yield allantoin with a half-time of 20 min. It seems unlikely that the uncatalysed chemistry is physiologically relevant, however. If the conversion of HIU to allantoin occurred without catalysis, there would be no physiological or genetic control of HIU degradation mediated by enzyme synthesis or activation. Perhaps more significantly, the non-enzymatic decomposition of HIU generates a racemic mixture of allantoin. Soybean allantoinase is specific for S-allantoin in vitro (Lee and Roush, 1964), and studies tracing the fate of [14C]urate in soybean root nodules demonstrated that allantoin is produced stereospecifically (Kahn and Tipton, 2000), and that no allantoin racemase activity is present in the root nodule (A Xu and PA Tipton, unpublished results).
Degradation of urate to allantoin in soybean nodules. Urate oxidase (uricase; EC 1.7.3.3) catalyses the addition of molecular oxygen to urate to form HIU which has an asymmetric carbon (Modric et al., 1992; Kahn et al., 1997). Though HIU is relatively unstable, forming a racemic mixture of allantoin with a short half-time, a nodule HIU hydrolase (EC 3.5.2.17) was purified which catalyses the hydrolysis of HIU to OHCU (Sarma et al., 1999). Optically pure HIU and OHCU are probably produced by urate oxidase and HIU hydrolase, respectively: Allantoin is produced stereospecifically in soybean root nodules (Kahn and Tipton, 2000) and soybean allantoinase is specific for S-allantoin in vitro (Lee and Roush, 1964). Abbreviations: HIU, 5-hydroxyisourate; OHCU, 2-oxo-4-hydroxy-4-carboxy-5-ureidoimidazolin.
The foregoing observations suggest that the pathway from HIU to allantoin is enzyme-catalysed. A novel enzyme that utilizes HIU as its substrate, hydroxyurate hydrolase, was purified from soybean root nodule extracts, and shown to catalyse the hydrolysis of HIU (Sarma et al., 1999). The product of the HIU hydrolase (HIUHase) reaction was identified by 13C-NMR as 2-oxo-4-hydroxy-4-carboxy-5-ureidoimidazoline (OHCU, Fig. 1), which is also relatively unstable. Cloning the gene for HIUHase yielded the surprising result that it has extensive relatedness to a family of β-glycosidases (Raychaudhuri and Tipton, 2002).
The β-glycosidases are characterized by conserved active site glutamate residues that play critical roles in catalysis. The conserved glutamates appear as E197 and E408 in HIUHase, and E197A and E408A mutants were essentially inactive (Raychaudhuri and Tipton, 2003). The concern that hydrolysis of HIU by HIUHase might be a spurious activity, and that the natural substrate for HIUHase is unrelated to ureide metabolism, was allayed by the observations that the protein is found predominantly in root nodules, is localized to the peroxisome, and is expressed only during N2-fixation (Raychaudhuri and Tipton, 2002).
Efforts to determine the pathway from OHCU to allantoin are ongoing, and recent results suggest that soybean root nodules contain a peroxisomal enzyme that utilizes OHCU as its substrate (A Raychaudhuri and PA Tipton, unpublished results). If, as seems likely, the novel enzyme catalyses the formation of allantoin from OHCU, the ureide pathway can be represented as shown in Fig. 1. What was once thought to be a simple one-step pathway is now revealed to require at least three enzymes.
Allantoin to allantoate
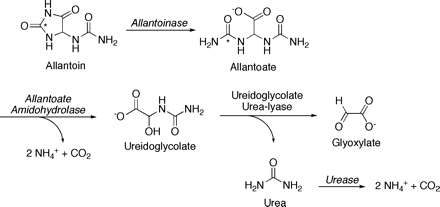
Allantoinases have been characterized from soybean (Lee and Roush, 1964; Webb and Lindell, 1993; Bell and Webb, 1995), French bean (Wells and Lees, 1992) and Vigna (Reddy et al., 1989). The French bean allantoinase has been cloned (Y Raso, P Piedras, and M Pineda, unpublished results). As noted above, ureide production and degradation also occurs in non-legumes. Arabidopsis has membrane-bound ureide transporters and can utilize ureides as the sole N source (Desimone et al., 2002). Its allantoin-degrading allantoinase is closely related to that of the tree legume, black locust. Indeed, the single ORF, expressed in yeast, corrects a dal1 mutant, deficient in yeast allantoinase (Yang and Han, 2004). It appears, then, that there is little controversy in invoking allantoinase as the single enzyme that catalyses the hydrolysis of allantoin to allantoate (Fig. 2).
