-
PDF
- Split View
-
Views
-
Cite
Cite
David J. Sharkey, Anne M. Macpherson, Kelton P. Tremellen, Sarah A. Robertson, Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells, Molecular Human Reproduction, Volume 13, Issue 7, July 2007, Pages 491–501, https://doi.org/10.1093/molehr/gam028
Close - Share Icon Share
Abstract
Exposure to semen elicits an inflammatory response in the female reproductive tract of rodents and other animals. The nature and regulation of any similar response in humans is poorly understood. This study investigated seminal plasma induction of inflammatory cytokine and chemokine gene regulation in human cervical and vaginal epithelial cells in vitro. Affymetrix microarray gene profiling revealed that inflammatory cytokine genes were prevalent among 317 known genes differentially expressed in immortalized ectocervical epithelial (Ect1) cells after incubation with pooled human seminal plasma. A dose- and time-dependent induction by seminal plasma of IL8, IL6, CSF2 and CCL2 mRNA expression in Ect1 cells was verified by quantitative RT–PCR. This was accompanied by increases in Ect1 secretion of immunoactive gene products IL-8, IL-6, GM-CSF and MCP-1. Similar cytokine responses were elicited in primary ectocervical epithelial cells. Endocervical epithelial (End1) and vaginal epithelial (Vk2) cells were less responsive to seminal fluid, with induction of IL-8 and MCP-1, but not GM-CSF or IL-6. In a panel of 10 seminal plasma samples, considerable variation in inflammatory cytokine-inducing activity was evident. These experiments show that seminal plasma can elicit expression of a range of inflammatory cytokines and chemokines in reproductive tract epithelia, and implicate the ectocervix as the primary site of responsiveness, with gene-specific differences in the kinetics and site-restrictedness of the response. Seminal factor regulation of inflammatory cytokines in the cervical epithelium is implicated in controlling the immune response to seminal antigens, and defence against infectious agents introduced at intercourse.
Introduction
In the reproductive process, seminal plasma is viewed primarily as a transport medium for spermatozoa traversing the female cervix and uterus after coitus (Mann, 1964; Aumuller and Riva, 1992). However, studies in animal species show that seminal plasma also delivers to the female an array of signalling molecules that interact with epithelial cells lining the female reproductive tract to trigger local cellular and molecular changes that resemble an inflammatory response (Robertson, 2005). In mouse and pig experiments, seminal fluid activates expression of several pro-inflammatory cytokines and chemokines in uterine epithelial cells (Robertson et al., 1992, 1996; O'Leary et al., 2004). In turn, these factors amplify the actions of seminal fluid chemotactic agents resulting in vascular changes and recruitment and activation of macrophages, granulocytes and dendritic cells which accumulate in the uterine endometrial tissue subjacent to the epithelial surface, and migrate between epithelial cells into the luminal cavity (Phillips and Mahler, 1975; McMaster et al., 1992; O'Leary et al., 2004). The infiltrating leukocytes are implicated in clearance of seminal debris from the female tissues and potentially selection of fertilizing sperm (Mattner, 1969; Roldan et al., 1992), and may also influence the female immune response to seminal antigens and evoke tissue remodelling changes to condition the endometrial environment in preparation for pregnancy (Robertson et al., 1997; Robertson, 2005).
In mice and pigs, the epithelial cells of the uterine endometrium are the primary site of seminal fluid interaction and in both species, the key cytokines induced are GM-CSF and IL-6, as well as the chemokines KC (mouse IL-8 homologue) and MCP-1 (Robertson et al., 1992, 1996; O'Leary et al., 2004). Experiments with male mice rendered deficient in sperm or seminal plasma, respectively, by vasectomy or surgical removal of the seminal vesicle gland show that the active signalling moieties are contained within the seminal plasma fraction of the ejaculate and are derived from the seminal vesicle (Robertson et al., 1996). Cytokines of the transforming growth factor-β (TGFβ) family of cytokines are identified as the major active factors in mouse seminal plasma (Tremellen et al., 1998). Seminal TGFβ is synthesized in the latent form in the seminal vesicle gland and is activated in the female tract upon deposition at mating (Tremellen et al., 1998).
In women, the ejaculate is deposited in the cervix and vagina, but the extent to which seminal fluid normally activates inflammatory cytokine synthesis or leukocyte infiltration in any compartment of the human female tract is unclear. Two previous in vivo studies in women have shown neutrophil exocytosis in the cervical tissues following either sexual intercourse or artificial insemination. Both reported that sperm, but not seminal plasma, is required to elicit this ‘leukocytic reaction’ (Pandya and Cohen, 1985; Thompson et al., 1992). There are preliminary indications that signalling activity is also associated with the cell-free, plasma fraction of semen. In vitro studies demonstrate that human female reproductive tract cells can respond to seminal plasma, with increased IL-8 and secretory leukocyte protease inhibitor (SLPI) secretion from cervical explants (Denison et al., 1999). Endometrial epithelial cells are reported to show elevated synthesis of IL-1β, IL-6 and leukaemia inhibitory factor (LIF) after culture with seminal plasma (Gutsche et al., 2003). Seminal fluid might also target infiltrating leukocytes directly, since IL-10 production in human monocyte U937 cells can be induced in response to seminal fluid constituents (Denison et al., 1999).
The aim of the current study was to undertake a comprehensive investigation of the seminal plasma regulation of inflammatory gene expression in female reproductive tract epithelial cells. After initially utilizing a gene array approach to evaluate the effect of seminal plasma of inflammatory cytokine gene expression in immortalized ectocervical epithelial (Ect1) cells, we then focused on seminal fluid regulation of four key cytokines IL-8, IL-6, GM-CSF and MCP-1. The relative responsiveness of cells from different locations in the female tract including vaginal, endocervical and ectocervical epithelial cells was evaluated, by comparing responses in Ect1 cells with endocervical (End1) and vaginal epithelial (Vk2) cells, which have been shown to retain the morphological, immunocytochemical and secretory characteristics of their respective tissues of origin (Fichorova et al., 1997; Fichorova and Anderson, 1999). To confirm the physiological relevance of the cell line data, similar experiments were undertaken using primary ectocervical epithelial cells prepared from healthy cervical tissue. Finally, we used a panel of semen samples from 10 proven fertile men to investigate variation in inflammatory cytokine-inducing activity and the difference in patterns of cytokines elicited. Our finding of seminal fluid regulation of an array of inflammatory cytokines and chemokines has implications for female reproductive immune function and defence against sexually transmitted pathogens.
Materials and Methods
Patient participation and tissue collection
Ethics approval for the collection of human cervical tissue and seminal plasma used in this study was granted by the combined human ethics committees of the University of Adelaide and North Western Adelaide Health Service (NWAHS). Signed informed consent was obtained from all participants. Human seminal plasma was obtained from 10 healthy, proven fertile males enrolled in an ongoing semen donation programme at Repromed Pty Ltd (Dulwich, Australia). Semen was produced by manual masturbation and following liquification, was centrifuged at 10 000g for 10 min at room temperature within 30 min of ejaculation. Seminal plasma was stored frozen in aliquots at −80°C until use. Sperm analyses were performed on all semen samples to confirm sperm number, motility and morphology parameters met WHO criteria for normality. Semen samples were excluded in the event of abnormal sperm parameters, high leukocytes or other symptoms of infection, or donor use of immune-modifying medications such as methotrexate or non-steroidal anti-inflammatory drugs (NSAIDs). Human cervical tissue was obtained from three women undergoing routine vaginal or total abdominal hysterectomy at the Lyell McEwin or Women's and Children's Hospitals (Adelaide), for benign non-cervical pathology (dysfunctional uterine bleeding, fibroids, endometriosis, pelvic pain or prolapse). All women were premenopausal, however no distinction was made regarding the stage of menstrual cycle at the time of surgery.
