-
PDF
- Split View
-
Views
-
Cite
Cite
Christine Stöhr, Stefanie Stremlau, Formation and possible roles of nitric oxide in plant roots, Journal of Experimental Botany, Volume 57, Issue 3, February 2006, Pages 463–470, https://doi.org/10.1093/jxb/erj058
Close - Share Icon Share
Abstract
Nitric oxide has been reported to act as a signalling molecule in different plant tissues and to participate in a variety of physiological processes. It is produced by different enzymes and sources. The root-specific plasma membrane-bound enzymes forming NO from the substrates nitrate and nitrite are of particular interest because roots serve as interfaces between plants and the soil. The co-ordinated activity of the root-specific plasma membrane-bound nitrate reductase (PM-NR) and nitrite:NO reductase (NI-NOR) suggests that NO might also be involved in root signalling and development. The rate of enzymatic production of this NO depends largely on the environmental conditions, mainly the availability of nitrate and oxygen and it is proposed that this NO plays a role during anoxia as an indicator of the external nitrate availability and in regulating symbiotic interactions at the root surface.
Introduction
Nitric oxide as a signalling molecule in plants has attracted much attention during recent years. Roles of NO have been elucidated in plant development processes, such as root growth and leaf expansion, photomorphogenesis and senescence (reviewed by Beligni and Lamattina, 2001; Neill et al., 2003), and also in rapid physiological reactions such as stomatal closure (Desikan et al., 2002, 2004) and the cytokinin signalling pathway (Tun et al., 2001). However, most data are available for the activity of NO during plant defence, the first of its functions that has been recognized (Delledonne et al., 1998, 2001; Durner et al., 1998; Klessig et al., 2000). Pathogen-derived elicitors stimulate an increase in cellular NO concentration (Foissner et al., 2000). It has been shown recently that lipopolysaccharides isolated from plant pathogens induce NO synthase (AtNOS1) and activate defence genes in Arabidopsis thaliana (Zeidler et al., 2004). The production of NO by roots has been underestimated for a long time and underground NO formation was associated solely with micro-organisms (Stöhr and Ullrich, 2002). Since NO is involved in many physiological reactions, the question arises how those different effects can be attained by the plant. Possible explanations could be the definite location of sources, targets and possible scavengers of NO in different organs, tissues, cells, or even cellular compartments.
In the present article these aspects will be considered with respect to the function of NO in roots. Roots are the plant organs that ensure nutrient and water supply for the whole organism. For this purpose root architecture has to be adapted to environmental conditions, including the development of root hairs and lateral roots. The surface of the active root zone is not protected by cell wall incrustations or even a cuticle that shields the leaf epidermis. This is necessary because the root surface has to remain permeable for water and nutrients and to establish symbiotic interactions, as in the case of mycorrhizal symbiosis. This implies that the root is much more exposed to pathogen infection than the upper plant parts. On the cellular level, it is the plasma membrane of the rhizodermis and cortex cells in the younger parts of the root where the uptake and signalling systems are located.
With respect to nutrients, phosphate and nitrogen availability in the soil are important factors to determine root morphology (Forde and Lorenzo, 2001; Forde, 2002). The local and systemic effects of nitrate on lateral root formation have been shown by Zhang and Forde (2000). However, a signal transduction, initiated by a putative nitrate receptor at the root plasma membrane, has not been clarified so far. Whether NO produced at the plasma membrane of root cells might play a role as a signal for environmental nitrate is under discussion.
