-
PDF
- Split View
-
Views
-
Cite
Cite
Rana Munns, Richard A. James, André Läuchli, Approaches to increasing the salt tolerance of wheat and other cereals, Journal of Experimental Botany, Volume 57, Issue 5, March 2006, Pages 1025–1043, https://doi.org/10.1093/jxb/erj100
Close - Share Icon Share
Abstract
This review describes physiological mechanisms and selectable indicators of gene action, with the aim of promoting new screening methods to identify genetic variation for increasing the salt tolerance of cereal crops. Physiological mechanisms that underlie traits for salt tolerance could be used to identify new genetic sources of salt tolerance. Important mechanisms of tolerance involve Na+ exclusion from the transpiration stream, sequestration of Na+ and Cl− in the vacuoles of root and leaf cells, and other processes that promote fast growth despite the osmotic stress of the salt outside the roots. Screening methods for these traits are discussed in relation to their use in breeding, particularly with respect to wheat. Precise phenotyping is the key to finding and introducing new genes for salt tolerance into crop plants.
Introduction
Increased salt tolerance of crops is needed to sustain food production in many regions in the world. In irrigated agriculture, improved salt tolerance of crops can lessen the leaching requirement, and so lessen the costs of an irrigation scheme, both in the need to import fresh water and to dispose of saline water (reviewed by Pitman and Läuchli, 2002). Salt-tolerant crops have a much lower leaching requirement than salt-sensitive crops. In dry-land agriculture, improved salt tolerance can increase yield on saline soils. In areas where the rainfall is low and the salt remains in the subsoil, increased salt tolerance will allow plants to extract more water. Salt tolerance may have its greatest impact on crops growing on soils with natural salinity as, when all the other agronomic constraints have been overcome (e.g. disease resistance and nutrient deficiency), subsoil salinity remains a major limitation to agriculture in all semi-arid regions. Even where clearing of land in higher rainfall zones has caused water-tables to rise and salt to move, improved salt tolerance of crops will have a place. The introduction of deep-rooted perennial species is necessary to lower the water-table, but salt tolerance will be required not only for the ‘de-watering’ species, but also for the annual crops that follow, as salt will be left in the soil when the water-table is lowered.
Wheat (Triticum aestivum) is a moderately salt-tolerant crop (Maas and Hoffman, 1977). In the field, where the salinity rises to 100 mM NaCl (about 10 dS m−1), rice (Oryza sativa) will die before maturity, while wheat will produce a reduced yield. Even barley (Hordeum vulgare), the most-tolerant cereal, dies after extended periods at salt concentrations higher than 250 mM NaCl (equivalent to 50% seawater). Durum wheat (Triticum turgidum ssp. durum) is less salt tolerant than bread wheat, as are maize (Zea mays) and sorghum (Sorghum bicolor) (Maas and Hoffman, 1977; Salt Tolerance Database reproduced on USDA-ARS, 2005).
Only halophytes (plants adapted to saline habitats) will continue to grow at salinities over 250 mM NaCl. Domestication of halophytes as new crops has been reviewed by Colmer et al. (2005), who point out that few species have reached the status of crop plant and none has a wide usage. However, some are useful forage species for saline land. Saltbushes (Atriplex spp.) are very salt tolerant, and can lower water-tables that have reached the surface, and restore saline land for animal production (Barrett-Lennard, 2002). The halophytic relative of wheat, tall wheatgrass (Thinopyrum ponticum, syn. Agropyron elongatum), is grown for forage on saline soils. Distant halophytic relatives of barley, such as sea barleygrass (Hordeum marinum), are even more salt tolerant (Garthwaite et al., 2005), but are not useful for forage. The salt tolerance in these halophytic relatives of wheat and barley has not yet been used to improve the tolerance of the related crop species, as the mechanisms conferring tolerance to the wild relatives are unknown, and the genomes do not recombine at meiosis, so a forward genetic approach cannot be used. The potential of wild relatives to improve the salt tolerance of wheat is reviewed by Colmer et al. (2006).
Relationship between salinity and yield in grain crops
Estimates of grain yield bring another complexity to the salinity response, not just because the crops must be grown in uncontrolled environments for long periods of time, but because the conversion of shoot biomass to grain biomass is complex. The harvest index (the proportion of total shoot mass that is found in grain) can vary from 0.2 to 0.5, depending on the timing and severity of the salt treatment (Francois et al., 1994; Husain et al., 2003). A low level of salinity may not reduce grain yield even though the leaf area and shoot biomass is reduced, which is reflected in a harvest index that increases with salinity, and the fact that grain yield may not decrease until a given (‘threshold’) salinity is reached. The comprehensive survey of salt tolerance of crops and pasture species published by the US Salinity Laboratory (Maas and Hoffman, 1977; USDA-ARS, 2005) presents for each species a threshold salinity below which there is no reduction in yield, and then a linear reduction in yield with increasing salinity (a ‘bent stick’ relationship). In that survey, the yield of rice starts to decline at 3 dS m−1 (30 mM NaCl) compared with 6–8 dS m−1 for wheat (60–80 mM NaCl), and the subsequent linear yield decline of rice with increasing salinity is double that of wheat. The survey highlights the genetic variation between species. However, in most cases, the data are for a single cultivar of the species or a limited number of cultivars at a single site, so they are not necessarily representative of the species, which can show a large genetic diversity. For example, large genetic variation was found with barley and durum wheat grown in the field with drip irrigation of different salinity levels up to 26 dS m−1 (Royo et al., 2000; Royo and Abió, 2003). These and other studies present a sigmoidal rather than a ‘bent stick’ relationship between yield and salinity, leading to the suggestion that an EC50 (EC that results in a 50% yield decline) is a more useful comparison between genotypes than a linear rate of decline (Royo et al., 2000; Steppuhn et al., 2005).
The studies of Royo et al. (2000) with barley, and of Royo and Abió (2003) with durum wheat, report a 30–40% lower salt tolerance than those of Maas and Hoffman (1977). This may have been due to treatments starting earlier in the former studies, and reducing the yield potential through the reduction in tiller formation, or it may have been due to the number of irrigations being less or the ambient conditions hotter and drier so that the salt concentration around the roots was greater (for a given EC) than in the experiments reported by Maas and Hoffman (1977). This raises the problem that screening in one environment may not select the right genotypes for a different environment. Field conditions vary from site to site, not only in soil salinity, but also in soil physical and chemical properties such as sodicity, high pH, and possibly toxic trace elements such as boron (Rengasamy, 2002). Further, there are differences across seasons in temperature and drought, which, particularly in dry-land agriculture, will directly affect the build-up of salts around the roots. Differences in flowering or maturity times between genotypes can cause large differences in yield if the ambient conditions are variable during the flowering or grain-filling periods.
For many soils, waterlogging and salinity are inextricably linked. In countries like Pakistan, the use of irrigation water of high ‘sodium hazard’ (high sodium absorption ratio) causes a decline in the structure of the fine-textured soils, and poor infiltration of water results in salinization (through evaporation of irrigation water at the soil surface) and waterlogging (Qureshi and Barrett-Lennard, 1998). In Australia, secondary salinity arises where water-tables rise to within 2 m of the soil surface, i.e. close to the root zone. Furthermore, as the soil has air-filled porosities of about 10%, it takes only 100 mm of rain for water-tables to rise to the surface, and plants to experience simultaneous salt and waterlogging stresses (Barrett-Lennard, 2002). Rarely are genotypes ranked for salt tolerance in contrasting environments. The extent of genotype×environment interactions is not fully understood, but is likely to be quite large.
Past approaches to improving salt tolerance in wheat
Various approaches have been taken to improve the salt tolerance of wheat by introducing genes for salt tolerance into adapted cultivars, including screens of large international collections, detailed field trials of selected cultivars, conventional breeding methods, and unconventional crosses with wheat relatives. The aim has been to exploit variation in salt tolerance within wheat and its progenitors or close relatives to produce new wheats with more tolerance than modern wheat cultivars.
Large international collections have been screened in hydroponic or sand culture by Kingsbury and Epstein (1984), Srivastava and Jana (1984), Sayed (1985), and Martin et al. (1994). For example, Kingsbury and Epstein (1984) screened 5000 accessions of bread wheat and found 29 that produced seed in 50% seawater. The results of these extensive screens have been summarized by Colmer et al. (2005), who comment that they were not followed by yield trials and that new cultivars have not been developed from the outstanding genotypes identified. Jafari-Shabestari et al. (1995) screened 400 Iranian wheats in an irrigated field site in California and identified several accessions that were consistently high for grain yield in both low and high salinity treatments, but no new salt-tolerant wheat cultivar was developed through breeding as a consequence of the screening work (CO Qualset, personal commmunication).
Little work has been done on breeding for salt tolerance in wheat. Many plant breeders in Australia are aware of the need for salt tolerance but this is only one of a number of constraints, the major one being drought, so salt tolerance is not specifically targeted. Targeted breeding has been largely confined to India and Pakistan. The most successful releases have been the Indian KRL1-4 and KRL 19, released by the Central Soil Salinity Research Institute (CSSRI) at Karnal, the Pakistani LU26S and SARC-1, released by the Saline Agriculture Research Cell (SARC) at Faisalabad, and the Egyptian Sakha 8, released by the Agricultural Research Centre at Giza.
In India, almost all salt-tolerant wheat germplasm is derived from Kharchia 65, a line developed from selections from farmers' fields in the sodic-saline soils of the Kharchi-Pali area of Rajasthan (Rana, 1986). KRL1-4, a cross of Kharchia 65 with WL711, has done well on the saline soils of northern India, but not in Pakistan, possibly because of the heavier soils and greater problems of waterlogging (Hollington, 2000). Another derivative of Kharchia 65 was developed in the UK by SA Quarrie and A Mahmood: a doubled haploid line, KTDH 19, from a cross of Kharchia 65 with a line identified with exceptional sodium exclusion, TW161. This derivative performed well in Spain (Hollington et al., 1994) but in India and Pakistan, although highly tolerant in terms of total dry matter, its grain yield was very low due to it maturing around 2 weeks later than local genotypes (Hollington, 2000). Mutation breeding has been used to reduce its time to maturity by 3 weeks without adverse effects on yield at 150 mM NaCl (Mahar et al., 2003). This material is now being further tested in southern Pakistan (PA Hollington, personal communication).