Primary ectocervical epithelial cell culture protocol
Human ectocervical epithelial cells were cultured using a modification of a technique described previously (Rheinwald and Green, 1975). Cervical tissue biopsies were washed twice in ice cold Hank's balanced salt solution (HBSS) (Invitrogen, Mount Waverley, Australia) then placed into 5 ml of Dulbecco's minimal essential medium (DMEM) (Invitrogen) containing 5 U dispase (Boehringer Mannheim, Mannheim, Germany) and incubated overnight at 4°C to dissociate the epithelial surface from the stromal tissue. The next morning, the biopsy/dispase solution was incubated at room temperature for 1 h, after which large sheets of epithelial cells were removed from the biopsy using sterile forceps. The sheets were then disaggregated by incubation in DMEM containing 0.25% trypsin and 0.01% collagenase type I (Sigma, Castle Hill, Australia) at 37°C for 30 min, followed by aspiration and extrusion through hypodermic needles of incrementally decreasing gauge. Epithelial cells were then treated with 0.01% EDTA in 10 ml DMEM at 37°C for 10 min, and finally were plated at 1 × 105 cells/well in culture medium consisting of 68% DMEM/22% Hams-F12/7% FCS/1% Nutridoma-SP (Boehringer Mannheim) with 0.02 mM glutamine and 5 mM hydrocortisone (Upjohn, Rydalmere, Australia) (ectocervical culture media/7% FCS), in 1.5 ml culture wells (Nunc, Weisbaden, Germany). Culture wells were seeded 24 h prior with murine 3T3 fibroblast cells (4 × 104 cells/well) previously rendered mitogenically inactive by incubation for 2 h with 4 µg/ml mitomycin C (Sigma). The 3T3 cells act as a feeder layer by secreting extracellular matrix components required for epithelial cell attachment.
Cervical epithelial cell cultures were incubated for 5–7 days to allow the majority of epithelial cells to attach to the culture well floor and to displace the 3T3 fibroblasts, which were not viable for more than 3–4 days. Once good attachment was observed, the culture supernatant containing desquamated epithelial cells and dead 3T3 cells was aspirated and replaced with 500 µl of fresh ectocervical culture medium/2% FCS. Twelve hours later, this ‘pretreatment’ supernatant was collected and replaced with 500 µl of fresh ectocervical culture medium/2% FCS containing various concentrations of pooled human seminal plasma (0.1, 1.0 or 10% v/v) or culture media alone (control). After 12 h, this treatment medium was discarded and replaced with fresh medium, which was in turn collected 24 h later as the ‘post-treatment’ supernatant. Supernatants were centrifuged to remove cellular debris and stored at −80°C until cytokine assay.
Immortalized epithelial cell culture conditions
Human Ect1, End1 and Vk2 cells were cultured according to protocols described previously (Fichorova et al., 1997; Fichorova and Anderson, 1999). All cell lines were propagated in keratinocyte serum-free media (KSFM) (Gibco, Mount Waverley, Australia) supplemented with 0.1 ng/ml recombinant human epidermal growth factor (EGF) (Gibco), 0.05 mg/ml bovine pituitary extract (Gibco) and 0.4 mM CaCl2. For experiments, 1 × 105 cells in 500 µl of KSFM were seeded in 1.5 ml culture wells (Nunc) and incubated at 37°C/5% CO2 for 2–3 days to generate a confluent monolayer, then the culture supernatant was carefully aspirated and replaced with 500 µl of fresh KSFM. Twelve hours later, this ‘pretreatment’ supernatant was collected and replaced with 500 µl of fresh media containing various concentrations of pooled human seminal plasma (0.01, 0.1, 1.0 or 10% v/v) or culture media alone (control). After 12 h, the treatment was discarded and replaced with fresh medium, which was in turn collected 24 h later as the ‘post-treatment’ supernatant. The supernatants were then centrifuged to remove cellular debris and stored at −80°C until cytokine assay.
Affymetrix GeneChip microarray
Quadruplicate wells of Ect1 cells were incubated with culture media alone (control) or with 10% seminal plasma (treatment) for a period of 10 h. Total RNA was extracted using Trizol® (Invitrogen, Carlsbad, USA) according to the manufacturer's instructions, and purified using RNeasy micro kits (Qiagen, Doncaster, AU). Ten micrograms of RNA from each replicate well was pooled for both groups, and sent to the Australian Genome Research Facility (AGRF) in Melbourne, Australia for single-cycle labelling and hybridization to Affymetrix GeneChip® Human Genome U133 plus 2.0 microarrays (Affymetrix, Santa Clara, USA). This microarray chip is comprised of 54 000 probe sets covering over 47 000 transcripts and variants (for complete list, see http://www.affymetrix.com). RNA integrity analysis, hybridization and washing were performed according to the manufacturer's instructions. Gene expression data were initially analysed using the algorithm GC-RMA from Bioconductor (open source software, www.bioconductor.org) with the affylmGUI package (Smyth, 2004), and ArrayAssist Expression v4.0 software (Stratagene, La Jolla, USA). Probe sets were classified as differentially expressed when both analyses showed M-values ≥1.0. Fold changes between probe sets were calculated as fold change = 2M. To apply more stringent criteria to the resulting list of differentially expressed probes, data were further analysed using the Affymetrix algorithms PLIER via ArrayAssist, and MAS5 (GCOS, Gene Chip® Operating Software v1.4) with filtering for ‘robust changes’ as recommended (Gene Chip® Expression Analysis. Data analysis Fundamentals: www.affymetrix.com/support). Pathway Express and OntoExpress analysis software (Draghici et al., 2003; Khatri et al., 2005) was utilized to assign genes to cytokine–cytokine receptor interaction, TGFβ-signalling, JAK / STAT signalling and inflammatory response pathways.
Quantitative real-time PCR
Ect1 cells were incubated with culture media alone (control), or with various doses (0.01–10%) of pooled human seminal plasma for a period of 4, 6, 8, 10 or 12 h. Total RNA was extracted using Trizol® solution (Invitrogen, Carlsbad, USA) according to the manufacturer's instructions. Following treatment with RNase-free DNase I (500 IU/ml; 60 min/37°C) (Roche, Basel, Switzerland), first strand cDNA was reverse transcribed from 5 µg RNA employing a Superscript-III Reverse Transcriptase kit (10 min/30°C, 45 min/42°C) (Invitrogen, Carlsbad, USA). Primer pairs specific for published cytokine and chemokine cDNA sequences were designed, and PCR reaction conditions were optimized for each primer pair as previously described (Jasper et al., 2006). Primer sequences, product sizes, gene target sites and Genbank accession numbers for target sequences are shown in Table 1.