Determination of NO in plants
One of the major obstacles in NO research is the exact determination and quantification of NO formation within plant tissues or extracts. Diaminofluoresceins (DAFs) have been designed and synthesized for the detection and imaging of NO (Yao et al., 2004) and have frequently been used to demonstrate NO production in roots (Stöhr and Ullrich, 2002; Guo et al., 2003; Zeidler et al., 2004). The fluorescent chemical transformation of DAFs is based on the reactivity of the aromatic vicinal diamines with N2O3, which itself is the reaction product of NO with dioxygen (Yao et al., 2004). Using fluorescence microscopy, NO formation of plant organs can be visualized directly in situ or in vivo. Although the advantages of this technique are obvious, it has to be considered that the formation of DAF-2T (triazofluorescein), which is the major fluorescent end-product of the reaction with NO, is highly dependent on pH, the availability of oxygen, and the presence of antioxidative substances. Even the presence of Ca2+ can disturb this reaction (Broillet et al., 2001). Additional reaction partners with DAF-2 (4,5-diaminofluorescein) have been also reported (Zhang et al., 2002) and are hardly under control in living objects. The compounds formed by DAF independently on NO have fluorescence emission profiles similar to DAF-2T (Zhang et al., 2002). In particular, the apoplastic acidic pH and the presence of dehydroascorbic acid or ascorbic acid, which have been proved to be present in the apoplast (Horemans et al., 1997), might be a source of false interpretation. The membrane impermeable DAF-2 in combination with fluorescence microscopy was used to study NO formation in tobacco (Nicotiana tabacum) root tips in planta. Distinct fluorescence of root hairs and cells located in the root apex has finally been confirmed to be unrelated to NO using the control substance 4-AF DA (4-aminofluoresceindiacetate) (Fig. 1). Córdoba-Pedregosa et al. (2003) reported differential zonal values for intra- and extracellular ascorbate (ASC) and dehydroascorbate (DHA) concentrations in whole onion (Allium cepa) roots. ASC and DHA concentrations were higher in the root apex and meristem and gradually decreased towards the root base. Zonal differences in ASC and DHA concentrations correspond to the observed DAF fluorescence alongside the root axis (Fig. 1). It seems likely that the observed DAF fluorescence might be a result of the reaction of DAF-2 and 4-AF DA with apoplastic ASC or DHA. Beligni et al. (2002) used 4-AF DA as a negative control to assess the NO fluorescence of injured barley aleurone cells detected with the membrane-permeable DAF-2 DA (4,5-diaminofluoresceindiacetate), but not with DAQ (1,2-diaminoanthraquinone). Since all aleurone protoplasts accumulated the negative control 4-AF DA and showed a bright fluorescence, DAF-2 DA was demonstrated to be unsuitable for monitoring NO production in barley aleurone cells.
Fluorescence of tobacco roots after treatment with diaminofluorescein in planta Fluorescence (excitation: 480 nm, emission: 520 nm) of tobacco roots visualized by fluorescence microscopy (Microscope: Olympus IX 51, Camera: Olympus CC 12) after incubation with DAF-2 (A, C) or 4-AF DA (B, D). Tobacco plants (Nicotiana tabacum L. cv. Samsun) were grown with 5 mM
To demonstrate the effect of ascorbate on the NO determination with DAF-2, the NO donor NONOate (diethylamine NONOate), that releases two moles of NO per mole compound, was used (Table 1). Without further additives the entire added NO could be detected. However, the presence of 1 mM ascorbate without any NO donor also resulted in fluorescence. Yet, NO in the presence of ascorbate could not be detected, since ascorbate inhibited the formation of the fluorescent DAF-2T. Ascorbate probably decreases the nitrosation of DAF-2 because of additional reducing activity that affects the amount of available N2O3 from NO (Zhang et al., 2002).
Effect of ascorbate on the reaction of DAF-2 with NO
A . | . | B . | . | . | A–B . | |||
|---|---|---|---|---|---|---|---|---|
| Substrate . | Calculated NO (μM) . | Substrate . | Addition after stop reaction . | Calculated NO (μM) . | Calculated NO (μM) . | |||
| 1. NONOate | 19.23±1.68 | – | NONOate | 1.35±0.09 | 17.88±1.63 | |||
| 2. Ascorbate | 2.63±0.21 | – | Ascorbate | 1.10±0.26 | 1.53±0.11 | |||
| 3. Ascorbate and NONOate | 2.82±0.11 | Ascorbate | NONOate | 2.78±0.41 | 0.04±0.41 | |||
| NONOate | Ascorbate | 18.15±0.56 | –15.33±0.56 | |||||
A . | . | B . | . | . | A–B . | |||
|---|---|---|---|---|---|---|---|---|
| Substrate . | Calculated NO (μM) . | Substrate . | Addition after stop reaction . | Calculated NO (μM) . | Calculated NO (μM) . | |||
| 1. NONOate | 19.23±1.68 | – | NONOate | 1.35±0.09 | 17.88±1.63 | |||
| 2. Ascorbate | 2.63±0.21 | – | Ascorbate | 1.10±0.26 | 1.53±0.11 | |||
| 3. Ascorbate and NONOate | 2.82±0.11 | Ascorbate | NONOate | 2.78±0.41 | 0.04±0.41 | |||
| NONOate | Ascorbate | 18.15±0.56 | –15.33±0.56 | |||||
(A) DAF-2 (10 μM) was incubated with (1) NONOate (10 μM) or (2) ascorbate (1 mM), or (3) both compounds for 2.5 h at 30 °C in buffer as indicated in Fig. 1. The reaction was terminated by the addition of NaOH (0.05 M). (B) In a second run NONOate or ascorbate was added after incubation under the same conditions as in (A) with DAF-2 and the addition of NaOH as indicated. Mean values ±SD (n=3). The DAF-2T fluorescence was determined in each sample at 495 nm excitation and 515 nm emission wave length.