The Pakistan selection LU26S showed improved yields on saline soils in Pakistan (Qureshi et al., 1980), but it is susceptible to rust and not adapted to dense saline-sodic soils where there is the possibility of waterlogging (PA Hollington, personal communication). LU26S was crossed with Kharchia, and two salt-tolerant genotypes, S24 and S36, were selected from F3 seed at salinity levels of 24 and 36 dS m−1, respectively (Ashraf and O'Leary, 1996). S24 had high salt tolerance, as high as Kharchia and SARC-1, possibly due to its low leaf Na+ accumulation (Ashraf, 2002).
Other approaches to improving salt tolerance in wheat are based on mechanisms for salt tolerance, using physiological traits to select germplasm. In wheat, salt tolerance is associated with low rates of transport of Na+ to shoots, with high selectivity for K+ over Na+ (Gorham et al., 1987, 1990). Bread wheats (hexaploid, ABD genomes) have a low rate of Na+ accumulation and enhanced K+/Na+ discrimination, a character controlled by a locus (Kna1) on chromosome 4D (Dubcovsky et al., 1996). The gene or genes associated with this locus have not been identified. Durum wheats (tetraploid, AB genomes) have higher rates of Na+ accumulation and poor K+/Na+ discrimination (Gorham et al., 1987; Munns et al., 2000b) and are less salt tolerant than bread wheat. A locus (Nax1) on chromosome 2A controlling Na+ accumulation has been found in an unusual durum genotype (Lindsay et al., 2004), and a tightly linked molecular marker is being used to introduce the trait for low Na+ accumulation into durum cultivars in a breeding programme, as described in a following section.
Correlations between grain yield and Na+ exclusion from leaves, along with the associated enhanced K+/Na+ discrimination, have been shown in wheat (Chhipa and Lal, 1995; Ashraf and O'Leary, 1996; Ashraf and Khanum, 1997), although the relationship does not hold across all genotypes (Ashraf and McNeilly, 1988; El-Hendawy et al., 2005), showing that Na+ exclusion is not the only mechanism of salt tolerance.
Improving salt tolerance in other cereals
Barley is one of the most salt-tolerant crops (Maas and Hoffman, 1977). Its greater salt tolerance in the field may derive partly from its rapid growth and fast phenological development, leading to an early maturity date. When developmental differences are eliminated, the difference in salt tolerance between barley and wheat becomes less clear. In a glasshouse comparison of the response to salinity in various cereals at the vegetative stage, Rawson et al. (1988b) found only small differences in biomass production in salinity between several barley, wheat, and triticale cultivars. All the same, the barley cultivars were, on the whole, more salt tolerant than the bread-wheat cultivars in conditions of both normal and accelerated development, and some barley cultivars were more salt-tolerant than others (Rawson et al., 1988b). Varietal differences for yield in saline conditions have been shown in several studies in both glasshouse (Greenway, 1962) and field (Richards et al., 1987; Slavich et al., 1990).
The Kna1 locus appears to be absent in barley, as judged by the high Na+ and low K+ concentrations compared with wheat (Gorham et al., 1990). There are varietal differences in the extent of accumulation of Na+ and Cl− in leaves (Greenway, 1962; Forster et al., 1994), but the relationship between Na+ or Cl− accumulation and salt tolerance has not been established in barley to the same extent as in wheat and rice (Colmer et al., 2005).
Progress in breeding for salt tolerance in rice has been reviewed by Gregorio et al. (2002). Salt-tolerant rice varieties such as Pokkali and Nona Bokra originated in coastal areas of India, and are the source of salt tolerance in most rice breeding programmes. Recent approaches to improve the salt tolerance of modern rice cultivars include somaclonal variants, anther culture-derived lines, and molecular marker-assisted selection (Gregorio et al., 2002). A region on the long arm of chromosome 1 has been shown to contain a quantitative trailt locus (QTL) for Na+ exclusion and K+/Na+ discrimination (Koyoma et al., 2001; Bonilla et al., 2002; Lin et al., 2004), independently designated Saltol and SKC1. Just recently, OsHKT8 has been identified as a candidate gene for SKC1 by Ren et al. (2005).
Major increases in salt tolerance of modern cereal cultivars will come from introducing new genes by either crossing with new donor germplasm or by transformation with single genes. In either case, the progeny must be back-crossed into adapted cultivars before the donor genes are of use to farmers. An understanding of physiological and genetic mechanisms is necessary for a breeding programme, in order to select the desired trait in the different genetic backgrounds. With either method of introducing new genes for salt tolerance, the expressed trait must be selected in back-crossed lines that undergo seed multiplication before field trials can take place.
Characteristics of a salt-tolerant variety include Na+ ‘exclusion’, K+/Na+ discrimination, retention of ions in the leaf sheath, tissue tolerance, ion partitioning into different-aged leaves, osmotic adjustment, transpiration efficiency, early vigour and early flowering leading to a shorter growing season, the latter increasing water use efficiency (summarized by Colmer et al., 2005). This paper discusses the physiological mechanisms; the individual genes that regulate these processes have been reviewed recently (Munns, 2005).
Processes involved in the osmotic versus salt-specific effects on growth
The two phases of the growth response
Salt in the soil water inhibits plant growth for two reasons. First, the presence of salt in the soil solution reduces the ability of the plant to take up water, and this leads to slower growth. This is the osmotic or water-deficit effect of salinity. Second, excessive amounts of salt entering the transpiration stream will eventually injure cells in the transpiring leaves and this may further reduce growth. This is the salt-specific or ion-excess effect of salinity.
In wheat, the two responses occur sequentially, giving rise to a two-phase growth response to salinity (Munns, 1993). For example, comparisons between genotypes with contrasting rates of Na+ uptake, and long-term differences in salt tolerance (Schachtman et al., 1991), showed that both genotypes had the same growth reduction for 4 weeks in 150 mM NaCl, and it was not until afterwards that a growth difference between the genotypes was clearly seen (Munns et al., 1995). However, within 2 weeks, dead leaves were seen on the more sensitive genotype, and the rate of leaf death of old leaves was clearly greater on the sensitive than the tolerant genotype. Once the number of dead leaves increased above about 20% of the total, plant growth slowed down and many individuals started to die (Munns et al., 1995).
The first phase of the growth response results from the effect of salt outside the plant. The salt in the soil solution (the ‘osmotic stress’) reduces leaf growth and, to a lesser extent, root growth (Munns, 1993). The cellular and metabolic processes involved are common to drought. The rate at which new leaves are produced depends largely on the water potential of the soil solution, in the same way as for a drought-stressed plant. Salts themselves do not build up in the growing tissues at concentrations that inhibit growth, as the rapidly elongating cells can accommodate the salt that arrives in the xylem within their expanding vacuoles. So, the salt taken up by the plant does not directly inhibit the growth of new leaves.
The second phase of the growth response results from the toxic effect of salt inside the plant. The salt taken up by the plant concentrates in the old leaves; continued transport of salt into transpiring leaves over a long period of time eventually results in very high Na+ and Cl− concentrations, and the leaves die. The cause of the injury is probably due to the salt load exceeding the ability of the cells to compartmentalize salts in the vacuole. Salts then would rapidly build up in the cytoplasm and inhibit enzyme activity. Alternatively, they might build up in the cell walls and dehydrate the cell (Flowers et al., 1991), but Mühling and Läuchli (2002) found no evidence for this in maize cultivars that differed in salt tolerance.
The rate that leaves die is crucial for the survival of a plant. If new leaves are continually produced at a rate greater than that at which old leaves die, then there are enough photosynthesizing leaves for the plant to produce flowers and seeds, although in reduced numbers. However, if old leaves die faster than new ones develop, then the plant may not survive to produce seed. For an annual plant there is a race against time to initiate flowers and form seeds while the leaf area is still adequate to supply the necessary photosynthate. For perennial species, there is an opportunity to become dormant, and thus survive a period of stress that will be relieved later by rainfall. These results illustrate the principle that the initial growth reduction is due to the osmotic effect of the salt outside the roots, and that what distinguishes a salt-sensitive plant from a more tolerant one is its inability to prevent salt from reaching toxic levels in the transpiring leaves.
Cause of the Phase 1 response: water stress, not salt toxicity
The mechanisms controlling this phase of the growth response are not specific to salinity. Reductions in the rate of leaf and root growth are probably due to factors associated with water stress rather than a salt-specific effect (Munns, 2002). This is supported by the evidence that Na+ and Cl− are below toxic concentrations in the growing cells themselves. For example, in wheat growing in 120 mM NaCl, Na+ in the growing tissues of leaves was at most only 20 mM, and only 10 mM in the rapidly expanding zones, and Cl− only about 50 mM (Hu et al., 2005). Similarly, in maize growing in 80 mM NaCl, Neves-Piestun and Bernstein (2005) found that Na+ and Cl− were, at most, only 40 mM in the most rapidly growing tissues (20 mm from the leaf base), and that the extent of inhibition by salinity of either the elongation rate or the total volume expansion rate did not correlate with the Na+ or Cl− in the tissues. Further, Fricke (2004) found only 38 and 49 mM Na+ in mesophyll and epidermal cells, respectively, in the growing cells of barley after 24 h of exposure to 100 mM NaCl. That this Na+ was not inhibitory to growth, but was probably beneficial as it might be taken up into the expanding vacuole for osmotic adjustment, was indicated by the fact that the growth rate increased with time over 24 h (after a temporary decline when the salt was applied) while the cellular Na+ increased (Fricke, 2004). In roots also, there is evidence that Na+ concentrations in dividing or rapidly elongating cells are low and well below toxic levels (Jeschke, 1984; Jeschke et al., 1986). For example, in root tips of saltbush (Atriplex amnicola), Na+ was only 40 mM at external NaCl concentrations of 400 mM (Jeschke et al., 1986). The rapid expansion of the growing cells would help to keep the salt from building up to high concentrations.
