-
PDF
- Split View
-
Views
-
Cite
Cite
Emilio Rolán-Alvarez, Sympatric speciation as a by-product of ecological adaptation in the Galician Littorina saxatilis hybrid zone, Journal of Molluscan Studies, Volume 73, Issue 1, February 2007, Pages 1–10, https://doi.org/10.1093/mollus/eyl023
Close - Share Icon Share
ABSTRACT
On exposed rocky shores in northwestern Spain there is a striking polymorphism of Littorina saxatilis that has been claimed as an example of a putative sympatric ecological speciation process. Two ecotypes of this species have evolved that are adapted to different shore levels and habitats, although they meet and hybridize on the mid shore where their two habitats overlap. As a by-product of adaptation these ecotypes have evolved an incomplete premating reproductive isolation where they meet on the mid shore. Although they are not yet true species, and the final outcome of the process cannot be predicted, the ecological mechanisms responsible for this polymorphism could cause sympatric speciation in similar situations. Here, I review all data in support of these claims and discuss the interest of such a model system in microevolutionary studies.
SPECIATION MECHANISMS
The study of speciation has been one of the most active areas in evolutionary biology, because many biologists believe that this is one of the keys to fully understand the process of evolution (Turelli, Barton & Coyne, 2001; Coyne & Orr, 2004). Under the biological species concept (the most popular and well established definition), species are groups of interbreeding populations that are reproductively isolated (by an isolation barrier) from other such groups (Coyne & Orr, 2004). Under such a definition, speciation is the process by which the isolation barriers arose in the ancestral population (a review of different isolation barriers is provided in Coyne & Orr, 2004: 28–29). One of the most important mechanisms contributing to speciation is when the isolation barrier emerges as a by-product of the process of adaptation (Schluter, 2001). A scenario of how natural selection can contribute to speciation during adaptation to a new environment can be given. For example, it is possible to imagine a widely distributed species in which a particular population is geographically isolated by a physical barrier (a desert, a lake, a mountain, a river, etc.), which maintains it in effective isolation from the ancestral population for a long evolutionary time. The particular evolutionary changes which affect this isolated population, as a consequence of both adaptation to the new environment and random fixation of alleles by genetic drift, could produce such important genetic, behavioural or life-history differences with respect to the ancestral population, that when the geographical barrier disappears and the two incipient species meet after secondary contact, they cannot mate and/or produce fertile hybrids. At that moment they become independent evolutionary entities. This is an example of the allopatric mode of speciation, which is considered the most plausible and frequent mode of speciation (Coyne & Orr, 2004), although almost nothing is known of the particular isolation barriers that are involved during the origin of such new species (Turelli, Barton & Coyne, 2001). There is, however, a controversial possibility, whether this process can occur when the incipient species and the ancestral species still maintain some gene flow between them. This is controversial because gene flow is a homogenizing evolutionary force which erodes the genetic differentiation between the connected subpopulations, apparently making it more difficult to maintain differentiation between the pair of incipient species. There is, however, no theoretical objection to the possibility that speciation can proceed independently of the strength of gene flow between the original divergent subpopulations (Turelli, Barton & Coyne, 2001).
Two extremes can be defined from a continuum of natural situations, depending on the particular distribution of the originally divergent subpopulations and the relative strength of gene flow between them. One is the parapatric mode of speciation, when the two incipient species are partially geographically separated, but maintain some contact by hybridization. The other is the sympatric mode of speciation, when the two incipient species live in the same geographical range, allowing them to meet and mate (Futuyma & Mayer, 1980; Coyne & Orr, 2004). This simplistic situation can change somewhat at a later time in the speciation process, because incipient sympatric species will probably find alternative niches (for example different microhabitats) in order to avoid competition (Futuyma & Mayer, 1980). In that case, they may show different microgeographical distributions, although the case can still be considered as a sympatric mode of speciation if they have a dispersal ability which (in the absence of any mechanism of habitat choice) allows them to meet and mate (Futuyma & Mayer, 1980). There are a few known putative cases of sympatric speciation (reviewed in Coyne & Orr, 2004; Barluenga et al., 2006; Savolainen et al., 2006), although in general little is known about the details of how the isolation barriers are produced in each case (as in any mode of speciation). Here, I review the published information in relation to a particular hybrid zone in a marine snail, because the existing knowledge on this ecological model system allows us to understand how adaptation occurs and, as a by-product of that, how some isolation (premating) barriers can evolve in sympatry across an environmental gradient of only a few meters in length.
LITTORINA SAXATILIS, A REPRESENTATIVE SPECIES FROM INTERTIDAL ROCKY SHORES
The intertidal rocky shore is an extreme habitat which represents a borderline between marine and terrestrial realms. Plant and animals living in this area need to resist extreme, although predictable, changes in many physical and biological parameters, because of the existence of a tidal cycle (one to two times every day; Raffaelli & Hawkins, 1996). The tides have the side effect of creating abrupt gradients (typically on a scale of a few metres) in environmental and ecological conditions. Such gradients affect many environmental variables, such as temperature, salinity and desiccation, determining the vertical distribution of many species (zonation patterns). However, the amplitude of tides can change considerably from a few centimetres on Mediterranean shores to more than 10 m in some parts of the Bristol Channel (Raffaelli & Hawkins, 1996). In some species it is possible to observe extreme intraspecific polymorphisms, presumed to originate by the different adaptation of each population to its particular shore level and habitat (Reid, 1996). In addition to the vertical gradient, there is also an horizontal gradient of variation, i.e. different localities show differentiation in physical (heat, waves, type of rock, etc.) and/or ecological (absence/presence of a competing species, a predator, food, etc.) parameters.