Effect of ascorbate on the reaction of DAF-2 with NO
A . | . | B . | . | . | A–B . | |||
|---|---|---|---|---|---|---|---|---|
| Substrate . | Calculated NO (μM) . | Substrate . | Addition after stop reaction . | Calculated NO (μM) . | Calculated NO (μM) . | |||
| 1. NONOate | 19.23±1.68 | – | NONOate | 1.35±0.09 | 17.88±1.63 | |||
| 2. Ascorbate | 2.63±0.21 | – | Ascorbate | 1.10±0.26 | 1.53±0.11 | |||
| 3. Ascorbate and NONOate | 2.82±0.11 | Ascorbate | NONOate | 2.78±0.41 | 0.04±0.41 | |||
| NONOate | Ascorbate | 18.15±0.56 | –15.33±0.56 | |||||
A . | . | B . | . | . | A–B . | |||
|---|---|---|---|---|---|---|---|---|
| Substrate . | Calculated NO (μM) . | Substrate . | Addition after stop reaction . | Calculated NO (μM) . | Calculated NO (μM) . | |||
| 1. NONOate | 19.23±1.68 | – | NONOate | 1.35±0.09 | 17.88±1.63 | |||
| 2. Ascorbate | 2.63±0.21 | – | Ascorbate | 1.10±0.26 | 1.53±0.11 | |||
| 3. Ascorbate and NONOate | 2.82±0.11 | Ascorbate | NONOate | 2.78±0.41 | 0.04±0.41 | |||
| NONOate | Ascorbate | 18.15±0.56 | –15.33±0.56 | |||||
(A) DAF-2 (10 μM) was incubated with (1) NONOate (10 μM) or (2) ascorbate (1 mM), or (3) both compounds for 2.5 h at 30 °C in buffer as indicated in Fig. 1. The reaction was terminated by the addition of NaOH (0.05 M). (B) In a second run NONOate or ascorbate was added after incubation under the same conditions as in (A) with DAF-2 and the addition of NaOH as indicated. Mean values ±SD (n=3). The DAF-2T fluorescence was determined in each sample at 495 nm excitation and 515 nm emission wave length.
As an alternative, the chemiluminescence assay has been applied to plant samples (Wildt et al., 1997; Stöhr et al., 2001; Rockel et al., 2002; Planchet et al., 2005). It is considered to be the most useful method because of its high sensitivity and real-time monitoring of NO (Yao et al., 2004). The reaction of NO with ozone (O3) yields nitrogen dioxide (
Sources of NO in plant roots
With regard to the plant organ and substrates, arginine or nitrite, different pathways for the formation of NO in plants have been described. Plants produce NO via the oxidation of arginine to citrulline (Neill et al., 2003), yet, proteomic analysis did not indicate any plant NO synthases (NOS) similar to those found in bacteria or mammals (Butt et al., 2003). Guo et al. (2003) reported a plant protein (AtNOS1) with sequence homology to a NO-producing enzyme in the snail Helix pomatia that also has homology to GTP-binding or GTPase domains. Homozygous mutants of Arabidopsis revealed that AtNOS1 is required for NO synthesis by the oxidation of arginine to citrulline in plants. This protein also seems to be present in roots since, in mutant seedlings, root NO-production was greatly diminished, and it is obviously involved in plant growth, fertility, stomatal movement, and hormone signalling as shown by the ABA-induced NO production in roots. NADPH, calmodulin, and Ca2+ are required as the sole cofactors. The reports about the cellular localization of NOS activity differ with the detection techniques applied, yet it seems to be located in the cytoplasm and peroxisomes (reviewed by del Río et al., 2004).
Nitrite as a substrate is used by cytosolic nitrate reductase (cNR) and by the root-specific plasma membrane-bound nitrite:NO reductase (NI-NOR). In vitro NO production by purified cNR with NADH as the electron source was measured by Yamasaki et al. (1999). In planta the production of NO by cNR is dependent on enzyme activity and the availability of the substrate nitrite. The amount of active cNR in root tissues is highly regulated by environmental factors such as the availability of nitrate (Stöhr, 1999) and oxygen. Root cNR activity is increased under anoxia (Stoimenova et al., 2003), and, as a consequence, the usually low level of cytosolic nitrite is built up and often excreted into the apoplast (Botrel et al., 1996). Extracts prepared from non-elicited leaves (Rockel et al., 2002) exhibit NO formation dependent on the relation between the concentration of nitrate and nitrite. However, a possible contribution of NO produced by cNR during pathogen infection has also been reported by Yamamoto et al. (2003).