Results of experimental manipulation of shoot water- relations suggest that hormonal signals, probably induced by the osmotic effect of the salt outside the roots, are controlling the rate of cell elongation growth (Munns et al., 2000a). These results were obtained using specialized root pressure chambers, and are described in a later section.
Cause of the Phase 2 response: salt toxicity
Species which cannot effectively exclude salt from the transpiration stream must have ways to handle the salt arriving in leaves as the water evaporates and salt gradually builds up with time. The salt concentrations in older leaves are much higher than in younger leaves at a given time. In the older leaves, the salt concentration eventually becomes high enough to kill the cells, unless they can compartmentalize the salt in vacuoles, thereby protecting the cytoplasm from ion toxicity.
The concept that salt must either be excluded from tissues or compartmentalized in cell vacuoles, derives from the early discovery by biochemists that enzymes of halophytes are no more tolerant of high concentrations of NaCl than are those of non-halophytes (also called glycophytes, or plants requiring sweet water). For example, in vitro activities of enzymes extracted from the halophytes Atriplex spongiosa or Suaeda maritima were just as sensitive to NaCl as were those extracted from beans (Phaseolus vulgaris) or peas (Pisum sativum) (Flowers, 1972; Greenway and Osmond, 1972; Flowers et al., 1977). Even enzymes from the pink salt-lake alga Dunaliella parva, which can grow at salinities 10-fold higher than those of seawater, are as sensitive to NaCl as those of the most-sensitive glycophytes (reviewed by Munns et al., 1983). Generally, Na+ severely inhibits most enzymes at a concentration above 100 mM. More than 50 enzymes require K+ as a cofactor, and these are particularly susceptible to high Na+ and high Na+/K+ ratios. The concentration at which Cl− becomes toxic is even less well defined, but is probably in the same range as that for Na+. Even K+ salts inhibit enzymes when at high concentrations (Greenway and Osmond, 1972). Some halophytes may have slightly modified forms of some enzymes (Flowers and Dalmond, 1992), but even so, most of the Na+ must be compartmentalized in vacuoles.
Mechanisms for the salt-specific features of salt tolerance are therefore of two main types: those minimizing the entry of salt into the plant, known as ‘salt exclusion’, and those minimizing the concentration of salt in the cytoplasm, known as ‘tissue tolerance’. Halophytes have both types of mechanisms; they ‘exclude’ salt well, but the cells can compartmentalize the salt in vacuoles. These mechanisms, together with salt glands or bladders that excrete salt, allow them to grow for long periods of time in saline soil. The physiological processes involved in ‘salt exclusion’ and ‘tissue tolerance’ are reviewed, followed by discussion of the processes involved in the response to the osmotic stress of the salt in the soil solution. Screening methods to identify genetic variation in these three processes will be suggested.
The mechanism of ‘salt exclusion’
There is a strong correlation between salt exclusion and salt tolerance in many species (reviewed by Läuchli, 1984; Flowers and Yeo, 1986; Munns and James, 2003), and recently reported for rice (Lee et al., 2003; Zhu et al., 2004) and wheat (Poustini and Siosemardeh, 2004). In those species that retain Na+ in woody roots or stems, there is a strong correlation between Cl− exclusion and salt tolerance; for example, citrus (Storey and Walker, 1999).
Figure 1 shows the relationship between leaf Na+ concentration and salt tolerance of a range of durum wheat genotypes. Salt tolerance was assessed as shoot dry matter after nearly 4 weeks of salt treatment (Munns and James, 2003). In general, the genotypes with the lowest Na+ concentrations produced the greatest dry matter. These low-Na+ genotypes had fewer injured leaves, and a greater proportion of living to dead leaves. The effect on growth was probably due to a better carbon balance in the genotypes with less Na+. A similar relationship between shoot dry matter and leaf Na+ was found in a population from a cross between high- and low-Na+ genotypes. There was a strong correlation between shoot dry matter production and Na+ concentration in leaves between families from a cross between the genotypes with the highest and lowest Na+ shown in Fig. 1 (S Husain, R Munns, unpublished results).
Relationship between salinity tolerance (% growth of controls) and leaf Na+ concentration in durum wheat (Triticum turgidum ssp. durum) selections. Na+ concentrations were measured on leaf 3 after 10 d in 150 mM NaCl and shoot biomass after 24 d. Values are expressed as a percentage of shoot biomass in control conditions (r2=0.74). All values are means (n=5). Reproduced from Munns and James (2003), with kind permission of Springer Science and Business Media.
Do all plants ‘exclude’?
A soil salinity of 100 mM NaCl or ∼10 dS m−1 is about as high as most crops will tolerate without a significant reduction in growth or yield, and a concentration of 100 mM NaCl on a whole shoot basis is about as high as is desirable because it will include some old leaves with much higher salt concentrations, as well as younger leaves or other tissues with lower concentrations. So for plants to grow for extended periods of time in soils with salinity of this order of magnitude, no more than 2% should get to the shoots.
Most plants, in fact, do exclude about 98% of the salt in the soil solution, allowing only 2% to be transported in the xylem to the shoots. Differences between cereal genotypes with contrasting rates of Na+ uptake, when grown in 50 mM NaCl, range from 99% for Janz to 98% for other bread wheats (Munns, 2005). Durum wheat, rice, and barley are not such good excluders, yet they still exclude at least 94% of the soil Na+ from the transpiration stream (Munns, 2005). Roots themselves do not accumulate excessively high concentrations of salt. The Na+ and Cl− concentration in roots is rarely much higher than in the external solution, and often is lower (Munns, 2005).
Control of salt exclusion at the whole-plant and cellular level
Physiological mechanisms conferring exclusion that operate at the cellular and whole-plant level have been described in previous reviews (Greenway and Munns, 1980; Läuchli, 1984; Pitman, 1984; Storey and Walker, 1999), and with particular reference to selectivity for K+ over Na+ (Jeschke, 1984; Jeschke and Hartung, 2000; Tester and Davenport, 2003). Salt tolerance of monocotyledonous species without salt glands depends on the control of Na+ transport at four major points: (i) selectivity of uptake by root cells in the cortex and stele; (ii) loading of the xylem by xylem parenchyma cells in roots; (iii) removal of salt from the xylem in the upper part of the roots, the stem, or leaf sheaths by xylem parenchyma cells; (iv) loading of the phloem. These four control points for Na+ exclusion were examined in a recent study with two genotypes of durum wheat (Triticum turgidum L. ssp. durum) known to differ in rates of Na+ accumulation, Line 149 and the cultivar Tamaroi (Munns et al., 2000b; see also below). Genetic studies had indicated two major gene loci controlling leaf-blade Na+ accumulation (Munns et al., 2003). The physiological traits determined by these genetic differences were investigated using measurements of unidirectional 22Na+ transport and net Na+ accumulation. The genotypes did not differ significantly at the first control point, i.e. in the unidirectional root uptake of Na+ (Davenport et al., 2005). The major differences in Na+ transport between the genotypes were at the second control point, the rate of transfer from the root to the shoot (xylem loading), which was much lower in the salt-tolerant genotype (Davenport et al., 2005), and the third control point, the capacity of the leaf sheath to extract and sequester Na+ as it entered the leaf (Fig. 2). There was no substantial recirculation of Na+ from shoots to roots (Davenport et al., 2005). It is likely that xylem loading and leaf sheath sequestration are separate genetic traits that interact to control leaf-blade Na+.
Increase in Na+ concentrations in (A) leaf sheath, (B) leaf blade, and (C) roots of durum wheat Line 149 (closed symbols) and Tamaroi (open symbols) during 10 d of exposure to 50 mM NaCl. Data represent mean ±standard error of the mean. The leaf shown is the first leaf. Modified from Davenport et al. (2005). © American Society of Plant Biologists.
Root uptake and transport
Roots have a remarkable ability to control their Na+ and Cl− concentrations, which do not increase in proportion to the external concentration; rather, they seem to plateau at about 50 mM NaCl, as illustrated in Table 1. Roots also have the ability to regulate their turgor over a wide range of salinity levels, at about 0.6 MPa (Pritchard et al., 1991). In wheat, once the external NaCl exceeds 50 mM (0.25 MPa), organic solutes must make a significant contribution to turgor maintenance, because the internal Na+ and Cl− concentrations are not increasing in proportion to the external solution and there is not enough salt inside to balance the osmotic pressure of the salt outside (Table 1). K+ is usually only 100 mM or less in roots, and declines with salinity. Table 1 indicates that there must be increasing concentrations of organic solutes. Remarkably little work has been done on the production of organic solutes in roots. Proline and glycinebetaine concentrations on a root fresh-weight basis were only one-quarter of that in shoots of barley grown at 100–200 mM NaCl, even though the Na+ and Cl− concentrations were much lower in roots than shoots (Wyn Jones and Storey, 1978). This suggests that unknown organic solutes are involved, possibly sucrose and other sugar-related compounds; however, this would impose a real carbon cost on the plant especially as the production of photosynthates would have been reduced at 150 mM NaCl by both stomatal closure and smaller leaf area (James et al., 2002).