An abundant species on North Atlantic intertidal rocky shores is the marine snail Littorina saxatilis (Olivi, 1792), although it can be found in other habitats as well (Reid, 1996). It grazes directly on the rock or on some larger animals and plants (mussels, barnacles, seaweeds, etc.) functioning as a micro-detritivore, feeding on microalgae, diatoms and other organic matter (Reid, 1996; Otero-Schmitt et al., 1997). The adults show low mobility of about 1.5 m per month on average (Janson, 1983; Erlandsson, Rolán-Alvarez & Johannesson, 1998). Such estimates are probably too low as the snails show homing behaviour, usually returning to a well known crevice or area (Janson, 1983; Rolán-Alvarez, Johannesson & Erlandsson, 1997; Cruz et al., 2004b). The species has separate sexes (gonochoristic) and has direct development (ovoviviparous; the female carries dozens to hundreds of embryos in a brood pouch). The low mobility of adults and the direct development are responsible for the presumed low dispersal capability of this species. The high level of interpopulation molecular differentiation compared to other littorinid species supports this view (Ward, 1990). In fact, some studies have found significant genetic differentiation between L. saxatilis populations separated just a few metres (Janson & Ward, 1984; Johannesson & Johannesson, 1990; Johannesson, Johannesson & Rolán-Alvarez, 1993). The low dispersal capability, in addition to the existence of many available distinct habitats, such as exposed and sheltered shores, salt marshes, lagoons, etc., has produced extraordinary intraspecific polymorphism in shell morphology and other characteristics (reviewed in Reid, 1996; Johannesson, 2003).
There are three areas of northern Atlantic rocky shores in which extreme intraspecific polymorphisms of L. saxatilis related to the degree of wave exposure are well known. Similar polymorphisms are found on other exposed shores, such as in Iceland, Maine and Norway, but they are poorly known (D.G. Reid, personal communication). K. Johannesson (née Janson) has pioneered the study of the origin and maintenance of a shell and behavioural polymorphism of L. saxatilis associated with the degree of exposure on the Swedish coast (reviewed in Johannesson, 2003). The Atlantic Swedish rocky shore (typically of granite) is relatively long because of the existence of many islands, although the main environmental gradient here is horizontal rather than vertical, due to the short tide ranges (about 20–40 cm). However, wave-exposed sites can be separated just a few metres from wave-protected areas (depending on the shore orientation with regard to the main wave direction). On these shores a different ecotype has evolved in each habitat: the E (exposed) morph lives directly on the rock surface on wave-exposed sites, while the S (sheltered) ecotype lives on boulder beaches in wave-protected bays. Between these ecotypes there may be morphologically intermediate (I) populations (Johannesson, 2003). Ecological and genetic differences between ecotypes have been detected within islands, although molecular variation between ecotypes is of approximately similar magnitude to that within ecotypes between populations of different islands (Johannesson et al., 2004). The polymorphism is maintained by the strength of natural selection acting differentially in the different habitats (exposed versus protected; Johannesson, 2003). Partial premating (sexual) isolation (based on size differences) between the two ecotypes has been detected in the laboratory (Hollander, Lindergarth & Johannesson, 2005; but see Erlandsson & Rolán-Alvarez, 1998), although it does not appear to have evolutionary consequences, because the distinct ecotypes do not meet in the wild and gene flow could take place through the intermediate populations (Janson, 1983; Johannesson, 2003).
On British rocky shores, the shell polymorphism of L. saxatilis is even more extreme and variable, in part, because the shore is longer and with larger tide ranges (up to more than 10 m in some areas). There are some examples of extreme intraspecific polymorphisms in L. saxatilis, as in the cases of ‘neglecta’ versus typical forms (Johannesson & Johannesson, 1990; Reid, 1993, 1996), and also in the H versus M ecotypes (Hull, Grahame & Mill, 1996; Wilding, Butlin & Grahame, 2001; Grahame, Wilding & Butlin, 2006). In these cases, their physical isolation due to microgeographical separation and the adaptation of each ecotype to its particular habitat may be responsible for the maintenance of the polymorphism, although a formal proof of the adaptation mechanism responsible for the H versus M ecotype differentiation is still needed. As in the Swedish model system, the extreme ecotypes are found associated with different degrees of exposure: neglecta and H types are associated with the most wave-exposed habitats, while typical or M ecotypes are associated with more protected habitats (Hull, Grahame & Mill, 1996; Johannesson, 2003). In addition, it has been suggested that both postzygotic and prezygotic partial reproductive isolation may also be contributing to the maintenance of the ecological isolation between H and M forms (Hull, Grahame & Mill, 1996; Hull, 1998; Pickles & Grahame, 1999). However, as in the Swedish population, sexual isolation (prezygotic isolation) is not able to contribute to the maintenance of (or to reinforce) the polymorphism (differences in ecotype distributions apart) as there are no stable hybrid zones in which the different ecotypes can meet and mate (Hull, 1998).