Nitrite:NO reductase (NI-NOR), a root-specific enzyme whose activity markedly differs from cytosolic nitrate reductase, has been studied mainly in Nicotiana tabacum (Stöhr et al., 2001). It does not use reduced nicotine adenine nucleotides; instead reduced cytochrome c can serve as an electron donor in vitro. However, participation of cytochrome c at the plasma membrane in vivo seems unlikely and the physiological electron donor has not been identified so far. Its pH optimum is more acidic (pH 6.1 versus pH 8.0) corresponding to the observed pH in apoplast (Felle, 2001). Results of solubilization studies suggest a tight association between NI-NOR and plasma membrane-bound nitrate reductase (PM-NR) which reduces nitrate to nitrite in the root apoplast using succinate as electron donor (Meyer and Stöhr, 2002).
Root PM-NR was purified and subjected to mass spectroscopic analysis. However, the obtained data only indicate that it might represent an unknown protein and no indications for a true nitrate reductase protein were obtained. So far, the identity of root PM-NR still remains undetermined. The root PM-NR activity (PM-NRA), however, has been shown to be strongly enhanced by high external nitrate concentrations from 5 mM up to 25 mM in sand culture, indicating an almost linear relationship between PM-NRA and external nitrate concentration in this range (Fig. 2; Stöhr, 1999). Since tobacco plants grown with 2 mM nitrate in sand culture revealed strong nitrogen-deficiency symptoms, 10 mM nitrate was chosen as an optimal growth condition as it was proven by analysis of the growth parameter (Stöhr, 1999). Concentrations above 25 mM nitrate in the nutrient solution led to a decrease in growth as well as in root PM-NRA, demonstrating the detrimental effect of nitrate in such high concentrations. The NO production by root plasma membrane vesicles was also raised together with the external nitrate concentration (Fig. 2), yet, it did not vary as strongly as PM-NRA between 10 mM and 25 mM nitrate. The highest NI-NOR activity was detected at 35 mM nitrate supply, corresponding to a markedly reduced growth of the plant (Stöhr, 1999). This could indicate that, for plants, the toxicity of high nitrate concentrations might be a result of NO increment at the root plasma membrane. In denitrifying bacteria different membrane-bound nitrite reductases are known to produce NO from nitrite (reviewed by Zumft, 1997). Purification of the NI-NOR protein is under progress and will finally show whether there exists any homology to those bacterial enzymes.
Activities of plasma membrane-bound nitrite-NO reductase (NI-NOR) dependent on nitrate supply. Tobacco plants (Nicotiana tabacum L. cv. Samsun) were grown with nutrient solution ranging from 5–40 mM
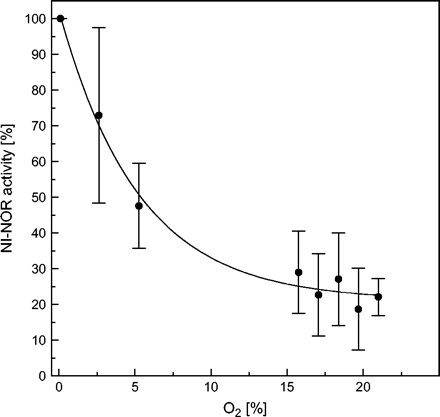
Effect of oxygen on in vitro activity of plasma membrane-bound nitrite-NO reductase (NI-NOR). Preparation of tobacco root plasma membrane vesicles as well as NO detection was performed as described for Fig. 1. To ensure the detection of the expected low NO-forming rates under air, 60 μg of PM protein were used (different from Fig. 1). NO quenching due to its possible reaction with oxygen was estimated with 10 μM NONOate for each oxygen concentrations. Correction factors have been included in the calculation of NI-NOR activity. The gas flow was adjusted by mixing compressed air and nitrogen as indicated. Mean values ±SD (n=3).
In the plant apoplast, a non-enzymatic production of NO has also to be considered. It has been demonstrated that reducing agents such as ascorbic acid and also some phenolics can accelerate the rate of NO formation from nitrite (Bethke et al., 2004) dependent on the acidic apoplastic pH.