Root ion concentrations in wheat grown for 2 weeks in 1–150 mM NaCl
NaCl (mM) . | Na+ (mM) . | K+ (mM) . | Cl− (mM) . | Balancing anion (mM) . | Δ ions (mOsmol l−1) . | Unknown solutes (mOsmol l−1) . |
|---|---|---|---|---|---|---|
| 1 | 11 | 103 | 4 | 110 | +226 | 14 |
| 10 | 26 | 76 | 10 | 92 | +184 | 56 |
| 25 | 36 | 65 | 15 | 86 | +152 | 88 |
| 50 | 45 | 77 | 23 | 99 | +144 | 96 |
| 75 | 47 | 63 | 21 | 89 | +70 | 170 |
| 100 | 53 | 61 | 28 | 86 | +28 | 212 |
| 150 | 65 | 54 | 28 | 91 | –62 | 302 |
NaCl (mM) . | Na+ (mM) . | K+ (mM) . | Cl− (mM) . | Balancing anion (mM) . | Δ ions (mOsmol l−1) . | Unknown solutes (mOsmol l−1) . |
|---|---|---|---|---|---|---|
| 1 | 11 | 103 | 4 | 110 | +226 | 14 |
| 10 | 26 | 76 | 10 | 92 | +184 | 56 |
| 25 | 36 | 65 | 15 | 86 | +152 | 88 |
| 50 | 45 | 77 | 23 | 99 | +144 | 96 |
| 75 | 47 | 63 | 21 | 89 | +70 | 170 |
| 100 | 53 | 61 | 28 | 86 | +28 | 212 |
| 150 | 65 | 54 | 28 | 91 | –62 | 302 |
Data shown are for a durum landrace, Line 141, which was typical of other cereal genotypes measured (Husain et al., 2004). The balancing anion (assumed to be monovalent) is calculated from the negative charge to balance the Na+ and K+ (i.e. the concentration of Na+ plus K+ minus Cl−). ‘Δ ions’ is the difference between the internal concentration of those ions (i.e. Na+, K+, Cl−, and a balancing monovalent anion), and the external concentration of ions, in osmol l−1. The ‘unknown solutes’ are those extra solutes to generate 0.6 MPa of turgor, i.e. the organic solutes needed along with the ions to make up an internal concentration of 240 mOsmol l−1 above that of the external solution.
Root ion concentrations in wheat grown for 2 weeks in 1–150 mM NaCl
NaCl (mM) . | Na+ (mM) . | K+ (mM) . | Cl− (mM) . | Balancing anion (mM) . | Δ ions (mOsmol l−1) . | Unknown solutes (mOsmol l−1) . |
|---|---|---|---|---|---|---|
| 1 | 11 | 103 | 4 | 110 | +226 | 14 |
| 10 | 26 | 76 | 10 | 92 | +184 | 56 |
| 25 | 36 | 65 | 15 | 86 | +152 | 88 |
| 50 | 45 | 77 | 23 | 99 | +144 | 96 |
| 75 | 47 | 63 | 21 | 89 | +70 | 170 |
| 100 | 53 | 61 | 28 | 86 | +28 | 212 |
| 150 | 65 | 54 | 28 | 91 | –62 | 302 |
NaCl (mM) . | Na+ (mM) . | K+ (mM) . | Cl− (mM) . | Balancing anion (mM) . | Δ ions (mOsmol l−1) . | Unknown solutes (mOsmol l−1) . |
|---|---|---|---|---|---|---|
| 1 | 11 | 103 | 4 | 110 | +226 | 14 |
| 10 | 26 | 76 | 10 | 92 | +184 | 56 |
| 25 | 36 | 65 | 15 | 86 | +152 | 88 |
| 50 | 45 | 77 | 23 | 99 | +144 | 96 |
| 75 | 47 | 63 | 21 | 89 | +70 | 170 |
| 100 | 53 | 61 | 28 | 86 | +28 | 212 |
| 150 | 65 | 54 | 28 | 91 | –62 | 302 |
Data shown are for a durum landrace, Line 141, which was typical of other cereal genotypes measured (Husain et al., 2004). The balancing anion (assumed to be monovalent) is calculated from the negative charge to balance the Na+ and K+ (i.e. the concentration of Na+ plus K+ minus Cl−). ‘Δ ions’ is the difference between the internal concentration of those ions (i.e. Na+, K+, Cl−, and a balancing monovalent anion), and the external concentration of ions, in osmol l−1. The ‘unknown solutes’ are those extra solutes to generate 0.6 MPa of turgor, i.e. the organic solutes needed along with the ions to make up an internal concentration of 240 mOsmol l−1 above that of the external solution.
Cell-specific localization of Na+ in the root can provide insight into the major control points that limit Na+ loading of the xylem. Cryo-SEM X-ray microanalysis was used to characterize cell-specific ion profiles in roots of the two durum wheat genotypes Line 149 and Tamaroi that differ in Na+ exclusion (Läuchli et al., 2005). The results from this X-ray microanalysis study indicate that the root cortex is the barrier to Na+ transport into the stele, rather than the endodermis, and that the major control of Na+ entry was from the outer two layers of cells in the cortex, the epidermis and hypodermis. The localization pattern for the Na+-excluding durum Line 149 is shown in Table 2. The highest concentration of Na+ was in the cells of the pericycle (the outermost cell layer of the stele, immediately within the endodermis), indicating that it may provide a major control point in limiting xylem loading of Na+, especially as this accumulation was less marked in cv. Tamaroi, which is less able to exclude Na+ from the xylem. The K+-accumulation distribution pattern was the reverse of the Na+ distribution (Läuchli et al., 2005).
Cell-specific Na+ profiles across the seminal root of durum wheat genotype Line 149, an efficient Na+ excluder
Cell type . | Na+ concentration (mM) . |
|---|---|
| Epidermis | 48±9 |
| Sub-epidermis (hypodermis) | 51±5 |
| Cortex (outer layer) | 35±9 |
| Cortex (middle layer) | 23±5 |
| Cortex (inner layer) | 22±5 |
| Endodermis | 39±10 |
| Pericycle | 85±14 |
| Xylem parenchyma | 34±5 |
| Metaxylem vessels | Not detectable |
Cell type . | Na+ concentration (mM) . |
|---|---|
| Epidermis | 48±9 |
| Sub-epidermis (hypodermis) | 51±5 |
| Cortex (outer layer) | 35±9 |
| Cortex (middle layer) | 23±5 |
| Cortex (inner layer) | 22±5 |
| Endodermis | 39±10 |
| Pericycle | 85±14 |
| Xylem parenchyma | 34±5 |
| Metaxylem vessels | Not detectable |
Plants were grown at 50 mM NaCl for 9 d. The Na+ profiles were determined by quantitative cryo-SEM X-ray microanalysis (n=16). Detection limit for Na+ was 5–10 mM (Läuchli et al., 2005).
Cell-specific Na+ profiles across the seminal root of durum wheat genotype Line 149, an efficient Na+ excluder
Cell type . | Na+ concentration (mM) . |
|---|---|
| Epidermis | 48±9 |
| Sub-epidermis (hypodermis) | 51±5 |
| Cortex (outer layer) | 35±9 |
| Cortex (middle layer) | 23±5 |
| Cortex (inner layer) | 22±5 |
| Endodermis | 39±10 |
| Pericycle | 85±14 |
| Xylem parenchyma | 34±5 |
| Metaxylem vessels | Not detectable |
Cell type . | Na+ concentration (mM) . |
|---|---|
| Epidermis | 48±9 |
| Sub-epidermis (hypodermis) | 51±5 |
| Cortex (outer layer) | 35±9 |
| Cortex (middle layer) | 23±5 |
| Cortex (inner layer) | 22±5 |
| Endodermis | 39±10 |
| Pericycle | 85±14 |
| Xylem parenchyma | 34±5 |
| Metaxylem vessels | Not detectable |
Plants were grown at 50 mM NaCl for 9 d. The Na+ profiles were determined by quantitative cryo-SEM X-ray microanalysis (n=16). Detection limit for Na+ was 5–10 mM (Läuchli et al., 2005).
Very high Na+ in the pericycle was also found by Storey et al. (2003) in grapevine roots grown in 25 mM NaCl, much higher than in the endodermis, again suggesting that the pericycle is an important control point in the radial transport of Na+.
Phloem export
Rates of retranslocation of salt from leaves are low in relation to rates of import in the transpiration stream, as shown by the continued presence of salt in leaves long after the salt around the roots is removed. Estimates of fluxes of Na+ from phloem sap collected through aphid stylets indicated that, in barley, phloem export from a leaf was only about 10% of the import in the xylem (Munns et al., 1986; Wolf et al., 1990).
Measurements of ions in phloem sap indicate that the more-salt-tolerant species exclude Na+ and Cl− from the phloem to a greater extent than less-tolerant ones (reviewed in Wolf et al., 1991). Exclusion of salt from the phloem ensures that salt is not redirected to growing tissues of the shoot. As shown by [14C]urea feeding studies, lower leaves supply the root, upper leaves feed the shoot apex, and mid-leaves feed both shoot apex and root (Layzell et al., 1981), and the same has been shown for K+ and Na+ (Wolf et al., 1991, and references therein). Na+ and Cl− concentrations in shoot apices and reproductive tissue of wheat and barley were very low compared with the leaves, and K+/Na+ ratios were particularly high (Munns and Rawson, 1999). As phloem sap is difficult to obtain, analyses of apical or reproductive tissues are a good indication of whether or not salt is excluded from the phloem.
Other factors—growth rates and shoot:root ratios
Other factors that contribute to low rates of salt accumulation in leaves are high shoot:root ratios and high relative growth rates (Pitman, 1984; Munns, 2005).
The shoot ion uptake rate is not determined by the transpiration rate. The flux of ions to the shoot is largely independent of the flux of water; the transport pathways for water and ions are separate. Water moves across root membranes through aquaporins (Tyerman et al., 2002) and ions move across root membranes through ion channels or transporters (Amtmann and Sanders, 1999), so when transpiration rates fall, ion concentrations in the xylem sap increase (Fig. 3). It is well established that K+ uptake is not influenced by transpiration rates in non-saline soil (Smith, 1991), and Na+ and Cl− fluxes are also independent of transpiration rates in saline soil (Munns, 1985; Ball, 1988). The independence of Cl− flux over a wide range of transpiration rates is shown in Fig. 3, and a similar relationship was found for Na+ and K+ (Munns, 1985). However, there is evidence for a ‘bypass’ pathway in rice roots, in which water can bypass all membranes and enter the xylem by an apoplastic route, which can account for a large part of the Na+ delivered to the shoot (Garcia et al., 1997).
The relationship between ion concentrations in the xylem, ion fluxes to the shoot, and transpiration rates (Munns, 1985). The data shown are for Cl−, but Na+ and K+ concentrations and fluxes showed a similar relationship to transpiration rate (Munns, 1985).