PCR primers used to quantify cytokine and chemokine mRNA expression in Ect1 cells
| Factor . | Gene . | Nt position . | Primer sequence . | Product size (bp) . | Genbank accession . |
|---|---|---|---|---|---|
| GM-CSF | CSF2 | 91 (fwd) | 5′ AGCCCTGGGAGCATGTGA | 77 | NM_000758 |
| 167 (rev) | 3′ CATCTCAGCAGCAGTGTCTCTACTC | ||||
| IL-6 | IL6 | 188 (fwd) | 5′ ACTCACCTCTTCAGAAGC | 283 | NM_000600 |
| 470 (rev) | 3′ GGCTTGTTCCTCACTACT | ||||
| IL-8 | IL8 | 131 (fwd) | 5′ GGCAGCTTCCTGATTTCTG | 155 | NM_000584 |
| 285 (rev) | 3′ CGCAGTGTGGTCCACTCTCA | ||||
| MCP-1 (CCL2) | CCL2 | 64 (fwd) | 5′ CGCCTCCAGCATGAAAGTCT | 67 | NM_0002982 |
| 130 (rev) | 3′ GGGAATGAAGGTGGCTGCTA | ||||
| β-Actin | ACTB | 1350 (fwd) | 5′ TGTGATGGTGGGTATGGGTC | 162 | NM_001101 |
| 1511 (rev) | 3′ ACACGCAGCTCATTGTA |
| Factor . | Gene . | Nt position . | Primer sequence . | Product size (bp) . | Genbank accession . |
|---|---|---|---|---|---|
| GM-CSF | CSF2 | 91 (fwd) | 5′ AGCCCTGGGAGCATGTGA | 77 | NM_000758 |
| 167 (rev) | 3′ CATCTCAGCAGCAGTGTCTCTACTC | ||||
| IL-6 | IL6 | 188 (fwd) | 5′ ACTCACCTCTTCAGAAGC | 283 | NM_000600 |
| 470 (rev) | 3′ GGCTTGTTCCTCACTACT | ||||
| IL-8 | IL8 | 131 (fwd) | 5′ GGCAGCTTCCTGATTTCTG | 155 | NM_000584 |
| 285 (rev) | 3′ CGCAGTGTGGTCCACTCTCA | ||||
| MCP-1 (CCL2) | CCL2 | 64 (fwd) | 5′ CGCCTCCAGCATGAAAGTCT | 67 | NM_0002982 |
| 130 (rev) | 3′ GGGAATGAAGGTGGCTGCTA | ||||
| β-Actin | ACTB | 1350 (fwd) | 5′ TGTGATGGTGGGTATGGGTC | 162 | NM_001101 |
| 1511 (rev) | 3′ ACACGCAGCTCATTGTA |
PCR primers used to quantify cytokine and chemokine mRNA expression in Ect1 cells
| Factor . | Gene . | Nt position . | Primer sequence . | Product size (bp) . | Genbank accession . |
|---|---|---|---|---|---|
| GM-CSF | CSF2 | 91 (fwd) | 5′ AGCCCTGGGAGCATGTGA | 77 | NM_000758 |
| 167 (rev) | 3′ CATCTCAGCAGCAGTGTCTCTACTC | ||||
| IL-6 | IL6 | 188 (fwd) | 5′ ACTCACCTCTTCAGAAGC | 283 | NM_000600 |
| 470 (rev) | 3′ GGCTTGTTCCTCACTACT | ||||
| IL-8 | IL8 | 131 (fwd) | 5′ GGCAGCTTCCTGATTTCTG | 155 | NM_000584 |
| 285 (rev) | 3′ CGCAGTGTGGTCCACTCTCA | ||||
| MCP-1 (CCL2) | CCL2 | 64 (fwd) | 5′ CGCCTCCAGCATGAAAGTCT | 67 | NM_0002982 |
| 130 (rev) | 3′ GGGAATGAAGGTGGCTGCTA | ||||
| β-Actin | ACTB | 1350 (fwd) | 5′ TGTGATGGTGGGTATGGGTC | 162 | NM_001101 |
| 1511 (rev) | 3′ ACACGCAGCTCATTGTA |
| Factor . | Gene . | Nt position . | Primer sequence . | Product size (bp) . | Genbank accession . |
|---|---|---|---|---|---|
| GM-CSF | CSF2 | 91 (fwd) | 5′ AGCCCTGGGAGCATGTGA | 77 | NM_000758 |
| 167 (rev) | 3′ CATCTCAGCAGCAGTGTCTCTACTC | ||||
| IL-6 | IL6 | 188 (fwd) | 5′ ACTCACCTCTTCAGAAGC | 283 | NM_000600 |
| 470 (rev) | 3′ GGCTTGTTCCTCACTACT | ||||
| IL-8 | IL8 | 131 (fwd) | 5′ GGCAGCTTCCTGATTTCTG | 155 | NM_000584 |
| 285 (rev) | 3′ CGCAGTGTGGTCCACTCTCA | ||||
| MCP-1 (CCL2) | CCL2 | 64 (fwd) | 5′ CGCCTCCAGCATGAAAGTCT | 67 | NM_0002982 |
| 130 (rev) | 3′ GGGAATGAAGGTGGCTGCTA | ||||
| β-Actin | ACTB | 1350 (fwd) | 5′ TGTGATGGTGGGTATGGGTC | 162 | NM_001101 |
| 1511 (rev) | 3′ ACACGCAGCTCATTGTA |
All Ect1 ectocervical epithelial cell samples were reverse transcribed in a single batch and were analysed for a given primer set in the same PCR run. The PCR amplification employed reagents supplied in a 2 × SYBR Green PCR Master Mix (Applied Biosystems), and each reaction volume (20 µl total) contained 3 µl of cDNA, and 5′ and 3′ primers at concentrations of 0.1–1.0 µM as previously described (Jasper et al., 2006). All cytokine primer sets were used with reaction cycle conditions of 95°C for 15 s and 60°C for 1 min, except for IL-6 primers which were used at 95°C for 20 s, 60°C for 20 s and 72°C for 1 min. The negative control included in each reaction consisted of H2O substituted for cDNA. PCR amplification was performed in an ABI Prism 7000 Sequence Detection System (Applied Biosystems) to allow amplicon quantification using the arithmetic equation 2ΔCt × 100 K−1 according to the manufacturer's instructions (Applied Biosystems User Bulletin #2), where Ct is the cycle number at which 50% maximal amplicon synthesis is achieved. Reaction products were analysed by dissociation curve profile, and by electrophoresis in 2% agarose (Promega, Madison, Wisconsin, USA) gel containing 0.5 µg/ml ethidium bromide followed by visualization over an ultra-violet light box and image capture using a Kodak digital camera. Representative PCR products were purified and sequenced at the Institute of Medical and Veterinary Science (Adelaide, Australia) using Big Dye version 2 or 3 (Applied Biosystems) to confirm primer specificity.
Cytokine ELISAs
The concentrations of IL-8, IL-6, GM-CSF and MCP-1 in both pretreatment and 24 h post-treatment epithelial cell supernatants were determined using commercial ELISA kits (R&D Systems, Minneapolis, US). All ELISAs were performed according to the manufacturer's instructions, with all samples analysed in duplicate. For GM-CSF and MCP-1 ELISAs, the minimum detectable threshold was 15.6 pg/ml, and the intra- and inter-assay coefficients of variation were less than 5%. The IL-6 ELISA had a minimum detectable threshold of 9 pg/ml, and intra- and inter-assay coefficients of variation of less than 5%, and the IL-8 ELISA had a minimum detectable threshold of 31.2 pg/ml, and intra- and inter-assay coefficients of variation less than 5%.
Data analysis and statistics
Cytokine output data in 24 h post-treatment supernatants were expressed as cytokine concentration in pg/105 cells per 24 h. Data were excluded from the analysis when comparable cytokine values (within 10% of control) were not obtained for pretreatment supernatants from control and treatment wells. SPSS version 12 (SPSS, Chicago, USA) was used to analyse complete data sets. One-way ANOVA followed by Sidak t-test for multiple groups were used to compare differences between treatment groups. Relationships between the content of different cytokines in supernatants were analysed by bivariate correlation using Pearson's correlation coefficient (R). Statistical significance in differences between groups or correlation was concluded when P < 0.05.
Results
Seminal plasma activates inflammatory response gene expression in Ect1 cells in vitro
A microarray experiment was undertaken to evaluate the effect of seminal plasma on gene expression in cervical epithelial cells. A 10% dilution of seminal plasma was chosen to approximate the physiological situation in the female reproductive tract after intercourse. Pooled aliquots of RNA recovered from immortalized ectocervical epithelial Ect1 cells incubated with or without 10% pooled human seminal plasma were reverse transcribed into cDNA and hybridized to Affymetrix Human Genome U133 plus 2.0 microarray chips. MAS5 (GCOS; Gene Chip Operating Software, Affymetrix) analysis identified 43.4% of probe sets as present in Ect1 ectocervical epithelial cells cultured in media alone, whereas 42.1% of probe sets were present in Ect1 cells incubated with seminal plasma. A total of 444 probe sets were classified as differentially expressed (>2.0-fold changes) on the basis of GC-RMA analyses, representing 317 known genes, of which 125 were up-regulated and 192 were down-regulated by seminal plasma. Of the 317 genes, several were associated with cytokine–cytokine receptor interaction, TGFβ signaling, JAK/STAT signaling or inflammatory response pathways, including 15/125 (12%) of those up-regulated and 4/192 (2%) of those down-regulated (Table 2). After additional analyses with the Affymetrix PLIER and MAS 5.0 with robust changes algorithms, 61 probe sets representing 46 of the initial 317 known genes were classified as exhibiting robust change. Of the 33 genes up-regulated in response to seminal plasma, 10 (30%) were identified as cytokine signalling or inflammation response genes (Table 2). When genes exhibiting robust change were subjected to Pathway Express analysis, ‘cytokine–cytokine receptor interaction’ ranked as the pathway most affected by treatment (corrected P-value = 0.00001).