Conversion of allantoin to glyoxylate and ammonia in soybean leaf and immature seed coat. Allantoinase (EC 3.5.2.2.5) has been characterized in soybean and in other legumes. Hydrolysis of its allantoate product is catalysed by allantoate amidohydrolase (EC 3.5.3.9) rather than urea-releasing allantoate amidinohydrolase (EC 3.5.3.4): Partially purified ammonia and CO2-releasing activities are not inhibited by urease (Winkler et al., 1985, 1987; Todd and Polacco, 2004), consistent with the ability of urease-negative mutant plants to assimilate allantoin (Stebbins and Polacco, 1995). Further, a ureidoglycine intermediate between allantoate and ureidoglycolate was identified as a possible precursor to a conjugation product with 2-mercaptoethanol in the assay reaction (Winkler et al., 1985). Urea production from ureidoglycolate via ureidoglycolate urea-lyase (EC 4.3.2.3) is consistent with inhibition, by urease inhibitor PPD, of half of the release of ammonia and CO2 from allantoin in leaf homogenates (Todd and Polacco, 2004). Ureidoglycolate urea-lyase has been purified from chickpea (Muñoz et al., 2001). Abbreviation: PPD, phenylphosphorodiamidate.
Allantoate to glyoxylate: ureide groups released as urea?
Complete N utilization of allantoate requires that its two ureido groups, which contain all allantoate N, be converted to ammonia. The controversy arises as to whether the production of ammonia and CO2 from allantoate is direct or by way of a urea intermediate. The only allantoate degrading activity purified from a photosynthetic organism was recovered from C. reinhardtii (Piedras et al., 2000). This activity is induced in cells using ureides as the sole N source and is undetectable in cells grown with ammonium or nitrate N (Piedras et al., 1998). The electrophoretically pure enzyme catalyses the degradation of allantoate to (−)ureidoglycolate and of (+)ureidoglycolate to glyoxylate, in both cases with urea as the nitrogenous product. It consists of six identical or similarly sized subunits of 34 kDa, arranged in two trimers of 100 kDa, with the subunits in the trimer bound by disulphide bridges (Piedras et al., 2000). Manganese binding in vitro activates this allantoicase (Piedras and Pineda, 2003). It has recently been shown that yeast allantoicase, a product of the dal2 gene, and which also generates urea from allantoate, is also a dimer of trimers (Leulliot et al., 2004).
By contrast, a soybean allantoicase in the seed coat (Winkler et al., 1985) and leaves (Winkler et al., 1987) was demonstrated to be an amidohydrolase, i.e. catalysing the release of ammonia and CO2 directly from allantoate. The evidence was lack of inhibition of ammonia and CO2 release by phenylphosphorodiamidate (PPD), a potent and specific urease inhibitor (Liao and Raines, 1985). This allantoate amidohydrolase required Mn2+ and was inhibited by EDTA and by acetohydroxamate, both chelators of divalent cations, although the latter has been reported to inhibit urease (Fishbein and Carbone, 1965). The operation of at least one amidohydrolase activity was consistent with in vivo evidence that urease-inhibited soybean cells in culture could utilize allantoin as the sole N source (Stahlhut and Widholm, 1989), without releasing urea, and that urease-negative soybean plants exhibited no growth or yield depression when dependent on fixed N (Stebbins and Polacco, 1995).
Shelp and Ireland (1985) examined the complete degradation of allantoin in soybean leaf sections. They reported that acetohydroxamate completely inhibited ammonia and CO2 release, thus favouring the release of both ureido groups as urea. As pointed out above, acetohydroxamate also inhibits, directly, the allantoate amidohydrolase (as well as allantoinase: Reddy et al., 1989) thus obfuscating its effects on urease. In an attempt to repeat the experiments on intact leaves, Todd and Polacco (2004) employed lightly homogenized leaves of Maple Arrow, the variety reported to liberate urea exclusively from allantoin (Shelp and Ireland, 1985) and Williams 82, the variety reported to release CO2 and ammonia directly (Winkler et al., 1987). No differences were found between the varieties. However, in both varieties, it was observed that half of the CO2 release was inhibited by PPD. This observation is consistent with an allantoate amidohydrolase, demonstrated in leaf extracts of both varieties, and urea release from ureidoglycolate (via an amidinohydrolase or a urea lyase) (Fig. 2). Consistent with urea release were whole plant studies in which urea accumulated in both varieties systemically fed PPD: urea accumulation was greater under N2-fixing conditions. In the urease-null mutant, eu3-e1/eu3-e1 (Freyermuth et al., 2000), fed [2-14C]allantoin through the petiole, there was an accumulation of [14C]urea not seen in the isogenic urease-positive Williams 82 (Todd and Polacco, 2004).