Improvements in ion exclusion could be made by selecting genotypes with lower rates of transport from root to shoot, higher relative growth rates or, in the case of rice, minimization of the bypass pathway through which ions leak into the xylem.
Genetic improvement in salt tolerance of durum wheat using the trait for sodium exclusion
Cultivated durum (pasta) wheat (Triticum turgidum ssp. durum) is more salt sensitive than bread wheat (Triticum aestivum), a feature that restricts the production of durum wheat on farms with sodic or saline soils. To increase the salt tolerance of durum wheat, attempts have been made to improve its sodium exclusion, building on the earlier work of John Gorham and Jan Dvořák (Gorham et al., 1990; Dvořák et al., 1994). In the bread and durum wheat, salt tolerance is associated with low rates of transport of Na+ to shoots with high selectivity for K+ over Na+; there is little genotypic variation in rates of Cl− transport (Gorham et al., 1990; Husain et al., 2004). In order to introduce the trait of Na+ exclusion into current durum wheat varieties, genetic variation in salt tolerance was investigated across a wide range of ancient durum-related accessions and landraces representing five Triticum turgidum subspecies. Selections were screened non-destructively for low Na+ concentration in leaves, and the associated enhanced K+/Na+ discrimination (Munns et al., 2000b). Wide genetic variation in Na+ accumulation (and K+/Na+ discrimination) was found, and a particular landrace named Line 149 was selected for breeding.
Proof of the concept that Line 149 would provide a source of salt tolerance for modern durum wheat was obtained by comparing it with another durum landrace, Line 141 with a high rate of sodium transport to leaves, to assess the effects of the sodium exclusion trait on preventing leaf injury and death in saline soil (Husain et al., 2003). Leaves of Line 149 lived longer than leaves of Line 141, the start of leaf senescence being delayed by 1 week or more. Figure 4 illustrates that the high Na+ lines lost chlorophyll more rapidly and died earlier than the low Na+ lines. Other leaves showed similar results (Husain et al., 2003), so by the time the grain was developing, all the leaves of Line 141 were dead but some were still alive in Line 149.
Effect of 150 mM NaCl on chlorophyll content in leaf 6 of low sodium (circles) and high sodium (squares) genotypes. Leaf 6 emerged 21 d after the salt treatment started. Bars show the standard error of the mean. Modified from Husain et al. (2003).
The low sodium trait improved yield by over 20% in saline soil in glasshouse trials at moderate salinity (75 mM NaCl); the low Na+ Line 149 suffered a yield reduction of 12% but the high Na+ Line 149 suffered a reduction of 30% compared with their respective controls (Husain et al., 2003). However, at high salinity (150 mM NaCl), there was no advantage to having the Na+-excluding trait; the yield was severely reduced in both genotypes (Husain et al., 2003). The differences in yield were similar to the differences between bread and durum wheat reported by Maas and Grieve (1990). At a salinity level equivalent to about 100 mM NaCl (0.45 MPa) they showed that the yield of the (low Na+) bread wheat was reduced by only 7% compared with a 38% reduction for the (high Na+) durum wheat. At a higher salinity, equivalent to about 150 mM NaCl (0.65 MPa), there was less difference between genotypes, the bread wheat being reduced by 43% and the durum wheat by 54% (Maas and Grieve, 1990)
Does Na+ exclusion pose a problem for turgor maintenance? The low Na+ trait did not restrict turgor maintenance as K+ uptake was enhanced (Rivelli et al., 2002). Four wheat genotypes with contrasting degrees of Na+ exclusion were selected to see if low Na+ uptake adversely affected water relationships or growth rates during exposure to saline conditions. Plants were grown in supported hydroponics, with and without 150 mM NaCl, and sampled for measurements of water relationships, biomass, stomatal conductance, and ion accumulation. After 4 weeks of exposure to salt, there was little difference between genotypes in the effect of salinity on water relationships, as indicated by their relative water content (RWC) and estimated turgor. Osmotic adjustment occurred in all genotypes. In the low Na+ genotypes, osmotic adjustment depended on higher K+ and high organic solute accumulation. These data indicate that selecting lines with low Na+ accumulation for the purpose of improving salt tolerance is unlikely to introduce adverse effects on plant–water relationships or growth (Rivelli et al., 2002). Thus the Na+ exclusion trait in Line 149 reduces the rate of leaf death, and improves plant growth and grain yield under saline conditions.
Genetic analysis of the segregating populations developed from crosses between genotypes with low and high rates of Na+ uptake indicated two dominant and interacting genes of major effect (Munns et al., 2003). It is likely that one gene controls the loading of Na+ in the xylem in the roots, while the other controls the retrieval of Na+ from the xylem in the lower part of the leaves (Davenport et al., 2005). These processes would work together to produce very low Na+ concentrations in the leaf blades, and so fit the Mendelian analysis of two interacting genes.
A locus for one gene, designated Nax1 (Na+ exclusion), was mapped to the long arm of chromosome 2A using a QTL approach with AFLP, RFLP, and microsatellite markers (Lindsay et al., 2004). This locus had a LOD score of 7.5 and accounted for 38% of the phenotypic variation in the mapping population. One particular microsatellite marker, Xgwm312, was closely linked to the low Na+ trait in other populations with different genetic backgrounds, and is being used to select low Na+ progeny in a durum breeding programme (Lindsay et al., 2004).
The mechanism known as ‘tissue tolerance’
Species which cannot exclude 98% of the salt from the transpiration stream must have ways to handle the salt arriving in leaves as the water evaporates, and salt gradually builds up with time. In the older leaves, the salt concentration will soon become high enough to kill the cells, unless they can compartmentalize the salt in vacuoles, thereby protecting the cytoplasm from ion toxicity.
This compartmentation is exemplified by halophytes, which hold concentrations of over 500 mM on a leaf tissue basis, but which show no sign of injury. Barley leaves can tolerate concentrations close to this without showing injury (Greenway, 1962; Rawson et al., 1988a), as can numerous other species. In these species, salt must be sequestered in vacuoles. This is difficult to measure directly, but it must happen when the leaf contains at least 100 mM on a tissue water basis (i.e. 0.5 mmol g−1 DW), as this concentration cannot be tolerated by most enzymes, as described above.
To understand the physiology of tolerance to high internal salt concentrations, two genotypes that differ in the degree of salt-induced leaf injury, the durum cultivar Wollaroi and the durum-related landrace Line 455, were grown in 150 mM NaCl for 4 weeks (James et al., 2002). The shoot biomass of both genotypes was substantially reduced by salinity, but genotypic differences appeared after 3 weeks, when the durum cultivar Wollaroi showed greater leaf injury and a greater reduction in biomass than the landrace Line 455. Ion accumulation, water relationships, chlorophyll fluorescence, and gas exchange were followed throughout the life of a leaf. Salinity caused a large decrease in stomatal conductance in both genotypes. This was not due to poor water relationships, as leaf turgor of both genotypes was not affected by the salt treatment (James et al., 2002), so was presumably due to ‘root signals’ as discussed in the following section. Photosynthesis per unit leaf area was not initially reduced by salinity, particularly in the more-tolerant Line 455, as the chlorophyll per unit area was higher in saline than non-saline conditions (the leaves were narrower, the cells were smaller, and so the chloroplast density was greater), but photosynthesis per plant was reduced as the leaves were smaller in area. With time, photosynthesis per unit area decreased in both genotypes due to reductions in stomatal conductance, and later there were non-stomatal limitations associated with a build-up of Na+ and Cl− in the whole tissue above 250 mM. Chlorophyll fluorescence measurements showed that the efficiency of PSII photochemistry in Line 455 was unaffected throughout. However, in Wollaroi, the potential and actual quantum yield of PSII photochemistry began to decline as the leaf aged and the thermal energy dissipation of excess light energy (NPQ) increased. This coincided with high Na+ and Cl− concentrations in the leaf (250 mm) and with chlorophyll degradation, indicating that these later reductions in CO2 assimilation in Wollaroi were a likely consequence of a direct toxic ion effect. The fluorescence parameters, other than NPQ, were no more sensitive than chlorophyll itself. The more easily measured fluorescence parameter Fv/Fm decreased only when chlorophyll content decreased. The physiological mechanism of tolerance in Line 455 was in delaying the onset of non-stomatal effects on photosynthesis, probably by delaying the time at which Na+ or Cl− reached a critical toxic level.
In a recent study, the relationship between photosynthetic capacity and the cellular and subcellular distribution of Na+, K+, and Cl− was studied in a salt-sensitive durum wheat, as well as a salt-tolerant barley, to see if barley's superior salt tolerance was associated with compartmentalization of salt in the vacuole (R James and R Munns, unpublished results). Vacuolar concentrations were measured in mesophyll and epidermal cells using cryo-SEM X-ray microanalysis. Cytoplasmic Na+ and K+ concentrations were calculated from these data, and from whole tissue analyses and volume fractions of vacuoles in different cell types. Efficient cellular and subcellular partitioning of both Na+ and K+ in barley led to the preservation of a favourable K+:Na+ ratio in the cytoplasm at high leaf Na+ concentrations (200–300 mM) by contrast to durum wheat (Fig. 5). The photosynthetic capacity of durum wheat declined at lower leaf Na+ concentrations than barley (R James and R Munns, unpublished results), indicating that the maintenance of photosynthetic capacity (and the resulting greater salt tolerance) was due to the maintenance of high K+, low Na+, and the resulting high K+:Na+ in the cytoplasm of mesophyll cells.
Relationship between leaf Na+ concentration and the estimated K+:Na+ ratio in the cytoplasm of leaves from barley (cv. Franklin) and durum wheat (cv. Wollaroi) grown in a range of high salinities leading to different leaf Na+ concentrations. Cytoplasmic Na+ and K+ concentrations were estimated from vacuolar concentrations measured using cryo-SEM X-ray microanalysis, whole tissue analyses, and volume fractions of cell compartments in different cell types (R James, R Munns, unpublished results).