Cytokine and chemokine genes regulated by seminal plasma in Ect1 cells
| Accession number . | Genea . | Gene description . | Fold change . |
|---|---|---|---|
| Cytokine-cytokine receptor interaction | |||
| NM_001511 | CXCL1b | CXCL1; melanoma growth stimulating activity α | 5.03 |
| NM_000584 | IL8b | IL-8 | 4.81 |
| NM_004591 | CCL20b | CCL20, MIP-3α | 4.20 |
| NM_176891 | IFNE1 | IFNε1 | 3.51 |
| NM_005228 | EGFRb | Epidermal growth factor receptor | 3.55 |
| NM_002089 | CXCL2b | CXCL2, MIP-2α | 3.09 |
| NM_000600 | IL6 | IL-6 | 2.82 |
| NM_004633 | IL1R2 | IL-1RII | 2.53 |
| NM_002090 | CXCL3 | CXCL3, MIP-2β | 2.49 |
| NM_000575 | IL1Ab | IL-1α | 2.42 |
| NM_000576 | IL1B | IL-1β | 2.14 |
| NM_004887 | CXCL14 | CXCL14 | −2.13 |
| TGFβ signalling | |||
| NM_006350 | FSTb | Follistatin | 9.16 |
| NM_000095 | THBS1b | Thrombospondin 1 | 3.81 |
| NM_020547 | BMPR2 | Bone morphogenic protein receptor, type 2 | −2.48 |
| NM_145259 | TGFBR1 | TGFβRI | −2.25 |
| JAK/STAT signalling | |||
| NM_003955 | SOCS3b | Suppressor of cytokine signalling 3 (SOCS3) | 3.16 |
| NM_004671 | PIAS2b | Protein inhibitor of activated STAT, 2 | −4.20 |
| Inflammatory response | |||
| NM_000963 | PTGS2b | Prostaglandin-endoperoxidase synthase 2; COX-2 | 4.40 |
| Accession number . | Genea . | Gene description . | Fold change . |
|---|---|---|---|
| Cytokine-cytokine receptor interaction | |||
| NM_001511 | CXCL1b | CXCL1; melanoma growth stimulating activity α | 5.03 |
| NM_000584 | IL8b | IL-8 | 4.81 |
| NM_004591 | CCL20b | CCL20, MIP-3α | 4.20 |
| NM_176891 | IFNE1 | IFNε1 | 3.51 |
| NM_005228 | EGFRb | Epidermal growth factor receptor | 3.55 |
| NM_002089 | CXCL2b | CXCL2, MIP-2α | 3.09 |
| NM_000600 | IL6 | IL-6 | 2.82 |
| NM_004633 | IL1R2 | IL-1RII | 2.53 |
| NM_002090 | CXCL3 | CXCL3, MIP-2β | 2.49 |
| NM_000575 | IL1Ab | IL-1α | 2.42 |
| NM_000576 | IL1B | IL-1β | 2.14 |
| NM_004887 | CXCL14 | CXCL14 | −2.13 |
| TGFβ signalling | |||
| NM_006350 | FSTb | Follistatin | 9.16 |
| NM_000095 | THBS1b | Thrombospondin 1 | 3.81 |
| NM_020547 | BMPR2 | Bone morphogenic protein receptor, type 2 | −2.48 |
| NM_145259 | TGFBR1 | TGFβRI | −2.25 |
| JAK/STAT signalling | |||
| NM_003955 | SOCS3b | Suppressor of cytokine signalling 3 (SOCS3) | 3.16 |
| NM_004671 | PIAS2b | Protein inhibitor of activated STAT, 2 | −4.20 |
| Inflammatory response | |||
| NM_000963 | PTGS2b | Prostaglandin-endoperoxidase synthase 2; COX-2 | 4.40 |
aListed genes are identified as differentially expressed by GC-RMA analyses and classified into gene pathways according to Pathway Express and OntoExpress gene ontology analysis.
bDenotes genes meeting additional criteria for ‘robust change’ with further analysis using PLIER from Array Assist and MAS 5.0 from GCOS software.
Cytokine and chemokine genes regulated by seminal plasma in Ect1 cells
| Accession number . | Genea . | Gene description . | Fold change . |
|---|---|---|---|
| Cytokine-cytokine receptor interaction | |||
| NM_001511 | CXCL1b | CXCL1; melanoma growth stimulating activity α | 5.03 |
| NM_000584 | IL8b | IL-8 | 4.81 |
| NM_004591 | CCL20b | CCL20, MIP-3α | 4.20 |
| NM_176891 | IFNE1 | IFNε1 | 3.51 |
| NM_005228 | EGFRb | Epidermal growth factor receptor | 3.55 |
| NM_002089 | CXCL2b | CXCL2, MIP-2α | 3.09 |
| NM_000600 | IL6 | IL-6 | 2.82 |
| NM_004633 | IL1R2 | IL-1RII | 2.53 |
| NM_002090 | CXCL3 | CXCL3, MIP-2β | 2.49 |
| NM_000575 | IL1Ab | IL-1α | 2.42 |
| NM_000576 | IL1B | IL-1β | 2.14 |
| NM_004887 | CXCL14 | CXCL14 | −2.13 |
| TGFβ signalling | |||
| NM_006350 | FSTb | Follistatin | 9.16 |
| NM_000095 | THBS1b | Thrombospondin 1 | 3.81 |
| NM_020547 | BMPR2 | Bone morphogenic protein receptor, type 2 | −2.48 |
| NM_145259 | TGFBR1 | TGFβRI | −2.25 |
| JAK/STAT signalling | |||
| NM_003955 | SOCS3b | Suppressor of cytokine signalling 3 (SOCS3) | 3.16 |
| NM_004671 | PIAS2b | Protein inhibitor of activated STAT, 2 | −4.20 |
| Inflammatory response | |||
| NM_000963 | PTGS2b | Prostaglandin-endoperoxidase synthase 2; COX-2 | 4.40 |
| Accession number . | Genea . | Gene description . | Fold change . |
|---|---|---|---|
| Cytokine-cytokine receptor interaction | |||
| NM_001511 | CXCL1b | CXCL1; melanoma growth stimulating activity α | 5.03 |
| NM_000584 | IL8b | IL-8 | 4.81 |
| NM_004591 | CCL20b | CCL20, MIP-3α | 4.20 |
| NM_176891 | IFNE1 | IFNε1 | 3.51 |
| NM_005228 | EGFRb | Epidermal growth factor receptor | 3.55 |
| NM_002089 | CXCL2b | CXCL2, MIP-2α | 3.09 |
| NM_000600 | IL6 | IL-6 | 2.82 |
| NM_004633 | IL1R2 | IL-1RII | 2.53 |
| NM_002090 | CXCL3 | CXCL3, MIP-2β | 2.49 |
| NM_000575 | IL1Ab | IL-1α | 2.42 |
| NM_000576 | IL1B | IL-1β | 2.14 |
| NM_004887 | CXCL14 | CXCL14 | −2.13 |
| TGFβ signalling | |||
| NM_006350 | FSTb | Follistatin | 9.16 |
| NM_000095 | THBS1b | Thrombospondin 1 | 3.81 |
| NM_020547 | BMPR2 | Bone morphogenic protein receptor, type 2 | −2.48 |
| NM_145259 | TGFBR1 | TGFβRI | −2.25 |
| JAK/STAT signalling | |||
| NM_003955 | SOCS3b | Suppressor of cytokine signalling 3 (SOCS3) | 3.16 |
| NM_004671 | PIAS2b | Protein inhibitor of activated STAT, 2 | −4.20 |
| Inflammatory response | |||
| NM_000963 | PTGS2b | Prostaglandin-endoperoxidase synthase 2; COX-2 | 4.40 |
aListed genes are identified as differentially expressed by GC-RMA analyses and classified into gene pathways according to Pathway Express and OntoExpress gene ontology analysis.
bDenotes genes meeting additional criteria for ‘robust change’ with further analysis using PLIER from Array Assist and MAS 5.0 from GCOS software.
Ect1 cells exposed to seminal plasma expressed several inflammatory cytokine genes encoding IL-6, IL-1α, IL-1β and interferon ε1 (IL6, IL1A, IL1B and IFNE1, respectively) and chemokine genes encoding macrophage inflammatory protein-3α (MIP-3α), CXCL2 (MIP-2α) and CXCL3 (MIP-2β), (IL8, CXCL1, CCL20, CXCL2 and CXCL3, respectively). Modulators of cytokine expression or signalling were also differentially expressed, with induction of SOCS3, IL1R2 and EGFR, and suppression of protein inhibitor of activated STAT, PIAS2. The TGFβ signalling pathway was notably represented with up-regulation of follistatin (FST) and thrombospondin-1 (THBS1) genes, and down-regulation of the bone morphogenic protein receptor type 2 (BMPR2) and TGFβ receptor 1 (TGFBR1). Seminal plasma also induced expression of the gene encoding prostaglandin synthesis enzyme cycloxygenase-2 (PTGS2) (Table 2).