Ureidoglycolate is an intermediate in the degradation of allantoin, irrespective of the enzyme catalysing allantoate degradation. Conversion of ureidoglycolate into glyoxylate can be catalysed by ureidoglycolate urea-lyase, which produces urea, or by ureidoglycolate amidohydrolase, which produces ammonium and CO2. The production of urea from ureidoglycolate is at variance with an earlier report of a ureidoglycolate amidohydrolase in soybean (Winkler et al., 1988). However, the recent results are in agreement with a ureidoglycolate urea-lyase purified from chickpea fruits (Muñoz et al., 2001). It is possible that urea release from ureidoglycolate cannot be generalized to all of the Leguminosae since Wells and Lees (1991) characterized an ammonia-generating ureidoglycolate amidohydrolase from developing French bean seeds (Phaseolus vulgaris). Chickpea (C. arietinum) is classified as an amide transporter and Phaseolus is usually considered to transport ureides. It remains to be established whether urea generation from ureidoglycolate is a feature of ureide-transporting legumes, of taxonomic groupings, or a combination of both (i.e. clustering of ureide transporters in specific clades). Phaseolus and Glycine have been grouped in one branch of the Papilionoid tree, while Cicer is in a separate, albeit closely related branch (Doyle and Luckow, 2003).
Ureides and Mn involvement in N2-fixation in drought-stressed soybean
N2-fixation is especially sensitive to water deficit in ureide transporters
Sinclair et al. (1987) reported that N2-fixation in soybean was more sensitive to water deficit than photosynthesis and respiration (reviewed by Serraj et al., 1999a). Sinclair and Serraj (1995) reported that ureide-exporting legumes shut down N2-fixation (acetylene reduction) during soil drying before there was an effect on relative transpiration rate, in contrast to amide exporters which exhibited less sensitivity of N2-fixation relative to transpiration. It has been estimated that the ATP cost per N assimilated for producing ureide transport molecules is half that of producing Gln and Asn (Schubert, 1986). The costs are calculated from known biochemical pathways, assuming that the starting materials are ammonium and phosphoglycerate (Schubert, 1986); the values deduced were 5 ATP per N for allantoin or allantoate compared with 12 for Asn. Further, ureides, with a C:N ratio of 1, are much more conserving of photosynthate than amides with C:N ratios of 2.0 (Asn) or 2.5 (Gln). Conservation of ATP and fixed C suggest that ureide exporters would exhibit more drought-resistant N2-fixation than amide exporters, since they could close stomata to conserve water while using less photosynthate to transport N.
However, there are other costs associated with the ureide ‘strategy:’ One is the induction of a de novo purine biosynthetic pathway in the nodule. Perhaps more critical is the release in the leaf of four ureido-derived
Water-deficit: the role of leaf ureide build-up
Allantoate accumulates in leaves when soybean plants experience drought, while N2-fixation in root nodules declines rapidly under these conditions (Sinclair and Serraj, 1995; Serraj and Sinclair, 1996; Serraj et al., 1999a, b). It has been proposed that the accumulation of nitrogenous metabolites, like allantoate, in leaves of leguminous plants experiencing drought may lead to feedback inhibition of N2-fixation in root nodules (Parsons et al., 1993; Serraj et al., 1999a).
The explanations of water stress-sensitive N2-fixation based on an aerial deficiency of C skeletons to accept fixed N as discussed above is preferred (Todd and Polacco, 2004). It is important to note that by discussing an aerial C deficit, this is only being considered as the reduced availability of C skeletons in a specific tissue and not in the plant as a whole. Therefore, a localized C deficit may still be consistent with reports (Sinclair et al., 1987) that biological N fixation is more sensitive to water limitation than carbon assimilation. This notion is supported by the observations of Serraj and Sinclair (2003) that increased CO2 alleviated inhibition of N2-fixation by ureides provided in the nutrient solution. At the highest ureide application (10 mM), no ureide accumulation was seen in leaf tissue of soybean subjected to 700 μmol mol−1 CO2, in contrast to control plants (350 μmol mol−1 CO2) which accumulated increasing leaf ureide with ureide supplements. Although the authors did not impose a water stress on the plants, they showed a significant interaction between ureide and CO2 concentrations: Under elevated CO2 there was less transpiration and increased total non-structural carbohydrates, or C-skeletons. Serraj and Sinclair (2003) present these results in support of the hypothesis that N2-fixation is subject to N-feedback regulation. Since imposing water-deficit also results in decreased N2-fixation and increased leaf ureide content, it seems likely that there is an underlying common cause for decreased N2-fixation due to ureide application or to water-stress.