Role of NO in roots
NO is released from soils in the order of a few mg N m−2 h−1 (as reviewed by Stöhr and Ullrich, 2002), however, variations are dependent on temperature, oxygen availability, pH, and N-fertilization rates. Although most of this release has been attributed to bacterial nitrification and denitrification, quantitative data on the contribution of soil bacteria to such rates vary a great deal. In spite of the increasing knowledge on NO and its role in root growth and developmental processes, a quantification of enzymatic NO formation by plant roots in soils and its distribution in the closer rhizosphere is still difficult, since any NO detected may result from microbial activity or from the plant root.
The involvement of NO in promoting root growth has been observed by Gouvêa et al. (1997). They found that NO induces cell elongation in a similar way to auxin. A transient increase in NO concentration was shown to be involved in adventious root development induced by indole acetic acid (Pagnussat et al., 2002). The authors suggest that NO could mediate the auxin response in this process. The participation of NO in gravitropic bending in soybean roots has been described by Hu et al. (2005). They found an asymmetric accumulation of NO in the primary root in response to gravistimulation. Similar to Pagnussat et al. (2002) it was observed that NO acts downstream of auxin leading to the accumulation of cGMP. The involvement of auxin during root development in response to nitrate has been demonstrated by Forde (2002). A possible role of NO in mediating root development, in response to nitrate and signalling at the root–environment interface, is inferred by the co-ordinated activity of the root-specific plasma membrane-bound enzymes, nitrate reductase (PM-NR) and nitrite:NO reductase (NI-NOR).
The availability of oxygen to plant roots might also be limited by environmental conditions. The soil might be well drained or, quite the reverse, subjected to flooding periods causing hypoxic or even anoxic conditions. The positive effect of nitrate assimilation under hypoxia has been frequently discussed and its effect on pH regulation, carbohydrate utilization, and regeneration of NAD+ has been considered in this context (Stoimenova et al., 2003; Igamberdiev and Hill, 2004). NO production by cNR is higher under hypoxic condition than in well-aerated tissue. This is a consequence of accumulated nitrite due to NR activity. Since NOS-type enzymes require oxygen for NO production, perhaps additional NO-producing enzymes had to be developed in roots that remain active under oxygen deprivation. Gupta et al. (2005) demonstrated the hypoxic/anoxic nitrite-dependent NO production by root mitochondria in the presence of NADH. Inhibitor studies suggest that both terminal oxidases of mitochondria are involved in the electron flow to nitrite. However, the authors cannot exclude the participation of further enzymes.
NI-NOR activity is reversibly inhibited by the presence of oxygen (Fig. 3). It loses about 78% of activity in ambient air corresponding to 21% oxygen. This indicates that, in the root apoplast, nitrite-dependent NO formation might also be regulated by the availability of oxygen in vivo. A high apoplastic NO production might also function as a signal for oxygen deficiency. Since a function of NO during programmed cell death has been described (Neill et al., 2003), its role in the development of aerenchyma under hypoxic condition in roots should be considered. In agreement with this idea is the report of Drew et al. (2000) demonstrating that inhibitors of NO signal transduction via cGMP in mammalian cells also affected aerenchym formation in maize roots. Igamberdiev and Hill (2004) discussed the role of nitrate, NO, and haemoglobin in maintaining plant cell viability under anoxic stress. Nitrate as an intermediate electron acceptor under oxygen deficiency leads finally to the production of NO by the root plasma membrane-bound enzymes or by cytosolic nitrate reductase. A cycle is proposed by Igamberdiev and Hill (2004) whereby the NO is oxygenated by hypoxically-induced class 1 haemoglobin. The turnover of this reaction is maintained by a methaemoglobin reductase. This cyclic reaction may help to maintain the redox status of the cell at very low oxygen tension as an alternative to fermentative pathways.
Regarding the dependence on the nitrate availability of the plasma membrane-bound root NO-forming system, this system could also represent a mechanism in regulating mycorrhizal symbiosis related to plant demand for nitrogen. Endosymbiotic interactions between plant and fungi include parasitic interactions, and mutualistic symbiosis as established between plant roots and arbuscular mycorrhizal fungi or nitrogen-fixing bacteria. The mutualistic association constitutes a strategy to improve the nutritional status of both partners. In the case of mycorrhiza, the fungi receive fixed carbon compounds from the host plant, while the plant benefits from the association by increased nutrient uptake, enhanced tolerance to abiotic stress and resistance to pests (Smith and Read, 1997). The mechanisms controlling the development of arbuscular mycorrhiza are the subject of current studies. Similar to plant–pathogen interactions, the induction or suppression of plant defence mechanisms including the hypersensitive response and cell death seem to play a very important role in the colonization by arbuscular mycorrhizal fungi (Allen et al., 1989; Douds et al., 1998; Salzer and Boller, 2000; Bonanomi et al., 2001; Bilou et al., 2000a, b; García-Garrido and Ocampo, 2002; Medina et al., 2003; Vieweg et al., 2005). It might be that the NO production by the plasma membrane-bound enzymes in roots, whose activity is highly dependent on nitrate availability in the soil, affects the development of pathogens as well as of arbuscular mycorrhiza. This might lead to poor or even no colonization of the plant at higher nitrate supply. In agreement with this idea is the function of haemoglobins as NO scavengers during root colonization by mycorrhizal fungi, which have been shown to be necessary to avoid programmed cell death (Vieweg et al., 2005).