Seminal plasma stimulates Ect1 cytokine mRNA expression in a dose-responsive and time-dependent manner
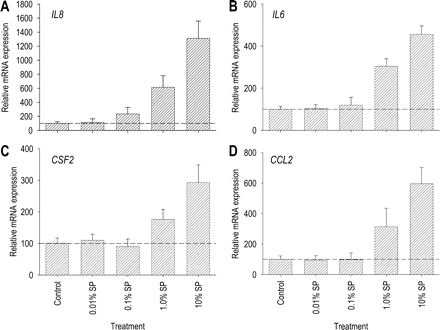
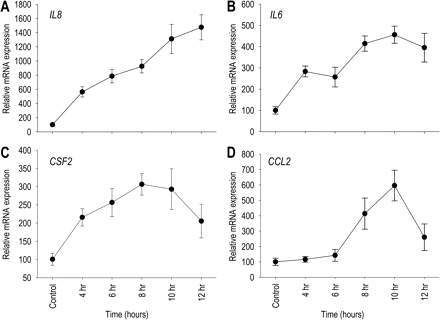
We next used quantitative RT–PCR to confirm the activation in Ect1 inflammatory cytokine gene expression revealed by microarray. IL8 and IL6 genes were chosen for further evaluation on the basis of their central role in the inflammatory response and their well-characterized contribution to the post-mating inflammatory response in mice and pigs (Robertson et al., 1996, 1998; O'Leary et al., 2004). Ect-1 cells responded to 10% seminal plasma with increases in IL8 and IL6 mRNA expression of 13.1- and 4.6-fold, respectively (Fig. 1A and B), both higher fold-changes than those seen in the microarray experiment (Table 2). IL8 and IL6 mRNA induction was responsive to seminal plasma concentration, with maximal responses at 10% seminal plasma and progressively declining response to higher dilutions (Fig. 1A and B). In a time course study, the effects of 10% seminal plasma on IL8 and IL6 mRNA synthesis were evident from as early as 4 h after co-culture with seminal fluid, and progressively increased until 10 h (Fig. 2A and B).
Dose response to seminal plasma in Ect1 ectocervical epithelial cell expression of IL8, IL6, CSF2 and CCL2 mRNAs. Ect1 cells were cultured to confluence before adding culture media alone (control) or culture media containing seminal plasma at concentrations of 0.01, 0.1, 1.0 or 10% (v/v). Cells were then incubated for 10 h, when total cellular RNA was extracted and reverse transcribed for quantitative RT–PCR analysis of (A) IL8, (B) IL6, (C) CSF2 and (D) CCL2 mRNA expression. All mRNA data were normalized to the housekeeping gene β-actin. Post-treatment data are expressed in arbitrary mRNA units as a per cent of the mean value of the control group, calibrated so that the mean value of the control group = 100. All measurements are the mean ± SEM of quadruplicate wells in each experimental group. The data shown are representative of three replicate experiments.
Time course of effect of 10% seminal plasma on IL8, IL6, CSF2 and CCL2 mRNA expression by Ect1 ectocervical epithelial cells. Ect1 cells were cultured to confluence before adding culture media alone (control) or culture media containing 10% (v/v) seminal plasma. Cells were then incubated for a further 4, 6, 8, 10 or 12 h, when total cellular RNA was extracted and reverse transcribed for quantitative RT–PCR analysis of (A) IL8, (B) IL6, (C) CSF2 and (D) CCL2 mRNA expression. All mRNA data were normalized to the housekeeping gene β-actin. Post-treatment data are expressed in arbitrary mRNA units as a per cent of the mean value of the control group, calibrated so that the mean value of the control group = 100. All measurements are the mean ± SEM of quadruplicate wells in each experimental group. The data shown are representative of three replicate experiments.
Two additional genes, CSF2 encoding GM-CSF and CCL2 encoding MCP-1, were also evaluated. Although these genes were not identified as differentially regulated in the microarray experiment, their cytokine products are well-characterized mediators of the post-mating inflammatory response in mice and pigs (Robertson et al., 1996, 1998; O'Leary et al., 2004) and known to be expressed by human cervical epithelial cells (Woodworth and Simpson, 1993; Hubert et al., 1999; Kleine-Lowinski et al., 2003; Fahey et al., 2005). Ect-1 cells responded to seminal plasma with increases in CSF2 and CCL2 mRNA expression of 2.9- and 5.9-fold, respectively (Fig. 1C and D). CSF2 and CCL2 mRNA induction was also responsive to seminal plasma concentration, with maximal responses at 10% seminal plasma and a lower response at 1.0% seminal plasma (Fig. 1C and D). The effect of 10% seminal plasma on CSF2 mRNA synthesis was evident from 4 h and maximal by 8 h, whereas CCL2 mRNA induction was not evident until 8 h and maximal at 10 h (Fig. 2C and D).
Seminal plasma stimulates Ect1 cytokine secretion in a dose-responsive manner
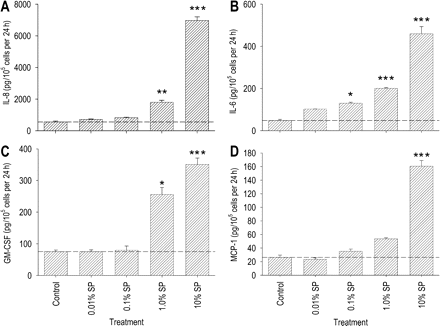
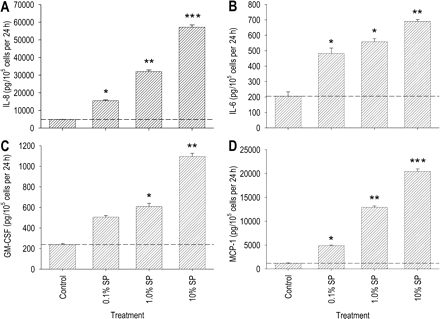
We next evaluated whether the seminal plasma-elicited increase in Ect-1 cytokine mRNA expression was accompanied by increased cytokine protein secretion. Immunoassay of culture supernatants collected from Ect-1 cells after 12 h incubation with seminal plasma showed increased secretion of IL-8, IL-6, GM-CSF and MCP-1. A concentration of 10% seminal plasma was required to elicit the full response for each cytokine, with a 12.5-fold increase in IL-8, a 9.5-fold increase in IL-6, a 4.7-fold increase in GM-CSF and a 6.1-fold increase in MCP-1 output (Fig. 3A–D). Higher dilutions also elicited cytokine responses. The threshold dilution of seminal plasma for IL-8, GM-CSF and MCP-1 stimulation was 1.0%, which induced increases of 3.2-, 3.4- and 2.0-fold, respectively, compared with the control wells (Fig. 3A,C and D). As little as 0.1% seminal plasma induced a 2.7-fold increase in IL-6 production (Fig. 3B).
Effect of seminal plasma on IL-8, IL-6, GM-CSF and MCP-1 secretion by Ect1 ectocervical epithelial cells. Ect1 cells were cultured to confluence before adding culture media (control) or diluted seminal plasma at concentrations of 0.01, 0.1, 1.0 or 10% (v/v). Cytokine content was measured in the 24 h post-treatment supernatants by ELISA. Data are expressed as (A) IL-8, (B) IL-6, (C) GM-CSF or (D) MCP-1 output in pg/105 cells per 24 h. All measurements are the mean ± SEM of triplicate wells in each experimental group. The data shown are representative of three replicate experiments. Data were analysed by one-way ANOVA and Sidak t-test to compare differences between the control and treatment groups. *P < 0.05; **P < 0.01; ***P < 0.001
Seminal plasma elicits different cytokine responses in endocervical, ectocervical and vaginal epithelial cell lines
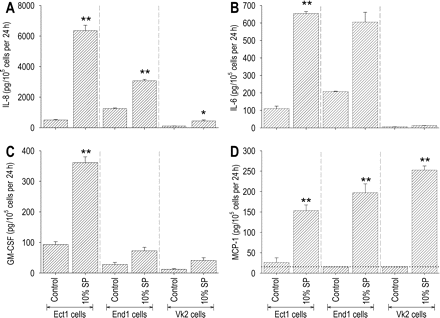
To evaluate whether epithelial cells from different regions of the reproductive tract respond differently to seminal plasma, we then compared the cytokine secretion profiles of Ect1 cells with immortalized endocervical (End1) and vaginal epithelial (Vk2) cells after stimulation with 10% pooled seminal plasma. Each cell line was found to be responsive to seminal plasma, but with a reproducibly different pattern of production of IL-8, IL-6, GM-CSF and MCP-1.