These results are consistent with the model of Todd and Polacco (2004), i.e. that under water-deficit there is a deficiency of leaf C skeletons, a situation remedied by provision of higher [CO2]. With more C-skeletons, leaf tissue is able to assimilate the ureide N provided in the nutrient solution and N2-fixation is not subject to feedback inhibition from the shoot. Under normal CO2 levels and water deficit, as carbon becomes limiting, leaves may make more efficient use of existing carbon skeletons by channelling N into Asn. King and Purcell (2005) showed that total leaf amino acids accumulate under water limitation, with Asn showing the largest single increase. Asn has been implicated as a potential candidate for feedback inhibition of N2-fixation (Vadez et al., 2000; Todd and Polacco, 2004) and can directly inhibit ureide catabolism (Lukaszewski et al., 1992), providing a possible mechanism for ureide accumulation. Asn and other nitrogenous compounds may then move through the phloem to play a role, directly or indirectly, in the feedback inhibition of N2-fixation.
Water-deficit: the role of Mn
It has been well established that supplemental Mn can have stimulatory effects on N2-fixation in soybean under water deficit and that there are genotypic differences in cultivar responses to soil-applied Mn (Purcell et al., 2000; Vadez et al., 2000; Sinclair et al., 2003). In addition, there are soybean genotypic differences in the sensitivity of N2-fixation to water-deficit, and the possibility of urea versus ammonia-generating activities distinguishing soybean varieties during drought was raised by Vadez and Sinclair (2001). This fit a hypothesis that complete allantoin degradation in sensitive Williams 82 required Mn2+ while that in relatively tolerant Maple Arrow did not. Mn-deficiency was offered as the basis of drought-sensitivity in Williams 82, since reduced xylem flow under low water potential delivered less Mn to ureide-degrading enzymes, resulting in a build-up of ureides and the subsequent feedback inhibition of fixation (Sinclair et al., 2003). However, as discussed above, Maple Arrow and Williams 82 have identical activities in the conversion of allantoate to glyoxylate, at least in well-watered plants (Todd and Polacco 2004). Indeed, ALL ureide-degrading enzymes (from allantoate to glyoxylate) described to date require Mn. Differences in response to Mn applications could as easily be explained by more efficient utilization, movement or storage of available Mn in Maple Arrow.
Low leaf Mn concentrations can be the result of low Mn supply or the presence of chelators such as Asn (Lukaszewski et al., 1992); in each case there is a lowered allantoate degradation rate in leaves thus blocking utilization of fixed N. However, Mn has other roles important to the metabolism of plants supported by N2-fixation. Chelation of Mn in the root nodule by Asn or other molecules can lower the activity of the NAD-malic enzyme, which is important in providing energy and carbon for N2-fixation and the incorporation of fixed N into amino acids, amides, and ureides (Chen et al., 1998; Driscoll and Finan, 1993). These possibilities are also consistent with the ideas recently discussed by King and Purcell (2005), who reported that ureide accumulation in leaves may not be directly responsible for the feedback inhibition of N2-fixation during drought, but that accumulation and translocation of specific amino acids or amides may be involved. Streeter (2003) suggested that, under drought conditions, bacteroids may be deficient specifically in malate, their source of reduced carbon, even though nodule glucose and sucrose levels increased under severe drought stress. These factors make for a seemingly complicated mode of regulation potentially involving interplay between N and C in different plant tissues with Mn supply and bioavailability. In addition, the full explanation of sensitivity of N2-fixation to water-deficit in ureide-exporting legumes will need to incorporate differences in ureide versus amide solubility (although allantoate, as opposed to allantoin, is very soluble), and in indeterminate versus determinate nodules, the latter characteristic of ureide producers.