Protection of plants against high NO concentration
The observed effects of elevated NO levels are caused by its high reactivity as a radical with oxygen, radical oxygen species (ROS), and metals. High concentrations of NO can either be beneficial (e.g. it activates defence responses or, together with ROS, it directly kills the pathogen) or detrimental for the plant cell itself (Beligni and Lamattina, 1999). Among the deleterious effects are lipid peroxidation, oxidation of tyrosine, as well as S-nitrosylation. The interaction of NO with iron-containing proteins can lead to the inhibition of enzymes such as the cytochrome c oxidase of mitochondrial respiration (Millar and Day, 1997; Yamasaki et al., 2001).
During the hypersensitive response, death of certain plant cells is beneficial for the plant. However, plants may also have developed protective mechanisms against high intracellular NO concentrations. The presence of various molecules acting as NO scavengers could help to prevent unwanted NO reactions. Urate has been shown to prevent the toxic effects of peroxynitrite in Arabidopsis (Alamillo and García-Olmedo, 2001). Recently, several studies have indicated a role for haemoglobins in the detoxification process of NO in plants (Dordas et al., 2003; Perazzolli et al., 2004; Vieweg et al., 2005). In plants, three types of haemoglobins have been discovered, which have been categorized as symbiotic, non-symbiotic, and truncated haemoglobins (Perazzolli et al., 2004). Non-symbiotic haemoglobins seem to exist in the entire plant kingdom. Their expression patterns vary in different tissues as well as in response to different types of stress (Hunt et al., 2001). Perazzolli et al. (2004) provided evidence that Arabidopsis non-symbiotic haemoglobin AHb1 functions as a NO dioxygenase, metabolizing NO to nitrate. Moreover they could show that S-nitrosohaemoglobin is endogenously produced in plants, indicating that Cys residue(s) play a conserved role in processing of NO and S-nitrosothiols (SNO). AHb1 reduces NO emission under hypoxic stress, confirming its role in NO detoxification in planta.
Truncated haemoglobins also appear to be present throughout the plant kingdom and share some characteristics with non-symbiotic haemoglobins (Perazzolli et al., 2004). Two new genes have been identified in Medicago truncatula encoding two different truncated haemoglobins (MtTrHb1, MtTrHb2) specifically expressed in root nodules and VAM colonized root cells (Vieweg et al., 2005). Since Ouellet et al. (2002) showed a detoxifying effect on NO for the bacterial haemoglobins (TrHbs), it appears plausible that the plant haemoglobins might also repress NO accumulation during rhizobial and mycorrhizal symbioses in order to diminish defence gene induction and programmed cell death. In addition, haemoglobins are induced under hypoxic conditions in different tissues such as seeds, roots, and stem tissue (Dordas et al., 2003). Haemoglobins may support respiration under oxygen limitation by scavenging NO, which is known to inhibit respiration. NO affects the mitochondrial cytochrome c oxidase, but not the alternative oxidase, whose transcription and activity can even be stimulated by NO (Huang et al., 2002). In this context, the formation of extracellular NO, as observed in roots, might also be an adaptation to avoid a high concentration of NO within the plant cell.
Concluding remarks
The discovery of different NO-forming pathways in plants is the basis for understanding the different effects of NO found under various conditions in plants. It became evident that the location and nature of NO-forming enzymes is crucial. Further expression studies of those genes will reveal the events leading to NO synthesis in different plant organs, cells, and cell compartments. Further data on NO-induced gene expression will lead to an understanding of regulation and signal transduction. NO research in plants has entered the next phase in understanding the network of reactions leading to toxic or beneficial effects in plants.
This work was supported by the DFG (STO 311/2).
References
Alamillo JM, García-Olmedo F.
Allen MF, Allen EB, Friese CF.