The secretory profile of End1 cells and their responsiveness to seminal plasma was similar to Ect1 cells, except for their considerably lower baseline and stimulated GM-CSF production. End1 endocervical epithelial cells cultured without seminal plasma produced 245, 188 and 30% of the IL-8, IL-6 and GM-CSF secreted by Ect1 cells (Fig. 4A–D). Baseline secretion of MCP-1 by End1 cells was below the detectable limit of the assay (Fig. 4D). End1 cells responded to 10% seminal plasma with similar increases to Ect1 cells in IL-8 and MCP-1 output of 2.5-fold and >12.7-fold, respectively, and with an increase in GM-CSF output of 2.6-fold (Fig. 4A–D). A 2.9-fold increase in IL-6 secretion from End-1 cells was induced after exposure to seminal plasma, however this failed to reach statistical significance (P = 0.059) (Fig. 4B).
Effect of seminal plasma on IL-8, IL-6, GM-CSF and MCP-1 secretion by Ect1, End1 and Vk2 epithelial cell lines. Ect1, End1 and Vk2 cells were cultured to confluence before adding culture media alone (control) or culture media containing 10% (v/v) seminal plasma. Cytokine content was measured in the 24 h post-treatment supernatants by ELISA. Post-treatment data are expressed as (A) IL-8, (B) IL-6, (C) GM-CSF or (D) MCP-1 output in pg/105 cells per 24 h. All measurements are the mean ± SEM of triplicate wells in each experimental group. The data shown are representative of three replicate experiments. Data were analysed by ANOVA and Sidak t-test to compare differences between the control and treatment groups. *P < 0.05; **P < 0.001
The secretory profile of Vk2 vaginal epithelial cells was substantially different to both cervical epithelial cell lines, and their cytokine profile was less responsive to seminal plasma regulation. Unstimulated Vk2 cells secreted only 22, 6 and 13% of the IL-8, IL-6 and GM-CSF produced by Ect1 cells (Fig. 4A–C). Baseline secretion of MCP-1 by Vk2 cells was below the detectable limit of the assay (Fig. 4D). Vk2 cells responded to 10% seminal plasma with a similar increase to Ect1 and End1 cells in MCP-1 output of >16.2-fold and a smaller increase in IL-8 of 3.9-fold, however no change in GM-CSF and IL-6 output was elicited (Fig. 4A–D).
Primary ectocervical epithelial cells are responsive to seminal plasma
To substantiate the physiological relevance of the findings in Ect-1 cells, it was important to determine whether seminal plasma could induce similar changes in cytokine production in primary ectocervical epithelial cell cultures. The effect of seminal plasma on IL-8, IL-6, GM-CSF and MCP-1 secretion was evaluated in three individual preparations of primary ectocervical cells from three different women.
Each of the three preparations of ectocervical epithelial cells responded similarly with a dose-dependent effect of seminal plasma (Fig. 5) and comparable magnitude of increase in IL-8, IL-6, GM-CSF and MCP-1 synthesis in each of the three preparations (Table 3). The increases in each cytokine were similar to those observed in Ect1 cells, with 10% seminal plasma inducing average increases over the three experiments in IL-8, IL-6, GM-CSF and MCP-1 output of 9.5-, 3.1-, 3.8- and 15.8-fold, respectively. Compared to unstimulated Ect1 cells, all three preparations of primary ectocervical epithelial cells had higher basal levels of IL-8, IL-6, GM-CSF and MCP-1 production. On average, the three cell preparations cultured without seminal plasma secreted 9.1-, 1.9-, 2.6- and 50-fold greater amounts of IL-8, IL-6, GM-CSF and MCP-1 than Ect1 immortalized epithelial cells. Furthermore, primary ectocervical cells were more responsive to seminal plasma stimulation than Ect-1 cells, with >2-fold increases in all four cytokines elicited with only 0.1% seminal plasma (Fig. 5), a dose which elicited minimal response in Ect1 cells other than in IL-6 production (Fig. 3).
Effect of seminal plasma on IL-8, IL-6, GM-CSF and MCP-1 secretion by primary ectocervical epithelial cells. Cervical epithelial cells were cultured in combination with mouse 3T3 fibroblasts for 7 days before adding culture media (control) or diluted seminal plasma at concentrations of 0.1, 1.0 or 10% (v/v). Cytokine content was measured in the 24 h post-treatment supernatants by ELISA. Data are expressed as (A) IL-8, (B) IL-6, (C) GM-CSF or (D) MCP-1 output in pg/105 cells per 24 h. All measurements are the mean ± SEM of triplicate wells in each experimental group. The data shown are representative of three independent experiments in cells from three patients (Patient C is shown). Data were analysed by one-way ANOVA and Sidak t-test to compare differences between the control and treatment groups. *P < 0.05; **P < 0.01; ***P < 0.001
Effect of 10% seminal plasma on IL-8, IL-6, GM-CSF and MCP-1 secretion by primary ectocervical epithelial cells
| . | IL-8 . | IL-6 . | GM-CSF . | MCP-1 . |
|---|---|---|---|---|
| Patient Aa | 7.4 ± 1.1 | 2.5 ± 0.4 | 3.0 ± 0.6 | ND |
| Patient B | 9.3 ± 0.5 | 3.5 ± 0.1 | 3.7 ± 0.2 | 14.2 ± 0.6 |
| Patient C | 11.8 ± 0.4 | 3.3 ± 0.2 | 4.6 ± 0.1 | 17.3 ± 0.4 |
| . | IL-8 . | IL-6 . | GM-CSF . | MCP-1 . |
|---|---|---|---|---|
| Patient Aa | 7.4 ± 1.1 | 2.5 ± 0.4 | 3.0 ± 0.6 | ND |
| Patient B | 9.3 ± 0.5 | 3.5 ± 0.1 | 3.7 ± 0.2 | 14.2 ± 0.6 |
| Patient C | 11.8 ± 0.4 | 3.3 ± 0.2 | 4.6 ± 0.1 | 17.3 ± 0.4 |
aCervical epithelial cells from three different women (Patient A, B and C) were cultured with media alone (control) or 10% (v/v) seminal plasma. Cytokine content in the 24 h post-treatment supernatants was measured by ELISA. Data are expressed as mean ± SEM fold-increase in IL-8, IL-6, GM-CSF and MCP-1 output, calculated from triplicate wells as a ratio of cytokine output in control wells.
Effect of 10% seminal plasma on IL-8, IL-6, GM-CSF and MCP-1 secretion by primary ectocervical epithelial cells
| . | IL-8 . | IL-6 . | GM-CSF . | MCP-1 . |
|---|---|---|---|---|
| Patient Aa | 7.4 ± 1.1 | 2.5 ± 0.4 | 3.0 ± 0.6 | ND |
| Patient B | 9.3 ± 0.5 | 3.5 ± 0.1 | 3.7 ± 0.2 | 14.2 ± 0.6 |
| Patient C | 11.8 ± 0.4 | 3.3 ± 0.2 | 4.6 ± 0.1 | 17.3 ± 0.4 |
| . | IL-8 . | IL-6 . | GM-CSF . | MCP-1 . |
|---|---|---|---|---|
| Patient Aa | 7.4 ± 1.1 | 2.5 ± 0.4 | 3.0 ± 0.6 | ND |
| Patient B | 9.3 ± 0.5 | 3.5 ± 0.1 | 3.7 ± 0.2 | 14.2 ± 0.6 |
| Patient C | 11.8 ± 0.4 | 3.3 ± 0.2 | 4.6 ± 0.1 | 17.3 ± 0.4 |
aCervical epithelial cells from three different women (Patient A, B and C) were cultured with media alone (control) or 10% (v/v) seminal plasma. Cytokine content in the 24 h post-treatment supernatants was measured by ELISA. Data are expressed as mean ± SEM fold-increase in IL-8, IL-6, GM-CSF and MCP-1 output, calculated from triplicate wells as a ratio of cytokine output in control wells.