Much to be done
While the genes encoding the activities in Fig. 1, and the allantoinase of Fig. 2 (Yang and Han, 2004) have been cloned, nothing is known of the structural genes that encode allantoate amidohydrolase and ureidoglycolate amidinohydrolase/urea-lyase. Their identification will allow the subcellular location of the final steps in ureide degradation, the signals which turn on their expression, and possible relatedness of ureide degradation genes to either amide or ureide modes of transporting fixed N to be defined. Candidate genes from Arabidopsis and their orthologues from soybean are currently being examined.
In addition, there are unanswered questions with respect to the ureide transport strategy and sensitivity to water-deficit. One involves the fate of released glyoxylate in sink tissues. Do ureide transporters exhibit higher photorespiratory activity? While the energy balance has been calculated for synthesis of ureides, a study needs to be performed on the energy costs of ureide versus amide assimilation in source tissues.
Present address: Department of Biology, 112 Science Place, University of Saskatchewan, Saskatoon, SK S7N 5E2, Canada.
CDT was supported by a Natural Sciences and Engineering Research Council of Canada Post-Doctoral Fellowship and a University of Missouri Life Sciences Post Doctoral Fellowship. Also supported by funds from grant no. 2003–00779, NRI, CSREES, USDA awarded to JCP and DGB and grant BOS2003–01595 awarded to MP from the Ministerio de Ciencia y Tecnología (Spain).
References
Atkins CA.
Atkins CA, Smith PMC.
Bell JA, Webb MA.
Capote-Maínez N, Sánchez F.
Chen F, Okade Y, Osano K, Tanjima S.
Desimone M, Catoni E, Ludewig U, Hilpert M, Schneider A, Kunze R, Tegeder M, Frommer WB, Schumacher K.
Doyle JJ, Lucknow MA.
Driscoll BT, Finan TM.
Fishbein WN, Carbone PP.
Freyermuth SK, Bacanamwo M, Polacco JC.
Harris EH.
Kahn K, Serfozo P, Tipton PA.
Kahn K, Tipton PA.
King CA, Purcell LC.
Lee KW, Roush AH.
Leulliot N, Quevillon-Cheruel S, Sorel I, Graille M, Meyer P, Liger D, Blondeau K, Janin J, van Tilbeurgh H.
Leydecker M-T, Moureax T, Kraepiel Y, Schnorr K, Caboche M.
Liao CFH, Raines SG.
Lukaszewski KM, Blevins DG, Randall DD.
Modric N, Derome AE, Ashcroft SJH, Poje M.
Montalbini P, Redondo J, Caballero JL, Cárdenas J, Pineda M.
Muñoz A, Piedras P, Aguilar M, Pineda M.
Nguyen T, Zelechowska M, Foster V, Bergmann H, Verma DPS.
Parsons LC, Stanforth A, Raven JA, Sprent JL.
Piedras P, Aguilar M, Pineda M.
Piedras P, Muñoz A, Aguilar M, Pineda M.
Piedras P, Pineda M.
Pineda M, Cárdenas J.
Pineda M, Fernández E, Cárdenas J.
Purcell LB, King CA, Ball RA.
Raychaudhuri A, Tipton PA.
Raychaudhuri A, Tipton PA.
Reddy RS, Rao NV, Sastry KS.
Redondo-Nevado J, Aguilar M, Caballero JL, Pineda M.
Sánchez F, Campos F, Padilla J, Bonneville JM, Enríquez C, Caput D.
Sarma AD, Serfozo P, Kahn K, Tipton PA.
Schubert KR.
Serraj R, Sinclair TR.
Serraj R, Sinclair TR, Purcell LC.
Serraj R, Sinclair TR.
Serraj R, Vadez V, Denison RF, Sinclair TR.
Shelp BJ, Ireland RJ.
Sinclair TR, Muchow RC, Bennett JM, Hammond LC.
Sinclair TR, Vadez V, Chenu K.
Smith MC, Atkins CA.
Stahlhut RW, Widholm JM.
Stebbins NE, Polacco JC.
Streeter JG.
Streeter JG.
Takane K, Tanaka K, Tajima S, Okazaki K, Kouchi H.
Todd CD, Polacco JC.
Tracey MV.
Triplett EW, Blevins DG, Randall DD.
Vadez V, Sinclair TR.
Vadez V, Sinclair TR, Serraj R.
Webb MA, Lindell JS.
Wells XE, Lees EM.
Wells XE, Lees EM.
Winkler RG, Blevins DG, Polacco JC, Randall DD.
Winkler RG, Blevins DG, Randall DD.
Winkler RG, Polacco JC, Blevins DG, Randall DD.
Yang J, Han KH.




Comments