Beligni MV, Fath A, Bethke PC, Lamattina L, Jones RL.
Beligni MV, Lamattina L.
Beligni MV, Lamattina L.
Bethke PC, Badger MR, Jones RL.
Bilou I, Ocampo JA, Garcia-Garrido JM.
Bilou I, Bueno P, Ocampo JA, Garcia-Garrido JM.
Bonanomi A, Oetiker JH, Guggenheim R, Boller T, Wiemken A, Vogeli-Lange R.
Botrel A, Magné C, Kaiser WM.
Bradford MM.
Broillet M, Randin O, Chatton J.
Butt YKC, Lum JHK, Lo SCL.
Córdoba-Pedregosa MC, Córdoba F, Villalba JM, González-Reyes JA.
Delledonne M, Xia Y, Dixon RA, Lamb C.
Delledonne M, Zeier J, Marocco A, Lamb C.
del Río LA, Corpas FJ, Barroso JB.
Desikan R, Griffiths R, Hancock J, Neill S.
Desikan R, Cheung MK, Bright J, Henson D, Hancock JT, Neill S.
Dordas C, Rivoal J, Hill RD.
Douds DD, Galvez L, Bécard G, Kapulnik Y.
Drew MC, He I, Morgan PW.
Durner J, Wendehenne D, Klessig DF.
Foissner I, Wendehenne D, Langebartels C, Durner J.
Forde BG.
García-Garrido JM, Ocampo JA.
Gouvêa CMCP, Souza JF, Magalhães CAN, Martins IS.
Gupta KJ, Stoimenova M, Kaiser WM.
Guo FQ, Okamoto M, Crawford NM.
Horemans N, Asard H, Caubergs RJ.
Hu X, Neill S, Tang Z, Cai W.
Huang X, von Rad U, Durner J.
Hunt PW, Watts RA, Trevaskis B, Liewelyn DJ, Burnell J, Dennis ES, Peacock WJ.
Igamberdiev AU, Hill RD.
Klessig DF, Durner J, Noad R, et al.
Medina MJH, Gagnon H, Piché Y, Ocampo JA, Garcia-Garido JM, Vierheilig H.
Meyer C, Stöhr C.
Millar AH, Day DA.
Ouellet H, Ouellet Y, Richard C, Labarre M, Wittenberg B, Wittenberg J, Guertin M.
Pagnussat GC, Simontacchi M, Puntarulo S, Lamattina L.
Planchet E, Kapuganti JG, Sonoda M, Kaiser WM.
Perazzolli M, Dominici P, Romero-Puertas MC, Zago E, Zeier J, Sonoda M, Lamb C, Delledonne M.
Rockel P, Strube F, Rockel A, Wildt J, Kaiser WM.
Salzer P, Boller T.
Stöhr C.
Stöhr C, Strube F, Marx G, Ullrich WR, Rockel P.
Stöhr C, Ullrich WR.
Stöhr C, Ullrich WR.
Stoimenova M, Libourel IGL, Ratcliff RG, Kaiser WM.
Tun NN, Holk A, Scherer GF.
Vieweg MF, Hohnjec N, Küster H.
Wildt J, Kley D, Rockel A, Rockel P, Segschneider HJ.
Yamamoto A, Katou S, Yoshioka H, Doke N, Kawakita K.
Yamasaki H, Sakihama Y, Takahashi S.
Yamasaki H, Shimoji H, Ohshiro Y, Sakihama Y.
Yao D, Vlessidis AG, Evmiridis NP.
Zeidler D, Zähringer U, Gerber I, Dubery I, Hartung T, Bors W, Hutzler P, Durner J.
Zhang H, Forde BG.
Zhang X, Kim WS, Hatcher N, Potfieter K, Moroz LL, Gillette R, Sweedler JV.