Between-individual variation in seminal plasma regulation of Ect1 cytokine secretion
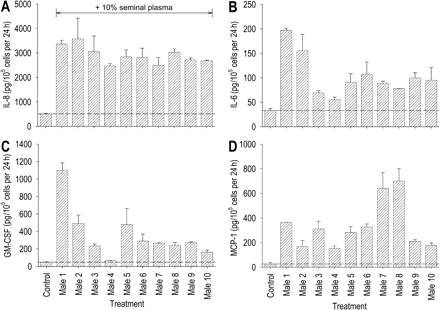
Seminal plasma samples collected from 10 healthy, proven fertile men were utilized to evaluate the extent of between-individual variation in seminal plasma regulation of Ect1 cytokine secretion. The effect on cytokine secretion profile of culturing Ect1 cells with 10% seminal plasma from each of the 10 males was examined. Relatively little variation was observed in the capacity of individual seminal plasma samples to stimulate IL-8 production from Ect1 cells. A mean 5.7-fold increase in IL-8 production was observed across the group, with individual increases ranging from 4.8- to 7.0-fold (85–123% of the mean; Fig. 6A). A similar level of variation occurred in stimulation of IL-6 production from Ect1 cells. A mean 3.1-fold increase in IL-6 production across the group was observed, with individual increases ranging from 1.7- to 6.0-fold (54–190% of the mean; Fig. 6B). Considerable variation was evident among individual seminal plasma samples in stimulating GM-CSF secretion, with a mean 8.9-fold increase and individual increases ranging from 1.8- to 23.4-fold (20–265% of the mean; Fig. 6C). Similar to GM-CSF, there was considerable variation in the MCP-1 response, with a mean 12.7-fold increase observed across the group, and individual increases ranging from 5.8- to 26.6-fold (46–210% of the mean; Fig. 6D).
Between-individual variation in seminal plasma cytokine-stimulating activity in Ect1 ectocervical epithelial cells. Ect1 cells were cultured to confluence before adding culture media alone (control) or culture media containing 10% seminal plasma collected from each of 10 healthy, proven fertile men (male 1–male 10). Cytokine content was measured in the 24 h post-treatment supernatants by ELISA. Data are expressed as (A) IL-8, (B) IL-6, (C) GM-CSF or (D) MCP-1 output in pg/105 cells per 24 h. All measurements are the mean ± SEM of triplicate wells in each experimental group. The data shown are representative of two replicate experiments with the same seminal plasma preparations.
Within individual seminal plasma samples, there was a strong correlation in the capacity to activate synthesis of GM-CSF and IL-6 (R = 0.950; P < 0.001). Induction of IL-8 was less strongly correlated with GM-CSF (R = 0.837; P = 0.024) and IL-6 (R = 0.855; P = 0.016). In contrast, regulation of MCP-1 production was not correlated with any of the other three cytokines.
Discussion
The experiments reported herein show that human seminal plasma is capable of interacting with human cervical and vaginal tissues to induce pro-inflammatory cytokine production. We have shown that epithelial cells of the female tract are a major responding cell lineage, consistent with findings in the mouse (Robertson et al., 1996), and that epithelial cells from different compartments of the tract are differentially responsive to seminal stimulation in terms of the range and strength of cytokine response. Ectocervical cells are identified as particularly sensitive to seminal activation. In immortalized cell lines, the magnitude of the response, as well as the range of cytokines produced, was greater in ectocervical cells than in either endocervical cells or vaginal cells. Primary ectocervical epithelial cells responded to seminal plasma stimulation in a similar manner to the Ect1 cell line, with similar fold increases in GM-CSF, IL-6, IL-8 and MCP-1 production, but exhibited a higher baseline and induced synthesis of each cytokine, and a greater sensitivity to low concentrations of seminal fluid.
Microarray analysis of the gene expression profile in Ect1 ectocervical cells showed that inflammatory cytokine genes are the predominant gene family activated following seminal fluid stimulation. Although it is important to note that the experiment was designed simply to provide insight on whether inflammatory genes are candidate targets of seminal fluid regulation rather than to conclusively define responding genes, it is interesting to note that many of the differentially expressed genes have previously been shown to be regulated during early pregnancy in reproductive tract epithelia of other species, with regulation attributed in some cases to seminal fluid. Elevated IL1A, IL1B, IL6 and IL8 expression occurs in both mouse and pig uterus after insemination (Robertson et al., 1992, 1996; Sanford et al., 1992; O'Leary et al., 2004). The type 1 interferon gene IFNE1 has been localized to mouse and human reproductive tract tissues but little is known about its regulation or function (Hardy et al., 2004). Several chemokine genes were induced by seminal fluid including CXCL1, CCL20 (MIP-3α), CXCL2 (MIP-2α) and CXCL3 (MIP-2β), which encode chemokines targeting monocytes, neutrophils, lymphocytes and dendritic cells via the CXCR1, CXCR2 and CCR6 receptor signalling pathways. Of these, MIP-2 has been reported as one of several chemokines induced by seminal fluid in mouse (Robertson et al., 1998). In addition, seminal plasma induced expression of the gene PTGS2, encoding the prostaglandin synthesis enzyme cyclooxygenase-2 (COX-2), previously shown to be induced by seminal fluid in the pig uterus (O'Leary et al., 2004).
On the basis of the microarray result and previous studies in animal models, we sought to study four cytokines in detail: IL-8, IL-6, GM-CSF and MCP-1. All four cytokines were induced at the transcriptional and protein level in cervical cells by exposure to seminal plasma. Each of these cytokines has previously been identified as a secretory product of epithelial cells in the cervix (Woodworth and Simpson, 1993; Fichorova and Anderson, 1999; Hubert et al., 1999; Kleine-Lowinski et al., 2003; Fahey et al., 2005), however induction of GM-CSF, IL-6 and MCP-1 synthesis in cervical cells by seminal factors has not previously been demonstrated. These results add to the evidence base for a signalling role for seminal plasma in the human female reproductive tract, expanding upon previous in vitro studies which suggest that seminal plasma can induce synthesis of IL-8 and SLPI secretion in cervical tissue explants (Denison et al., 1999) and activate expression of mRNAs encoding IL-1β, IL-6 and LIF in epithelial cells from the uterine endometrium (Gutsche et al., 2003). We were unable to detect IL10 mRNA expression in Ect-1 cells or IL-10 protein release by either immortalized or primary vaginal or cervical epithelial cells (data not shown), consistent with a previous report (Denison et al., 1999), where seminal plasma elicited IL-10 production from U937 monocyte cells but not cervical explants.
In comparison to the other transformed cell lines, ectocervical Ect1 cells were particularly responsive to seminal plasma. Ect1 cells responded with production of all four cytokines tested, whereas endocervical End1 cells failed to synthesize GM-CSF and Vk2 cells produced substantially less GM-CSF, IL-6 and IL-8 and matched the other cell lines only in terms of MCP-1 output. In view of the close similarity in morphology and gene expression characteristics between these cell lines and their primary parent cells (Fichorova et al., 1997), it seems reasonable to extrapolate from this data that the ectocervix is likely to be the primary site of seminal plasma responsiveness. Furthermore, there was a striking identity in the characteristics of the responses of Ect1 cells and primary endocervical cells, with the major difference being in the considerably more potent cytokine output in both unstimulated and seminal fluid-activated primary cells. This is consistent with previous reports that cervical keratinocytes immortalized with human papilloma virus DNAs secrete reduced amounts of GM-CSF and other cytokines compared with primary cells (Woodworth and Simpson, 1993). Reduction in MCP-1 expression in these same human papilloma cell lines is due to active suppression by viral oncogenes E6 and E7, acting in a cytokine selective manner since other chemokines IL-8, IL-10 and RANTES were unchanged in comparison with primary cells (Kleine-Lowinski et al., 2003).
Seminal plasma induction of these pro-inflammatory and chemotactic cytokines supports the interpretation that one function of seminal fluid is to activate an inflammatory cascade after deposition in the human female reproductive tract at intercourse, analogous to the consequences of mating in mice (Robertson et al., 1992) and in pigs (O'Leary et al., 2004). Since each of these factors also regulate leukocyte recruitment and activation in humans, it is likely that elevated production in the cervix elicits changes in local leukocytes, which manifest as the post-coital leukocytic reaction in women (Pandya and Cohen, 1985; Thompson et al., 1992). Epithelial cell regulation of this response is consistent with the notion that epithelial cytokines control accumulation and functional behaviour of local dendritic cell, macrophage and granulocyte populations in other epithelia (Barker et al., 1991). However, since we have not examined the effects of seminal fluid on leukocytes, we cannot exclude the possibility of direct seminal fluid signalling in these cells contributing to the response.
A pivotal function of GM-CSF in epithelial tissues is to regulate the proliferation and differentiation of dendritic cells from monocyte precursors (Sallusto and Lanzavecchia, 1994; Burnham et al., 2000). In the cervical epithelium, dendritic cells form an interconnecting network of sentinel cells, which initiate and regulate the quality of local immune responses through their professional antigen-presenting function (Hughes et al., 1988; Hubert et al., 1999). In vitro experiments show that keratinocytes from normal human cervix synthesize GM-CSF (Woodworth and Simpson, 1993) and that this cytokine is a potent regulator of dendritic cell colonization of cervical explants, accounting for the majority of the dendritic cell chemotactic activity released by cervical cells (Hubert et al., 1999). This is consistent with studies in the reproductive tract of mice, which identify GM-CSF as essential for maintaining normal uterine macrophages and dendritic cell populations, and as a regulator of activation markers including MHC class II (Robertson et al., 1998, 2000).