Screens for tissue tolerance of Na+
In an attempt to find genotypes of durum wheat as tolerant as barley to high salt concentrations in leaves, over 50 genotypes of durum wheat and its closely related tetraploid relatives were harvested, after 21 d of salt treatment, to identify genotypes with the least leaf injury associated with highest leaf Na+ concentration (Munns and James, 2003). Barley was included as a benchmark, because of its established reputation for salinity tolerance coupled with high rates of salt accumulation, and previous observations that it was slow to develop leaf injury. Significant variation in percentage dead leaf (weight of dead leaf as a proportion of total leaf dry weight) was found between individual tetraploid lines, the percentage dead leaf ranging from 2 to 29 (Munns and James, 2003). The barley cultivar had a low degree of leaf injury as expected, only 3%.
The total leaf Na+ content of individual genotypes did not correlate with the percentage dead leaf, suggesting there might be genotypic variation in the ability to tolerate the Na+ at the tissue or cellular level. The ratio of Na+ content to percentage dead leaf (whole shoot basis) was calculated as an index of tolerance to Na+ in the leaves. A higher Na+ content per percentage dead leaf might indicate a higher degree of tissue tolerance to Na+. This ratio ranged from 15 to 108 μmol Na+ per percentage dead leaf, with the barley cultivar Skiff at the high end of that range with a value of 107 (Fig. 6). The bread-wheat cultivars, while excluding 2–3 times the amount of Na+ from the leaves, displayed similar levels of leaf injury as a number of tetraploid selections, indicating greater sensitivity to tissue Na+ levels. This experiment revealed five tetraploid genotypes with an exceptional combination of high Na+ accumulation and low leaf injury, indicating they may have an exceptional ability to tolerate high Na+ levels in tissues (Munns and James, 2003). Other screening methods have been based on loss of chlorophyll with increasing salt concentration (Yeo and Flowers, 1983), but could assess the health of a leaf with ageing, such as its rate of photosynthesis or turgidity at a given age. However, most methods have drawbacks.
Frequency distribution of Na+ content per percentage dead leaf of 47 tetraploid wheat selections, grown in 150 mM NaCl for 21 d. The bars represents LSDs at P=0.05 for selection comparisons. Reproduced from Munns and James (2003), with kind permission of Springer Science and Business Media.
Photosynthesis
Screening methods based on gas exchange are not feasible as the measurements are too slow to handle large numbers. The chlorophyll fluorescence measurement of Fv/Fm can be made quickly and can handle larger numbers but, as mentioned earlier, it may not be significantly better than chlorophyll level as measured with a SPAD meter. The more easily measured fluorescence parameter Fv/Fm decreased only when chlorophyll content decreased, indicating that a simple meter for measuring chlorophyll density in leaves (such as the SPAD meter) is a more cost-effective measure of photosynthetic capacity than chlorophyll fluorescence.
Water relationships
Plant water status is difficult to assess as it can change so much from minute to minute, as much as stomatal conductance on which it is entirely dependent in the short term, and psychometric or pressure chamber measurements are tricky to do accurately. Relative water content (RWC) is easier to measure, but not valid when osmotic adjustment occurs (Lafitte, 2002). RWC, although a convenient and widely used method of assessing plant water status, is not useful for salt-treated plants, at least not with the conventional method of detaching leaves and rehydrating on distilled water. This is because in most plants, osmotic adaptation has occurred; i.e. the solute content of cells is higher in saline than non-saline conditions, due largely to the accumulation of Na+, Cl−, and also to organic solutes. The increased solute content of the cells in the salt-treated plants causes more water to be taken up than in the control leaves, resulting in an apparent low RWC in the salt treatment. This was shown in water relationship measurements in durum wheat when the turgor pressure (calculated from the difference between total water potential and osmotic potential) was unchanged by salinity, but the RWC was significantly decreased (James et al., 2002; Rivelli et al., 2002).
Recent work with isopiestic psychrometry on wheat and barley in a range of saline solutions confirmed that turgor was unchanged, but RWC decreased (JS Boyer, RA James, R Munns, unpublished results). This was due to abnormal water uptake by leaves with a high solute content when floated on distilled water, which caused a leakage of cytoplasmic solution into the apoplast. Thus, osmotic adjustment shifts the relationship between turgor and RWC, and so RWC is not an indicator of turgor in plants undergoing osmotic adjustment. Screening methods for leaf water status should use rehydrating conditions for the whole plant, such as a dark humidified atmosphere while roots are still in saline soil, rather than detached leaves floating in distilled water when abnormal water uptake occurs (JS Boyer, RA James, R Munns, unpublished results).
Osmotic tolerance: mechanisms and screening methods
The osmotic or water stress effect of salt in the soil quickly reduces the growth rate in proportion to the salinity level (the Phase 1 effect; see Fig. 7). In species that produce multiple stems such as wheat, the growth reduction occurs mainly in the reduction of tiller number (Maas and Grieve, 1990; Francois et al., 1994; Husain et al., 2003) which, for a farmer, means fewer spikes and therefore less potential yield per plant. Developing salt injury will cause the sensitive genotypes to grow even slower than the more tolerant (Phase 2 effect; see Fig. 7). In the example shown in Fig. 7, the osmotic effect of 150 mM NaCl reduces the biomass after 40 d by roughly 75% and the salt-specific effect by another 20%.
Two accessions of the D-genome diploid wheat progenitor Aegilops tauschii grown in control solution (closed symbols) and in 150 mM NaCl (open symbols). Circles denote the tolerant accession, triangles the sensitive one (modified from Munns et al., Australian Journal of Plant Physiology24, 1995, http://www.publish.csiro.au/journals.fpb). The osmotic effect of the NaCl in the soil reduces the biomass after 40 d by about 75% and the salt-specific effect by another 20%, as indicated by the arrows.
Despite the fact that the osmotic effect on growth of the more-tolerant species such as wheat and barley is much greater than the salt-specific effect, the mechanisms that regulate the growth rate are not understood. Whether water status, hormonal regulation, or supply of photosynthate exerts the dominant control over growth rate of plants in dry or saline soil is still unresolved. Over the timescale of days, hormonal signals rather than water relationships are controlling growth in saline soils. The evidence for this is that leaf expansion in saline soil at the timescale of days does not respond to an increase in leaf water status. Water status was manipulated by growing plants in sand in pots that could be placed in pressure chambers, watering with saline solution (100 mM NaCl), and then pressurizing the root systems in chambers with a pressure equal to that of the salt concentration, to compensate for the suction of the soil solution (Termaat et al., 1985). No lasting effect of pressurization on growth was found over periods up to 8 d, with species as diverse as barley, wheat, white lupin, Egyptian clover, and saltbush (summarized in Munns, 1993). More recently, pressurization was done at ‘balancing pressures’, where the chambers were pressurized sufficiently to keep the xylem at atmospheric pressure (i.e. a small cut on a leaf was kept on the point of bleeding). This meant that the leaf water potential was maintained close to its maximum during both day and night, while the soil was watered with 100 mM NaCl. This treatment also did not make plants grow faster (Munns et al., 2000a), indicating that hormonal signals, and not leaf water deficit or ion toxicity, were controlling growth. The results in every way were reminiscent of experiments done with plants in dry soil; these had shown no response of leaf growth to an increase in shoot water status brought about by pressurization at balancing pressures. Further, ‘split-root’ experiments with plants whose root systems were divided between wet and dry soils showed that leaf expansion decreased while leaf water status was unaffected (reviewed by Davies and Zhang, 1991; Munns, 2002).
These experiments indicated that there are chemical signals coming from roots in dry or saline soil that reduce leaf growth. Abscisic acid (ABA) has been considered the obvious candidate for this signal as it is found in xylem sap and increases after drought and salinity stress (reviewed by Munns and Cramer, 1996). However, there is still no conclusive proof that ABA is the only signal from the roots (reviewed by Dodd, 2005). Moreover, the origin of the ABA in the xylem sap is not known, for it moves readily in the phloem and recirculates from leaves to roots (reviewed by Munns and Cramer, 1996), and may be synthesized in situ in leaves. ABA could regulate leaf cell expansion through a signal transduction pathway that controls the activity of ion channels that take up essential solutes for growth, such as K+ and amino acids, or it could work through other hormones. ABA may affect the synthesis of gibberellins that control the rate of cell expansion, or it may affect the synthesis of other hormones such as cytokinins and auxins that are known to control cell division.
ABA's reputation as a ‘growth inhibitor’ under stress may be undeserved. There is strong evidence that the increased production of ABA under drought suppresses accumulation of ethylene that would otherwise inhibit growth (LeNoble et al., 2004). Further indication of a positive role of ABA under salt stress is indicated by studies with the ABA-deficient tomato mutant sitiens. The mutant grows slower under optimal conditions, probably because of excessive ethylene production (Sharp et al., 2000). To see whether ABA accumulation inhibits or promotes shoot growth under salinity stress, sitiens and its wild type were grown at 75 mM NaCl for 2 weeks under conditions of moderate or high relative humidity (Mäkelä et al., 2003). The major difference between genotypes was in the degree of desiccation injury suffered by older leaves. For instance, when plants were grown at 95% relative humidity to maximize the leaf water status of both genotypes, there was no significant effect of salt on shoot dry weight of either genotype. However, older leaves of sitiens died due to desiccation, whereas no visible injury appeared in the wild type (Mäkelä et al., 2003). These results confirm that ABA promotes rather than inhibits plant growth under stress, and has a major effect on preservation of older leaves.
That the hormonal control of cell division and differentiation is affected by salinity is clear from the appearance of leaves: leaves are smaller in area but greener, i.e. the density of chloroplasts has increased, indicating that cell size and shape has changed. Leaves have a higher specific leaf weight (higher dry weight:area ratio) which means that their transpiration efficiency is higher (more carbon fixed per water lost), a feature that is common in plants adapted to both dry or saline soil.