THE GALICIAN HYBRID ZONE
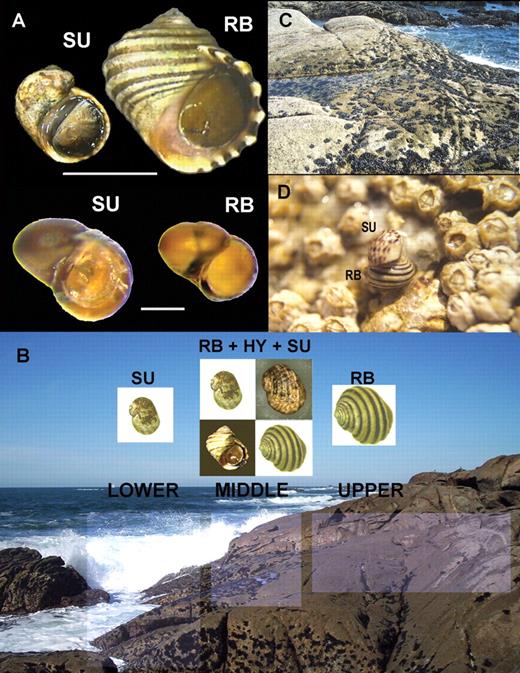
On exposed Galician rocky shores (northwestern Spain), a striking phenotypic and genetic polymorphism of L. saxatilis is found associated with different intertidal rocky shore levels and habitats (Johannesson, Johannesson & Rolán-Alvarez, 1993; Fig. 1A). The ridged and banded (RB) ecotype is found associated with the barnacle (Chthamalus stellatus and C. montagui) belt from the mid to upper shore, while the smooth and unbanded (SU) ecotype is found in the mussel (Mytilus galloprovincialis) belt from the mid to lower shore (Fig. 1B). The main morphological differences between these ecotypes are the size (on average RB is nearly twice the size of SU; Johannesson, Rolán-Alvarez & Ekendahl, 1995) and the relative area of the shell aperture (SU > RB; Carvajal-Rodríguez, Conde-Padín & Rolán-Alvarez, 2005), but they also differ in many morphological, behavioural and even physiological aspects (reviewed in Pérez-Figueroa et al., 2005). The rocky shore substratum is granite and so, in spite of the strength of the waves, the slope on these shores can be relatively low. Thus, although the tide range is relatively small, the linear distance from upper to lower shore can be up to 60 m. The barnacle belt is considerably wider than the mussel belt (and a small number of barnacles are always present even in the mussel belt). On the mid shore, the two habitats overlap, forming a mosaic distribution of mussel and barnacle patches (typical width of mid shore area is 1–5 m; personal observation; Fig. 1B, C). In some areas of the mid-shore habitat, the two ecotypes meet and occasionally mate, producing apparently fertile intermediate morphological forms (designated as hybrids) at variable frequencies (range 1–40%; Rolán-Alvarez et al., 1999), although each ecotype still shows preference for particular microhabitats (Kostylev, Erlandsson and Johannesson, 1997; Otero-Schmitt et al., 1997; Carballo, Caballero & Rolán-Alvarez, 2005; Fig. 1D). Thus, a stable hybrid zone is produced across a vertical shore gradient a few meters in length (Fig. 1B).
A. Typical specimens of Galician RB and SU ecotypes of Littorina saxatilis: adults (above) and shelled embryos (below). B. Vertical zonation patterns of mussels and barnacles in a characteristic hybrid zone of L. saxatilis. Associated with the different shore levels and habitats distinct ecotypes are found (RB, SU and hybrids). C. A detailed typical view of the mid shore, where both mussels and barnacles overlap and both ecotypes (RB and SU) and a variable number of morphologically intermediate forms (HY) appear. D. An example of the rare matings between RB and SU ecotypes that can be found on the mid shore.
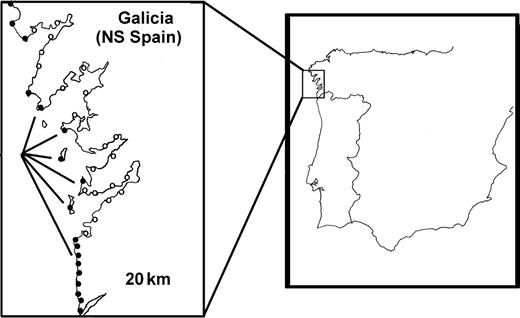
This distribution of habitats and ecotypes is found exclusively on the most exposed areas of the Galician coast. The exposed areas occupied by these two ecotypes is not continuous, but is interrupted by sheltered rías (large drowned river valleys) or (on islands) by open sea (Fig. 2). Because of this population distribution and also because this species shows an extremely low dispersal capability, the same polymorphism could evolve partially independently in many areas (Rolán-Alvarez et al., 2004). This polymorphism, however, does not exist in other shores in northern Spain (outside Galicia) or Portugal, probably because they have no granite substratum or similar environmental gradients. The granite rocky shore may provide an extremely stable vertical gradient which allows the differential adaptation of these two ecotypes to their particular shore levels. In summary, although this hybrid zone is a local polymorphism at the European scale, it can be studied in different geographical areas, thus permitting pseudo-replication. In addition to this geographical replication, some areas, especially in the south of Galicia, have large (up to 10 km) quasi-continuous hybrid zones, which can be considered as replicates on a smaller spatial scale (partially isolated in this case by long vertical crevices or small bays).
Geographical distribution of Littorina saxatilis in the hybrid zone (black circles) and outside the hybrid zone (open circles). Notice that outside the hybrid zone different ecotypes appear (often with some similarities to the RB ecotype).