IL-6 has previously been described as a constitutive secretory product of normal human cervical cells (Woodworth and Simpson, 1993), and studies in polarized endocervical cells show IL-6 is secreted primarily into the apical but also the basolateral compartment (Fahey et al., 2005). Like GM-CSF, IL-6 is a key regulator of the immune regulatory phenotype of macrophages and dendritic cells, influencing their capacity to selectively polarize type 2 T-helper cell (Th2) differentiation through induction of IL-4 synthesis, and concurrent inhibition of Th1 development (Diehl and Rincon, 2002).
IL-8 and MCP-1 are chemotactic cytokines identified as major regulators of neutrophil and macrophage recruitment, respectively, in inflammatory sites (Yoshimura and Leonard, 1992; Baggiolini, 1995). Several previous studies have shown IL-8 and MCP-1 are secreted by cervical epithelial cells (Woodworth and Simpson, 1993), and like IL-6 they are released predominantly into the apical compartment (Fahey et al., 2005), with the differential gradient across the epithelial surface presumably facilitating leukocyte exocytosis into the luminal space. In vitro studies using neutralizing antibodies show that IL-8 is the major neutrophil chemotactic agent secreted by cervical epithelial cells, and that GM-CSF acts synergistically with IL-8 to amplify its chemotactic actions (Shen et al., 2004). The current study confirms a previous report that IL-8 secretion from cervical explants is up-regulated by seminal plasma (Denison et al., 1999). In that report, the potency of seminal plasma IL-8-inducing activity was similar to the current results in primary epithelial cells, with dilutions equivalent to 0.05% seminal plasma inducing ∼2-fold increases in IL-8 output in each of 15 different cervical tissue biopsies.
The capacity for the epithelial lining of the female reproductive tract to synthesize inflammatory cytokines after intercourse is consistent with the immunological competence required of these tissues. It is reasonable to postulate that synthesis of different patterns of cytokines might act to differentially regulate the number and phenotypic character of inflammatory cells in the various compartments, in patterns that reflect the likely physiological challenges faced by each tissue. As a chemotactic agent for macrophages and dendritic cells, elevated MCP-1 in the vagina and throughout the cervix would be expected to recruit these cells into the epithelial surfaces of both tissues and would provide a first line of defence against any pathogenic agents introduced at intercourse. In contrast, the relative abundance of the neutrophil chemokines IL-8 and GM-CSF in the cervix compared with the vagina would explain why neutrophil efflux after insemination occurs in the cervix, and might reflect a special function of this tissue in sperm immobilization and selection. Active synthesis of a wider range of cytokines in the cervix after intercourse would facilitate optimal competence in protecting the higher reproductive tract from pathogen invasion and in reinforcing the defensive barrier function of this epithelial surface (Quayle, 2002).
Induction of GM-CSF and IL-6 in the cervical tissues would influence the activation status of local antigen-presenting cells, programming phenotypes that impact the ensuing response to antigens processed by those cells (Sallusto and Lanzavecchia, 1994; Burnham et al., 2000; Diehl and Rincon, 2002). The significance of these two cytokines being preferentially expressed in the ectocervix is consistent with this tissue being the primary site for female ‘sampling’ of paternal antigens. Their presence together with the action of the immune-deviating agents TGFβ and prostaglandin E2 (PGE2) in seminal fluid would be expected to ensure that the outcome of any antigen-specific immune response did not adversely affect female tolerance of any future exposure to semen. Similarly, uptake and processing of male antigens in semen may provide an opportunity for priming the maternal immune response in preparation for an ensuing pregnancy fathered by the same male, since the conceptus shares many of the same paternal antigens (Thaler, 1989; Robertson et al., 1997). Induction of PTGS2 expression resulted in increased COX-2 activity and PGE2 synthesis in cervical tissue following insemination would be expected to further amplify the immune regulatory effects of seminal fluid PGE2.
IL-6, together with LIF and IL-1β, can also be induced in uterine endometrial cells by seminal factors in vitro (Gutsche et al., 2003), where it appears to be a key determinant of uterine receptivity at embryo implantation (Lim et al., 2000; Jasper et al., 2006). This suggests that the action of seminal fluid in regulating the quality of female tract immune responses may extend higher into the female tract, after transport of active seminal constituents by uterine peristaltic contractions which transport macromolecular material as high as the fallopian tube (Kunz and Leyendecker, 2002). Whether GM-CSF, IL-8 and IL-6 can also be induced in endometrial cells remains to be examined.
The identity of the seminal constituents that activate cytokine production in the human female reproductive tract epithelia is not currently known. Consistent with the mouse where TGFβ family members comprise the major active moieties in seminal fluid (Tremellen et al., 1998), TGFβ has been shown to mimic some aspects of the seminal response (Denison et al., 1999; Robertson et al., 2002; Gutsche et al., 2003) and is present together with several other cell-signalling agents in human seminal fluid (Maegawa et al., 2002). In support of a role for seminal fluid TGFβ, it is relevant that TGFβ signalling pathway genes encoding TGFβ-signalling modulators follistatin and thrombospondin-1, and receptors for TGFβ (TGFBR1) and the related bone morphogenic protein cytokine family (BMPR2) were among those genes differentially expressed in the microarray experiment. Human semen is also unique in containing very high levels of PGE2 and 19-hydroxy PGE (Templeton et al., 1978), and both forms are active in inducing cytokine expression in female reproductive tract cells (Denison et al., 1999; Muller et al., 2006). Additional bioactive moieties with potential effects on epithelial cell cytokine synthesis include bacterial products such as endotoxin, and polyamines that are abundant in seminal fluid. Experiments are underway in our laboratory to investigate the extent to which these constituents each account for the activity of whole seminal plasma in regulating GM-CSF, IL-6, IL-8 and MCP-1. Interestingly, the differences between each of the 10 seminal plasma samples evaluated in the current study showed that while all 10 had cytokine-inducing activity, there were differences in the strength and the quality of the cytokine response elicited. There was a remarkable correlation between capacity to induce GM-CSF, IL-6 and IL-8 in individual seminal samples, suggesting these factors are co-regulated and there may be identity in the seminal constituents regulating their expression. However, since MCP-1 induction did not follow the same pattern, the presence of more than one active constituent is indicated. Our characterization of the responsiveness to seminal fluid of immortalized cervical epithelial cells, and their close replication of primary cell behaviour, provides a model to facilitate further analysis of the molecular regulation of the seminal signalling response and assist in overcoming the infrequent availability and limited quantity of cervical tissue from hysterectomy patients.
In conclusion, this study describes a regulatory action of seminal plasma in activating expression of pro-inflammatory and immune-regulating cytokines in human cervical cells, and begins to provide a molecular explanation for the observed leukocytic response to intercourse in women. Seminal fluid is thus identified as a potentially major determinant of the cytokine micro-environment existing in the cervix, contributing together with ovarian steroid hormones, inflammatory cytokine stimulation and microbial challenge via Toll-like receptors to regulate epithelial cell cytokine output (Tabibzadeh, 1994; Fichorova and Anderson, 1999; Wira et al., 2002, 2005). As the predominant influence on the abundance and functional phenotypes of local dendritic cell, macrophage and granulocyte populations (Barker et al., 1991), fluctuations in cervical epithelial cell cytokine synthesis would impact all of the downstream pathways in which cervical leukocytes engage—including the induction and quality control of immune responses to sperm antigens and bacterial and viral pathogens, clearance of excess sperm and seminal debris and tissue remodelling processes at parturition and menstruation. Exposure to seminal fluids could also play a role in the incidence and progression of cervical neoplasms, with altered cytokine synthesis in transformed cells potentially acting together with seminal plasma-induced expression of tumorigenic and angiogenic factors (Muller et al., 2006) to favour carcinoma growth. Further studies are required to investigate the molecular identity of the seminal constituents mediating cervical cytokine induction, and to explore the extent to which responses modelled in vitro are replicated in the physiological situation.
Acknowledgements
This study was supported by project and fellowship grants from the NHMRC (Australia).