Screens for tolerance of the osmotic effect of salt
The regulation of growth rates in leaves and roots under stress is so complex, and the mechanisms so little understood, that the idea of improving salt tolerance by the manipulation of individual genes or even pathways concerned with growth rates is not feasible at this stage. Screening methods that have been used or tested to select for genetic improvements in the growth response to osmotic stress are related to growth or survival and have the following disadvantages.
(i) Growth rate is difficult to replicate because of the large environmental effects and the length of time over which plants must be grown. The maintenance of optimal conditions for growth of plants in non-saline conditions is particularly difficult, especially when genotypes with different heights or developmental patterns are being compared, as the tallest or more vigorous ones compete successfully for light with those with dwarfing genes, thus giving a false idea of salt tolerance. It is not possible to accommodate large numbers of genotypes in greenhouses, especially if the effect on grain yield needs to be measured. On the other hand, the field is a complex and unpredictable environment.
(ii) Germination rate is by far the easiest thing to measure, but the least likely to predict the ability of plants to grow in saline soil. No correlation has been observed between salt tolerance at germination and the seedling stage (reviewed by Shannon, 1997; Munns and James, 2003), nor between germination and grain yield (Ashraf and McNeilly, 1988). Furthermore, in the field, germination rarely takes place in high salt concentrations. In irrigated agriculture, salt would normally be leached from the surface at sowing, and in dry-land agriculture, the crop is normally planted after rain. In those salt-affected situations where the crop is sown without rain or leaching irrigation, the soil in the top 10 cm is likely to be sodic as well as saline, and the main constraint to emergence might be the hardness of the soil as much as the salts in the soil solution. Seeds that germinate on filter paper wetted with a highly saline solution may be too weak to break a soil crust and establish as viable plants (Shannon, 1997). Emergence rate might be a more practical screening criterion than germination rate, and seedling vigour may also be a useful screening factor for soils that form hard crusts.
(iii) Desiccation tolerance is also easy to score: potted plants can be left to dry out and then rewatered so that selections can be made from those that recover best. Although plant biologists have given an enormous amount of attention to plant desiccation tolerance, these processes are largely irrelevant to crop yield. If plant cells desiccate, crop yields will be negligible and even if yield is doubled by plant manipulation, then it is still negligible (Serraj and Sinclair, 2002). One exception to this situation is the combination of responses that allow a perennial crop plant to stay alive under desiccating conditions. This capacity to ‘live to fight another day’ can be highly advantageous for forage yield in succeeding growth seasons. The capacity to survive is often irrelevant in annual grain crops as many are grown in such short seasons that a stress-induced delay in development can result in a complete loss of yield.
(iv) Survival as a selection criterion, while rapid and simple, needs additional assessment. Survival at high NaCl does not necessarily imply healthy growth at these high salinity levels, and it is important to evaluate yield and yield components of promising lines.
(v) Stomatal conductance measured by viscous flow porometry, or assessed by leaf temperature (thermal imaging) can measure several hundred genotypes per day, as long as ambient conditions are constant.
(vi) Yield needs to be measured in the field, as controlled environment chambers or glasshouses cannot provide the space required to maximize yield; inadequate lighting and pot size will always limit long-term growth and yield. Screening large numbers of genotypes for salt tolerance in the field is difficult, as discussed in the Introduction, due to variation and unpredictability of weather, but also of soil moisture, soil type, spatial heterogeneity of soil chemical and physical properties, and waterlogging. For example, Slavich et al. (1990), after carefully mapping the heterogeneity in soil conductivity with an EM meter, found that barley rankings differed on different soil types and in different seasons, presumably due to variation in soil moisture. Thus, field trials give information of performance only at a particular site, and in a particular season. Field experiments may be more appropriate at the final stages of a breeding programme, rather than at initial stages when screening and selection for novel germplasm or for specific traits is best done under controlled environments. This is particularly important when the putative donors of specific traits are foreign genotypes not adapted to local conditions.
The most extensive screen for salt tolerance in the field has been done by Jafari-Shabestari et al. (1995), who evaluated 400 Iranian wheats on one site in California over two seasons, irrigated with water at three salinity levels (1, 5, and 8 dS m−1). They measured final biomass and yield, and calculated a ‘stress susceptibility index’ that relates grain yield in saline versus non-saline soils. They found little correlation between grain yield at high salinity with biomass, harvest index, or stress susceptibility, and noted that some genotypes with low stress susceptibility (i.e. apparent tolerance to stress) had low yield potential. They concluded that the susceptibility index is highly subject to experimental errors, especially with small plots, and questioned its use. A lack of correlation between relative yield and absolute yield, in a comparison of 38 genotypes of wheat and other cereals, was also noted by Richards et al. (1987) who concluded that the most efficient way to increase yields at high salinity was to select for the best performers at low salinity.
These results suggest that the field is not appropriate for screening large numbers of genotypes, especially exotic or foreign genotypes, as their yield will be influenced strongly by flowering and maturity time, as well as by other factors such as height and disease resistance, and so may not compare well with adapted cultivars for this reason alone. Further, extensive experiments involving replicated sites and seasons is not cheap. A more cost-effective way may be to screen for specific traits in controlled environments, back-cross the traits (if from exotic germplasm) into adapted cultivars, and test these breeding lines in the field.
Taking salt tolerance from the laboratory to the field
Field performance is the ultimate test for salt tolerance, and must be done at replicated sites and seasons. The differences between seasons in temperature and drought which, particularly in dry-land agriculture, will directly affect the build-up of salts around the roots, mean that tests must be done over at least 3 years. Field tests may show up unexpected responses, as described earlier for derivatives of the salt-tolerant Indian landrace, Kharchia. KRL1-4 performed well in India but not in Pakistan, possibly because of the greater problems of waterlogging, and KTDH 19 performed well in Spain but not in India and Pakistan because it matured about 2 weeks later than local genotypes (Hollington, 2000).
As mentioned in the Introduction, field conditions vary from site to site, not only in soil salinity, but also in soil physical and chemical properties such as sodicity, high pH, and boron, and interactions between these stresses can occur. High pH can cause reduced K+ uptake even though it might not affect Na+ uptake (Ahmad, 2002), and boron can affect the subcellular distribution of salt in leaves and hence the salt tolerance of the plant (Wimmer et al., 2003). Waterlogging worsens the effects of salinity on wheat (Barrett-Lennard, 2003) and may be a major reason why wheat bred for salt tolerance has had little success in farmers' fields in some regions (Hollington et al., 2002). When O2 deficiency occurs in roots in waterlogged soils in species with little aerenchyma or adaptations such as shallow roots, respiration is impaired, and may be the cause of the higher salt uptake described by Barrett-Lennard (2003), especially now it is known that there are normally high rates of Na+ efflux from roots to the external solution (Davenport et al., 2005) which is an energy-demanding process. The roots of many wetland species contain a barrier to radial O2 loss in addition to having extensive aerenchyma, and introduction of these traits into wheat may increase its waterlogging tolerance and its ability to provide enough energy to exclude Na+ in waterlogged soils (Colmer et al., 2005).
All breeding lines developed with greater tolerance to salt or any soil constraint should have the ability to yield well under optimal conditions. Most farmers' fields are heterogeneous for soil physical and chemical properties, and most of the yield in the field comes from the least saline and most fertile areas, so yield potential is probably the most important trait of all. Richards (1993) argued that the best breeding strategy is to select for high yield on non-saline soils, and it certainly follows that in a breeding or backcrossing programme, the most productive genotype at the lowest salinity should always be used as the recurrent parent (Richards, 1993; Royo and Aragüés, 1999). That argument was made in the context of yield penalties associated with introducing new genes for salt tolerance from progenitors or wild relatives with little yield potential, and provides a caution to physiologists or cytogeneticists to ensure that no yield penalty or ‘linkage drag’ is associated with the trait under study.
Summary
To increase the salt tolerance of cereals, attention in most laboratories has focused on identifying new genetic sources of low rates of Na+ uptake to leaves. Less attention has been given to genetic sources of tolerance of high Na+ concentrations in leaves, probably because this trait is much more difficult to quantify. Potential sources of tissue tolerance to high internal Na+ concentrations have been identified in rice (Yeo and Flowers, 1983; Yeo et al., 1990) and durum wheat (Munns and James, 2003), but it has not been easy to transfer this into cultivars because of the lack of precise phenotyping techniques.
It is intriguing that so little is known about the physiology of the Indian landrace Kharchia 65, universally regarded as highly salt tolerant (Joshi, 1976; Kingsbury and Epstein, 1984; Sharma et al., 1984; Ashraf, 2002), apart from an observation by Sharma et al. (1984) that it combined low Na+ uptake rates with successful osmotic adjustment, and the finding of Richards and Lukacs (2002) that it has unusually high specific leaf area and early vigour. Yet it is still the mainstay of the Indian wheat breeding programme. Perhaps a combined physiological and molecular analysis of this and the other highly salt-tolerant genotypes such as SARC-1 and Sakha 8 could reveal important mechanisms that could be used to develop salt-tolerant cultivars for other countries.
Also little is known about the mechanism of tolerance of barley in comparison to wheat, other than that barley tolerates its high internal concentrations of salt almost as well as halophytes, and must be able to compartmentalize the salt in vacuoles. This raises the questions of whether barley could be even more tolerant of saline soil if it could exclude Na+ better, and what would happen if genes responsible for the low Na+ transport rates in wheat were introduced.
Other traits are needed to improve tolerance of the osmotic effect of the salt outside the roots, which can have a greater effect on growth and yield than the salt-specific component. Such traits include water use efficiency, osmotic adjustment, and morphological or developmental patterns that conserve water; such traits are more important for dry-land than irrigation agriculture.
The largest gains from diversity within a crop species could be made by selecting for specific traits, and recombining these from a series of donor parents, rather than selecting for salt tolerance per se, as discussed by Yeo and Flowers (1986). This pyramiding approach might enable improvements in tolerance beyond that presently available within a specific crop. This requires that the underlying traits be identified; that there is a reliable and precise screening method, and that the traits are heritable.