THE ROLE OF NATURAL SELECTION IN THE ORIGIN AND MAINTENANCE OF THE POLYMORPHISM
That two ecotypes differing in many morphological, physiological and behavioural characteristics are consistently found associated with their appropriate shore levels and habitats, itself indicates an important role for natural selection in maintaining this zonation pattern. There are, in addition, multiple independent sources of evidence that support this claim: the partial fitness components of viability, sexual selection and fecundity have each been directly estimated in the wild for these two ecotypes and their intermediate forms (hybrids).
First, viability differences between ecotypes were quantified after transplanting more than 8000 specimens of different ecotypes between shore levels and localities. One month after their release, the ecotype living in its own habitat showed the highest survival when compared with ecotypes from different shore levels and habitats (Rolán-Alvarez, Johannesson & Erlandsson, 1997). For example, on the upper shore, transplanted SU specimens from the lower shore showed 13–23% lower viability than transplanted RB specimens from the upper shore. On the lower shore site, RB specimens from the upper shore showed 10–59% lower viability than SU specimens from the lower shore. The same result was observed in a similar experiment when the design allowed disentangling of dispersal from viability effects (Cruz et al. 2004b). Furthermore, the strength of divergent viability selection (acting on different quantitative shell traits) was highly correlated with the degree of morphological differentiation between the ecotypes across a number of independent transects in the hybrid zone (Cruz et al., 2004c). All these results support the suggestion that viability selection causes the present ecotype differences.
Second, sexual selection favours extreme values of some morphological traits on the mid shore, contributing to the maintenance of the differences between ecotypes for some traits (Cruz, Rolán-Alvarez & García, 2001). Such an effect, however, does not produce a mean sexual advantage of RB and SU against hybrids in males, although a significant trend was observed in females (Johannesson, Rolan-Alvárez & Ekendahl, 1995; Rolán-Alvarez & Ekendahl, 1995; Rolan-Alvárez et al., 1999). However, the effect on females was only significant when the frequency of SU ecotypes was higher around the mating pairs (Rolán-Alvarez, Johannesson & Ekendahl, 1995). This pattern was interpreted as an indirect property of ecotypes living in different microhabitats rather than as a pure density- or frequency-dependent mechanism affecting mating behaviour (Rolán-Alvarez, Johannesson & Ekendahl, 1995). Thus, sexual selection is only secondarily contributing to the morph differentiation.
Third, systematic differences in fecundity between RB and SU ecotypes have been observed (RB > SU), although such differences do not contribute to the maintenance of the morphological differences between them across the shore gradient, except for some particular shell traits (Cruz & García, 2001, 2003). This is because the distinct fecundity rates represent different life history strategies adapting each ecotype to each particular habitat. Indeed, the low fecundity rate in the SU populations may represent a strategy to maximize the size (even at the cost of numbers) of newly born juveniles on the lower shore (a risky habitat due to waves), while on the upper shore the fitness of RB females may be maximized by higher progeny number (Cruz, Rolan-Alvárez & García, 1998).
These fitness estimates corroborate the main roles of natural selection, especially the viability component, in maintaining the present polymorphism. But what is it known in relation to the particular biological or ecological mechanisms causing such selective differences? On the lower shore, the main physical and ecological factor affecting survivorship is the strength of waves and so, as expected, the SU ecotype is smaller and has smooth (without ridges) shells to avoid dislodgement by waves (Johannesson, Johanneson & Rolan-Alvárez, 1993). Accordingly, the SU ecotype also shows a larger shell aperture (to accommodate a larger muscular foot for more effective attachment to the substratum) than the RB ecotype (Carvajal-Rodríguez, Conde-Padín & Rolan-Alvárez, 2005). This morphological adaptation of SU to better resist the effect of waves was verified in simple behavioural experiments; SU specimens better resisted detachment from a glass surface (94%) when vigorously shaken underwater than RB ones (24%; Rolán-Alvarez, Johannesson & Erlandsson, 1997).
On the other hand, a number of environmental and ecological factors act more strongly on the upper shore (Johannesson, Johannesson, Rolan-Alvárez, 1993). This shore level is exposed to the air for longer periods of time, which forces the RB ecotype better to resist desiccation, osmotic stress (rain is common during winter on these shores) or high temperatures. As an example of the strong selection pressures that can occur on these shores, up to one-quarter of the mussel population died on the mid shore after a few hot days in the summer of 1998 (personal observation). Again, upon laboratory conditions, RB specimens displayed a significantly higher capability to resist fresh water (86%) or sun exposure (98%) than SU specimens (0 and 12%, respectively) (Rolán-Alvarez, Johannesson & Erlandsson, 1997). Accordingly, the RB ecotype shows a relatively smaller shell aperture, to protect the body within from environmental stress (Carvajal-Rodríguez, Conde-Padín & Rolán-Alvarez, 2005). Another characteristic of the upper shore is that it has an abundant population of crabs (Pachygrapsus marmoratus) that can feed on Littorina saxatilis, preferentially on juveniles. When both ecotypes were placed in the same aquarium with crabs, the SU ecotypes were more frequently predated (48%) than larger (0%) or equally sized (20%) RB ones, suggesting that stronger and ridged shells may protect against crab attacks (Rolán-Alvarez, Johannesson & Erlandsson, 1997). The evolution of ridges on shells has been explained as an adaptation against crab predation in many other populations of this and related species (Johannesson & Johannesson, 1996; Reid, 1996). Another piece of (non-experimental) evidence of the differences between ecotypes is that SU specimens die sooner than RB ones when both are similarly maintained in laboratory or sent abroad by mail (personal observation), suggesting physiological adaptation to resist to environmental stressors in the former.