![Fluorescence of tobacco roots after treatment with diaminofluorescein in planta Fluorescence (excitation: 480 nm, emission: 520 nm) of tobacco roots visualized by fluorescence microscopy (Microscope: Olympus IX 51, Camera: Olympus CC 12) after incubation with DAF-2 (A, C) or 4-AF DA (B, D). Tobacco plants (Nicotiana tabacum L. cv. Samsun) were grown with 5 mM \batchmode \documentclass[fleqn,10pt,legalpaper]{article} \usepackage{amssymb} \usepackage{amsfonts} \usepackage{amsmath} \pagestyle{empty} \begin{document} \(\mathrm{NO}_{3}^{{-}}\) \end{document} in sand culture (according to Stöhr, 1999). Three days before the experiment, plants (4-weeks-old) were transferred to light-protected Petri dishes and daily irrigated with nutrient solution. Without further disturbance root fluorescence was detected in planta 30 min after the addition of 20 μM DAF-2 or 4-AF DA in (HEPES 0.025 M and MES 0.05 M buffer adjusted to pH 6.0 with NaOH). The detection of root autofluorescence had been reduced by technical means before the fluorescence dye was added. Fluorescence of roots after incubation (A) with 20 μM DAF-2 and (B) with 20 μM of the control dye 4-AF DA. In both cases, the strongest fluorescence signals were detected in the root tip, mainly in cells of the root cap and of the root meristem indicating that the DAF-2 fluorescence signal is not caused by NO production. Fluorescence signals derived from both compounds were also observed with root hairs, which were either incubated (C) with 20 μM DAF-2 or (D) with 20 μM 4-AF DA. In both cases a strong fluorescence signal became visible at the tip of the root hairs.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jxb/57/3/10.1093/jxb/erj058/2/m_jexboterj058f01_4c.gif?Expires=1716561052&Signature=Kv9LqQ7r804R57iCZf0obbrRmD~4QnTm-sPtlPxv0njKCOHQ5pEkKTGsg9mHYfYjDLQJRlp7u6TLHIkVcurkfHTC2br34vRbB7JheKckAYqLGREkRqj8yQdUuImuqiYwZ5MXeVLdlrGGVuvun4imoPBumedNz~8hOcKqPDbYQEFwW5TeD85T~KhaBHfhpzoCtDjmrmUaDWoYm4hl8y3orzrUw1MwRkfWECmL-X2ADEHiYvKzRubaGUZzKRf8TuaRhTeaUAl-XMmFcxo5Qo~7951eOd-jV7XO0A3Ap5GaxOOxZAvLCqSJYDzF28Q2ZR4XZd~w7XWR-MTLPFyHcL~vKg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Activities of plasma membrane-bound nitrite-NO reductase (NI-NOR) dependent on nitrate supply. Tobacco plants (Nicotiana tabacum L. cv. Samsun) were grown with nutrient solution ranging from 5–40 mM \batchmode \documentclass[fleqn,10pt,legalpaper]{article} \usepackage{amssymb} \usepackage{amsfonts} \usepackage{amsmath} \pagestyle{empty} \begin{document} \(\mathrm{NO}_{3}^{{-}}\) \end{document} as indicated (according to Stöhr, 1999). Homogenization of roots and preparation of plasma membrane vesicles with the two-polymer phase system were performed as reported by Stöhr and Ullrich (1997). Protein in the fractions was determined according to Bradford (1976) in the presence of 0.1% octylglucoside. Recording of NO formation by chemiluminescence detection was performed as described by Stöhr et al. (2001) under anoxic conditions. Nitrogen gas (5.0) was first perfused through deionized water and then through the glass chamber with a constant flow of 0.4 l min−1. The sample was continuously stirred in a final volume of 500 μl HEPES-buffer (0.025 M) with MES (0.05 M) adjusted to pH 6.0 with NaOH in presence of NaNO2 (1 mM) and reduced cytochrome c (final concentration 0.3 mg ml−1). The reaction was started by addition of 30 μg plasma membrane protein. NO was measured by chemiluminescence intensity (CLD 88 ep, ECO PHYSICS), fluxes were calculated from concentration differences before and after addition of plasma membrane vesicles (closed circles). Mean values ±SD (n=3). For comparison, root PM-NR activities are presented (dotted line) as published by Stöhr (1999) and reprinted by kind permission of Blackwell Publishing.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jxb/57/3/10.1093/jxb/erj058/2/m_jexboterj058f02_lw.gif?Expires=1716561052&Signature=Jwcunt0F83tV1p6Xx7vo6j3OfPw3AlT3eTKmmExE29iVXxzT3RS-inVNXSPmjD5xaKBE8hxGJtDnkO3BjnMpFH3Ofb2PdIbpX5CRJMwwnEETxiyWSP~f~ZaVlM9osJSLZ4pLyQYiroxJxbZSZ52lGcxXkDcNRMSG4KX80ax82pJTQ~Kmt1xCk0EOfmEkUz9pSTIZyiL7I~SWqvfvMt1SfbV9gjvRkBNODg2RYxZjTKzQnYyvZf0migXKKRdfS6tiegNNoUrmNNkZ7lxa0IG2P3SPaOJ-KpW0b19EP609hYgHf1soARdx~E2ETkaVNF8zOCWZ~We88wNW8FbO~tMKaw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

Comments