When insufficient natural variation exists with in a species or its close relatives, use of gene transformation technology can provide novel and useful genetic material. However, the novel gene has to be backcrossed into commercial cultivars that are adapted to the environment of interest, and the value of the trait has to be measured in different genetic backgrounds. This process will be accelerated by the knowledge of the physiological mechanisms and how to quantify their effect on salt tolerance. Selections of breeding lines can be done most cost-effectively in the glasshouse, and field trials reserved for lines that are known to express the gene or trait of interest. The field, with its inherent variability, can be used to assess the performance of the selected trait in adapted cultivars, and evaluate breeding lines for commercial release.
Further improvements in salt tolerance will undoubtedly result from close interactions between molecular geneticists and physiologists, and benefit from timely feedback from plant breeders and agronomists.
AL received a McMaster Fellowship from CSIRO. The work on durum wheat reported in this paper has been supported by the Grains Research and Development Corporation (GRDC) of Australia.
References
Ahmad M.
Amtmann A, Sanders D.
Ashraf M.
Ashraf M, Khanum A.
Ashraf M, McNeilly T.
Ashraf M, O'Leary JW.
Ball MC.
Barrett-Lennard EG.
Barrett-Lennard EG.
Bonilla P, Dvořák J, Mackill D, Deal K, Gregorio G.
Chhipa BR, Lal P.
Colmer TD, Flowers TJ, Munns R.
Colmer TD, Munns R, Flowers TJ.
Davenport R, James RA, Zakrisson-Plogander A, Tester M, Munns R.
Davies WJ, Zhang J.
Dodd IC.
Dubcovsky J, Santa Maria G, Epstein E, Luo MC, Dvořák J.
Dvořák J, Noaman MM, Goyal S, Gorham J.
El-Hendawy SE, Hu Y, Schmidhalter U.
Flowers TJ.
Flowers TJ, Dalmond D.
Flowers TJ, Hajibagheri MA, Yeo AR.
Flowers TJ, Troke PF, Yeo AR.
Flowers TJ, Yeo AR.
Forster BP, Pakniyat H, Macaulay M, Matheson W, Phillips MS, Thomas WTB, Powell W.
Francois LE, Grieve CM, Maas EV, Donovan TJ, Lesch SM.
Fricke W.
Garcia A, Rizzo CA, Ud-Din J, Bartos SL, Senadhira D, Flowers TJ, Yeo AR.
Garthwaite AJ, von Bothmer R, Colmer TD.
Gorham J, Bristol A, Young EM, Wyn Jones RG, Kashour G.
Gorham J, Hardy C, Wyn Jones RG, Joppa LR, Law CN.
Gorham J, Wyn Jones RG, Bristol A.
Greenway H.
Greenway H, Munns R.
Greenway H, Osmond CB. 1972. Salt responses of enzymes from species differing in salt tolerance.
Gregorio GB, Senadhira D, Mendoza RD, Manigbas NL, Roxas JP, Guerta CQ.
Hollington PA.
Hollington PA, Aktar J, Aragüés R, Hussain Z, Mahar AR, Quarrie SA, Qureshi RH, Royo A, Saqib M.
Hollington PA, Royo A, Miller TE, Quarrie SA, Mahmood A, Aragüés R.
Hu Y, Fricke W, Schmidhalter U.
Husain S, Munns R, Condon AG.
Husain S, von Caemmerer S, Munns R.
Jafari-Shabestari J, Corke H, Qualset CO.
James RA, Rivelli AR, Munns R, von Caemmerer S.
Jeschke WD.
Jeschke WD, Aslam Z, Greenway H.
Joshi YC.
Koyama ML, Levesley A, Koebner RMD, Flowers TJ, Yeo AR.
Lafitte R.
Läuchli A.
Läuchli A, James RA, Munns R.
Layzell, DB, Pate JS, Atkins CA, Canvin DT.
Lee K-S, Choi W-Y, Ko J-C, Kim T-S, Gregoria GB.
LeNoble ME, Spollen WG, Sharp RE.
Lin HX, Zhu MZ, Yano M, Gao JP, Liang ZW, Su WA, Hu XH, Ren ZH, Chao DY.
Lindsay MP, Lagudah ES, Hare RA, Munns R.
Maas EV, Grieve CM.
Maas EV, Hoffman GJ.
Mahar AR, Hollington PA, Virk DS, Witcombe JR.
Mäkelä P, Munns R, Colmer TD, Peltonen-Sainio P.
Martin PK, Ambrose MJ, Koebner RMD.
Mühling KH, Läuchli A.
Munns R.
Munns R.
Munns R.
Munns R, Cramer GR.
Munns R, Fisher DB, Tonnet ML.
Munns R, Greenway H, Kirst GO.
Munns R, Guo J, Passioura JB, Cramer GR.
Munns R, Hare RA, James RA, Rebetzke GJ.
Munns R, James RA.
Munns R, Rawson HM.
Munns R, Rebetzke GJ, Husain S, James RA, Hare RA.
Munns R, Schachtman DP, Condon AG.
Neves-Piestun BG, Bernstein N.
Pitman MG.
Pitman MG, Läuchli A.
Poustini K, Siosemardeh A.
Pritchard J, Wyn Jones RG, Tomos AD.
Qureshi RH, Ahmad R, Ilyas M, Aslam Z.
Qureshi RH, Barrett-Lennard EG.
Rana RS.
Rawson HM, Long MJ, Munns R.
Rawson HM, Richards RA, Munns R.
Ren ZH, Gao JP, Li LG, Cai XL, Huang W, Chao DY, Zhu MZ, Wang ZY, Luan S, Lin HL.
Rengasamy P.
Richards RA.
Richards RA, Dennett CW, Qualset CO, Epstein E, Norlyn JD, Winslow MD.
Richards RA, Lukacs Z.
Rivelli AR, James RA, Munns R, Condon AG.
Royo A, Abió D.
Royo A, Aragüés R.
Royo A, Aragüés R, Playán E, Ortiz R.
Sayed HI.
Schachtman DP, Munns R, Whitecross MI.
Serraj R, Sinclair TR.
Sharma SK, Joshi YC, Bal AR.
Sharp RE, LeNoble ME, Else MA, Thorne ET, Gherardi F.
Slavich PG, Read BJ, Cullis BR.
Srivastava JP, Jana S.
Steppuhn H, van Genuchten MT, Grieve CM.
Storey R, Schachtman DP, Thomas MR.
Termaat A, Passioura JB, Munns R.
Tester M, Davenport R.
Tyerman SD, Niemietz CM, Bramley H.
USDA-ARS.
Wimmer MA, Muhling KH, Läuchli A, Brown PH, Goldbach HE.
Wolf O, Munns R, Tonnet ML, Jeschke WD.
Wolf O, Munns R, Tonnet ML, Jeschke WD.
Wyn Jones RG, Storey R.
Yeo AR, Flowers TJ.
Yeo AR, Flowers TJ.
Yeo AR, Yeo ME, Flowers SA, Flowers TJ.



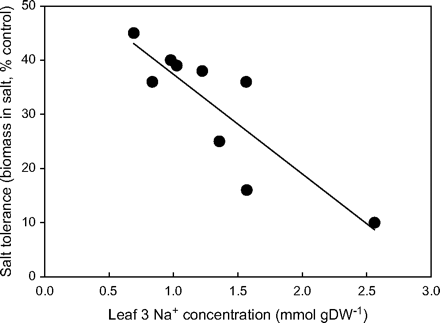
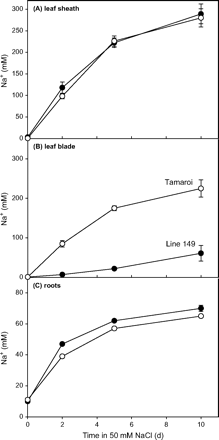
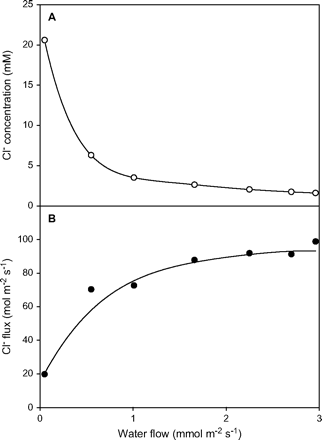
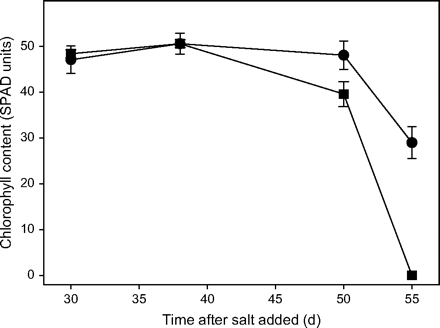
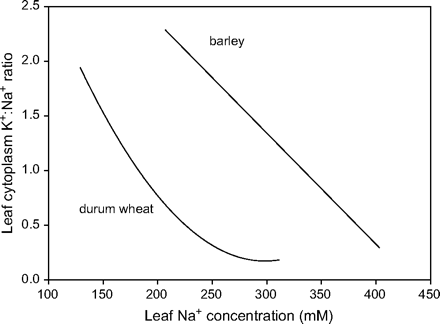
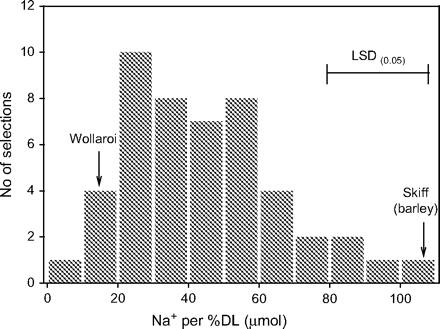
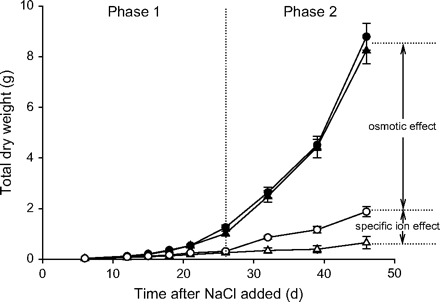

Comments