The final piece of evidence showing that different selection regimes affect populations living at different shore levels is that these ecotypes have evolved different habitat preferences, as was observed when they were experimentally transplanted to a different shore level (Rolán-Alvarez, Johannesson & Erlandsson, 1997; Erlandsson, Rolán-Alvarez, Johannesson, 1998; Cruz et al., 2004b). In addition, even on the mid shore they aggregate differently at a micro-scale, suggesting distinct preferences for slightly different microhabitats (Kostylev, Erlandsson and Johannesson, 1997; Otero-Schmitt et al., 1997; Carballo, Caballero & Rolán-Alvarez, 2005). The evolution of habitat preferences is a theoretical condition that facilitates the coexistence of any intraspecific polymorphism in sympatry (García-Dorado, 1986), or even favours sympatric speciation (Turelli, Barton & Coyne, 2001).
The above experiments show fitness differences between ecotypes, as well as some morphological/ecological/behavioural characteristics that can explain those fitness differences by natural selection. However, if natural selection is causing the differences in morphology, physiology or behaviour, there is another requisite: the existence of genetic variation for the traits being selected. This is fundamental to reject the alternative scenario that the above differences between ecotypes could result partially from phenotypic plasticity, a phenomenon that has been observed in other species of the same genus (e.g. L. obtusata; Trussell, 1996). First, I will review all indirect evidence in support of a genetic basis for the ecotypic differences. The first indirect evidence is that shelled embryos show the same kind of shape differentiation as adults (Conde-Padín et al., 2006): see for example the relative size of the shell aperture in Figure 1A. The second is that most ecotype characteristics are maintained at different shore levels; for example the morphology, growth rate and behaviour of the RB ecotype are similar at upper and mid-shore levels, and the same occurs with SU populations from lower and mid-shore levels (Johannesson, Johannesson & Rolán-Alvarez, 1993; Johannesson, Rolán-Alvarez & Erlandsson, 1997; Erlandsson, Rolán-Alvarez & Johannesson, 1998). The third is that the ecotypes maintained their morphological characteristics one month after transfer to a different habitat in a mark-recapture experiment (Rolán-Alvarez, Johannesson & Erlandsson, 1997). Finally, Johannesson, Johannesson & Rolán-Alvarez (1993) showed that juveniles bred in the laboratory were morphologically similar to their mothers for qualitative traits (as ridges and bands) when they had achieved 3 mm in shell length.
In addition, the percentage of additive genetic variation (heritability) that can explain the observed phenotypic variation has been directly estimated in laboratory and in the wild (Carballo, García & Rolán-Alvarez, 2001; Conde-Padín et al., 2006). The ovoviviparity of this species permits the use of groups of sibs from the field (using shelled embryos within pregnant females) for quantitative genetic estimation (Newkirk & Doyle, 1975). The heritability estimates are very important because the additive genetic component of a particular morphological trait is the genetic component directly influenced by natural selection affecting that trait, and so phenotypic plasticity only remains as a possible explanation for the environmental component of the morphological variability (Falconer & Mackay, 1996). Obviously, phenotypic plasticity could be partially genetic in origin, but its genetic component cannot be measured directly by the morphological trait which affected it. Carballo, García & Rolán-Alvarez (2001) showed that shell morphology (different distances and ratios) has a major additive genetic component (heritability >0.5) acting on phenotypic variation, which suggests that shell morphology could further evolve by natural selection. More recently, a similar result was found when shell morphology was decomposed into shell size and shape components using geometric morphometric methods (Conde-Padín et al., 2006). Furthermore, when compared with the genetic differentiation between ecotypes for both molecular (putatively neutral) and quantitative traits, the pattern found was only compatible with strong selection acting on the morphological (quantitative) traits across the vertical environmental gradient. In fact, the morphological trait showing the strongest trend was a shape variable that could be interpreted in terms of the relative size of the shell aperture. Another interesting trait was shell size (also with intermediate heritability) that it is directly involved in the process of incomplete speciation as a by-product of ecological adaptation (see below).
All these results taken together unquestionably support the conclusion that natural selection is responsible for the extreme shift in morphological, behavioural and physiological traits between these two ecotypes, because the populations living at different shore levels have adapted to survive in two extremely different habitats. However, just in case of any remaining doubt, computer simulations have provided further support for this interpretation (Pérez-Figueroa et al., 2005). These authors used a realistic model of the Galician hybrid zone to confirm that divergent natural selection, partial reproductive isolation and a relatively high gene flow between subpopulations from different shore levels are the key parameters that maintain this polymorphism.
THE EXISTENCE OF PARTIAL REPRODUCTIVE ISOLATION
The two ecotypes (RB and SU) live typically among barnacles or mussels, respectively, but in certain areas of the mid shore they can meet and hybridize in sympatry on a patchy mixture of these two habitats. There was previously no estimate of the level of introgression of the different hybrids (morphologically intermediate forms), although for most traits (even for most genetic markers) they show averages of the trait values of each of the pure ecotypes (Johannesson, Johannesson & Rolán-Alvarez, 1993; E. Rolán-Alvarez, unpubl.). Presently, more than 2000 AFLP loci are being studied in RB, SU and hybrids from three localities (J. Galindo, unpubl.); the preliminary results suggest that only a minor proportion of the intermediate forms were genetically intermediate for semi-diagnostic markers (and therefore probably true F1 hybrids or introgressed forms), while the rest were genetically identical to pure ecotypes (RB or SU). This suggests that most morphologically intermediate forms represent phenotypic variation from pure ecotype genotypes rather than introgressed forms. Thus, the low frequency of introgressed forms observed in the wild suggests two possibilities: true hybrids have problems surviving on the mid shore (because of a lower average fitness than pure ecotypes) or pure ecotypes have a strong (pre- or postzygotic) reproductive isolation which minimizes the chance of creating F1 hybrids.
First, I will discuss the possibility that poor adaptability of hybrids to available habitats is causing their low frequency. Hybrids (morphological intermediates), on average, show intermediate viabilities, sexual selection and fertilities compared with pure (RB and SU) ecotypes (Rolán-Alvarez, Johannesson & Erlandsson, 1997; Johannesson, et al., 2000; Cruz, Rolán-Alvarez & García, 2001). Thus, there is no evidence of such mechanism, but the fitness of F1 hybrids was never compared with that of pure ecotypes, and so the possibility that natural selection may somewhat work against F1 hybrids in the wild cannot be excluded. However, such a process, if it exists, does not seem to be fundamental for the maintenance of the present polymorphism, based on the results with computer simulations, (see Pérez-Figueroa et al., 2005). If such problems with the F1 hybrids exist, they may not be caused by postzygotic isolation because laboratory crosses between male SU and female RB give the same fertility (and abortion rates) as pure RB crosses (Rolán-Alvarez et al., 2004). The opposite cross could not be performed due to the difficulty of maintaining SU ecotypes in the laboratory.
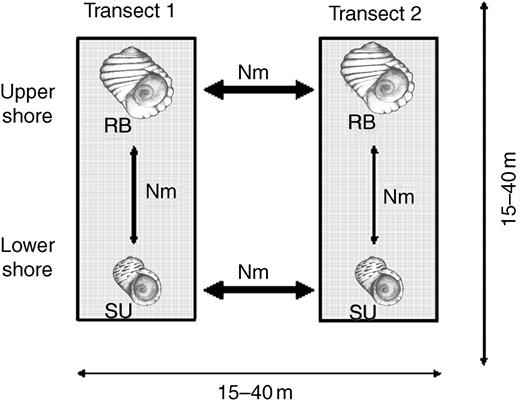
On the other hand, there is strong evidence that premating isolation barriers are contributing to the maintenance of the genetic cohesion of the ecotypes. First, the two ecotypes live preferentially in different microhabitats (RB on the upper shore and SU on the lower shore), which produces an effective ecological isolation (sensuCoyne & Orr, 2004) throughout most of the population range. How this mechanism arose is quite clear, as each ecotype has a preference for living in its particular shore level and habitat (Rolán-Alvarez, Johannesson & Erlandsson, 1997; Erlandsson, Rolán-Alvarez & Johannesson, 1998; Cruz et al., 2004b), and this is so because to be in the wrong habitat can be very costly in terms of fitness (Rolán-Alvarez, Johannesson & Erlandsson, 1997; Cruz et al., 2004b). Second, these ecotypes meet and mate over significant areas of the mid shore, and so a behavioural isolation (sexual isolation sensuCoyne & Orr, 2004) is also contributing to the genetic separation between them (Johannesson, Rolán-Alvarez & Ekendahl, 1995; Rolán-Alvarez, Rolán & Johannesson, 1996; Rolán-Alvarez et al., 1999, 2004; Fig. 3). Sexual isolation studies are typically done in laboratory conditions, and then used to infer what may be occurring in the wild (Coyne & Orr, 2004). However, in this hybrid zone it is possible to capture wild mating pairs and so estimate sexual isolation directly in the field, giving to this model system one of its main advantages. The study of mates in the field shows that these ecotypes mate partially assortatively (sexual isolation is 70% of the maximum possible, range 50–100%; Fig. 1D), but typically randomly with the intermediate forms, when they meet in sympatry (Johannesson, Rolán-Alvarez & Ekendahl, 1995; Rolán-Alvarez et al., 1999, 2004; Cruz et al., 2004a). The existence of an incomplete reproductive isolation between the Galician ecotypes can be independently inferred from molecular data: in every locality a small and significant genetic differentiation for allozymes, microsatellites and mtDNA variation is also observed between the ecotypes in strict sympatry (on the mid shore; Fig. 3), although such differentiation does not exist between populations of the same ecotypes separated by similar microgeographical distances (Rolán-Alvarez, Rolán & Johannesson, 1996; Rolán-Alvarez et al., 2004; Fernández et al., 2005). Obviously, it is not known whether these isolation barriers will be completed or not, but it gives the chance to understand the biological mechanisms causing the evolution of a partial reproductive isolation up to a level close to being definitive (70% of the maximum on average; Rolán-Alvarez et al., 1999). Thus, knowledge of this uncompleted speciation process will surely help to understand how speciation can ultimately be completed in this and similar organisms.
Schematic representation of the gene flow estimated between different subpopulations. Each box represent a particular transect studied on the vertical environmental gradient. At the two extremes of each transect a sample of each ecotype was studied and the genetic differentiation for different genetic markers inferred (from Rolán-Alvarez, Rolán & Johannesson, 1996; Fernández et al., 2005). The arrows of the figure represent the degree of gene flow estimated from the patterns of genetic differentiation (see Fernández et al., 2005), the wider the arrows the higher the gene flow estimated between subpopulations. The pattern of gene flow shows that some factors (habitat choice and partial reproductive isolation) are reducing the gene flow between ecotypes at a microgeographical scale (see text).
THE MODE OF SPECIATION
There are different alternative scenarios that can explain the origin and evolution of reproductive isolation (reviewed in Turelli, Barton & Coyne, 2001). One possibility is that the two ecotypes have evolved their differences in allopatry and then, after secondary contact, reproductive isolation emerged and could even have been reinforced. The allopatric mode is believed to be the most frequent mode of speciation, although it is assumed that speciation can be produced in the presence of gene flow (Turelli, Barton & Coyne, 2001; Coyne & Orr, 2004).
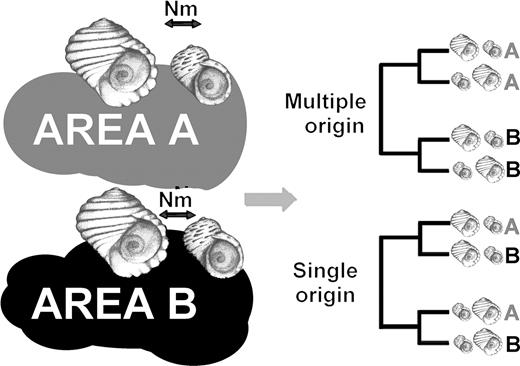
There are two kinds of arguments that make the allopatric mode of speciation rather implausible in L. saxatilis. The first is the observed pattern of genetic variation for all genetic markers (allozymes, microsatellite and mtDNA) studied (Rolán-Alvarez et al., 2004): the genetic differentiation between ecotypes found on the mid shore disappears when populations of the same ecotypes from different localities are pooled. Thus, because the former molecular variation studied was putatively neutral, the local differentiation between ecotypes appears as a side effect of the partial reproductive barrier existing at each site caused both by habitat preference and mate choice. This allows the particular differentiation between ecotypes at a microgeographical scale to be maintained independently at each locality. A limitation of such an interpretation, however, is that the pattern of genetic variation does not necessarily inform us about the history of past populations (Chan & Levin, 2005). However, when the phylogeny of the mtDNA haplotypes presented in four geographically isolated localities (with both RB and SU populations present) is studied, the tree inferred is only compatible with a sympatric and parallel origin of these ecotypes (H. Quesada, unpublished; Fig. 4). Even a model of allopatric differentiation followed by full gene flow after secondary contact is incompatible with a systematic monophyletic origin of haplotypes present in both ecotypes at each locality. In fact, the sympatric and parallel model of speciation suggested is easy to understand in a model system like this: with a low dispersal species living in exposed rocky shore habitats partially isolated on islands or separated by estuaries (Fig. 2). A plausible history of how this system could have originated can be advanced. The ancestral ecotype (probably more similar to the RB ecotype) arrived on these shores probably from the north (this species show a mainly northern distribution in Europe; Reid, 1996), and as soon as it arrived at each exposed site the ancestral form split into the two ecotypes. Because natural selection is extremely strong and local (the environmental gradients are not identical on every shore), the fixation of alleles at each locality (among the geographically partially isolated localities) was basically an independent process.
Two alternative scenarios (single allopatric versus multiple sympatric evolution of the ecotypes) predict different phylogenetic patterns of the haplotypes (not populations) present in the ecotypes from different localities (modified from H. Quesada, unpubl.). For example if both ecotypes are sampled in two geographically distant populations (with reduced gene flow between localities), allopatric speciation predicts that haplotypes are not clustered by localities, while under the alternative scenario they always appear clustered by localities (other alternative scenarios show the same predictions as the single allopatric; not shown).
A sympatric and multiple independent origin of these ecotypes, in localities separated by estuaries or on islands, is only compatible with the evolution of their premating isolation indirectly driven by natural selection. This is so because the ecotypes still maintain some gene flow, and so a sympatric origin can only be maintained under strong divergent natural selection (Turelli, Barton & Coyne, 2001), such as that described above. In fact, when the details of the biological mechanisms responsible for the sexual isolation are investigated (the ecological isolation mechanism described above is obviously an adaptive side effect), further support for the sympatric and parallel origin of the reproductive isolation is obtained. The strong but incomplete sexual isolation is produced by means of two independent processes: a non-random microhabitat distribution on the mid shore (related to the strong habitat preferences detected in upper- and lower-shore habitats) and true mate choice (Erlandsson, Kostylev & Rolán-Alvarez, 1999; Rolán-Alvarez et al., 1999, 2004; Cruz et al., 2004a). The first process is able to explain up to 50% of the sexual isolation observed, while mate choice is able to explain it all. The non-random micro-distribution could be caused by two complementary ecological processes: search for refuges, or micro-habitat choice (Erlandsson, Kostyler & Rolán-Alvarez, 1999; Carballo, Caballero & Rolán-Alvarez, 2005). Either of them could have originated as a by-product of adaptation of each ecotype to its particular habitat (Rolán-Alvarez et al., 2004; Cruz et al., 2004a).
In addition, the second mechanism of sexual isolation (mate choice) is a consequence of two independent processes as well: first, the existence of a widespread size-assortative mating that it is observed in most populations of L. saxatilis (Reid, 1996; Erlandsson & Rolán-Alvarez, 1998). Size-assortative mating is a very common phenomenon in many species and it is interpreted as a strategy to maximize the fertilization rate (Andersson, 1994). This phenomenon is not related to the existence of ecotypes or forms, and occurs within ecotypes as well, producing size-assortative mating in other littorinid populations of about 50% of the maximum possible (Erlandsson & Rolán-Alvarez, 1998; Conde-Padín et al., 2006). Second, the existence of significant size differences between adults of both ecotypes (RB being larger than SU; Fig. 1A), in addition to the pre-existence of assortative mating for size, can explain the observed sexual isolation observed (Rolán-Alvarez et al., 2004; Cruz et al., 2004a). This explanation assumes that the size has been affected by disruptive selection between upper- and lower-shore habitats (see Johannesson, Rolán-Alvarez & Ekendahl, 1995; Cruz, Rolán-Alvarez & García, 2001; Conde-Padín et al., 2006; see above). Thus, the size differences between ecotypes can be explained as different adaptive responses to each habitat: smaller sizes are suitable on the lower shore so they can fit into refuges that protect them from waves, while larger sizes are better on the upper shore because they have stronger shells which are able to resist crab attacks (Johannesson, Johannesson & Rolán-Alvarez, 1993; Rolán-Alvarez, Johannesson & Erlandsson, 1997).
Four main lines of evidence support the hypothesis that sexual isolation originated through the mean size difference between ecotypes in this model system. First, there is a similar positive correlation for size both within and between ecotypes, suggesting that size-assortative mating appeared before sexual isolation (Rolán-Alvarez et al., 2004). Second, the pattern of mate choice for the different mating pairs estimated directly in the wild is compatible with such mechanism (see Cruz et al., 2004a). Third, size is the morphological trait that explains the largest proportion (50%) of the variability in assortative mating in the hybrid zone (P. Conde-Padín, unpubl.). Fourth, the size-assortative mating observed in populations outside the hybrid zone shows the same biological characteristics as the sexual isolation estimated between the ecotypes. In fact, size variation alone could account for nearly 90% of the variation in size-assortative mating across geographically distant populations (P. Conde-Padín, unpubl.). The only available information that is partially incompatible with the above explanations is the existence of an inverse cline in the hybrid zone in relation to the partial reproductive isolation (see Johannesson, Rolán-Alvarez & Ekendahl, 1995; Rolán-Alvarez, Johannesson & Ekendahl, 1995): the partial reproductive isolation observed on the mid shore disappears on the lower shore when a small number of upper-show ecotypes can occasionally be found. Such kinds of inverse clines have been suggested as the most plausible evidence for the reinforcement by natural selection of reproductive isolation (Butlin, 1995). In this case, however, a more simple behavioural explanation can be proposed. The rare RB specimens found on the lower shore are typically adults from the mid shore that are exploring new sites or habitats. The virtual absence of RB juveniles on the lower shore supports this view (personal observation). Thus the inverse cline is not produced by different alleles in different subpopulations, but rather by specimens that change their mating behaviour in a new habitat. Such a change in mating behaviour is easy to understand as each RB specimen is surrounded by SU ones and so will finally mate with one of them (partial sexual isolation is not complete). When collecting mating pairs from the lower shore, the rare RB specimens will be found mated with SU ones, giving the impression that they mated randomly.
In summary, all available data support the hypothesis that the reproductive isolation has evolved as a by-product of adaptation. Furthermore, the key trait causing the increase of sexual isolation in this model system, the size, is able to evolve further as considerable genetic variation within populations has been observed for both ecotypes (Carballo, García & Rolán-Alvarez, 2001; Conde-Padín et al., 2006). Thus, this mechanism would be able to complete the speciation process as a by-product of the adaptation of each ecotype to its particular shore level and habitat if, for example, a change in the environmental gradient further increased the size differences between ecotypes. Obviously, to predict if this will happen or not is unrealistic, but at least a mechanism able to complete the speciation process in a marine intertidal rocky shore snail has been identified. More importantly, even under an allopatric mode of speciation the information obtained with this model system is relevant, whenever the mechanism affecting the evolution of the reproductive isolation has originated as a by-product of adaptation (Schluter, 2001). Thus, such a mechanism makes it easier to understand some pairs of sympatric species that live at different shore levels in intertidal habitats, as occurs in other species of Littorina (L. obtusata versus L. fabalis), other gastropods (Gibbula obliquata versus G. pennanti) or even barnacles (Chthamalus stellatus versus C. montagui).
CONCLUSIONS AND FUTURE OF THIS SYSTEM
Ecological speciation occurs when divergent selection on traits between populations or subpopulations in contrasting environments leads directly or indirectly to the evolution of reproductive isolation (Schluter, 2001). There are some studies which in one way or another find a relationship between traits that are being affected by divergent selection and their contribution to partial or complete reproductive isolation (reviewed in Rundle & Nosil, 2005). However, in the majority of cases reproductive isolation has been studied in the laboratory, and so it is difficult to know the real contribution of the ecological trait to reproductive isolation in the wild. A few exceptions exist, like the size-based speciation process described in natural populations of the threespine stickleback (Gasterosteus aculeatus), where it is known that size differences between ecotypes are caused by differential adaptation and also cause partial reproductive isolation (McKinnon et al., 2004; Boughman, Rundle & Schluter, 2005), and the ds2 locus in Drosophila, which simultaneously contributes to adaptation and sexual isolation (Greenberg et al., 2003). Here, I have reviewed the importance of natural selection indirectly contributing to the evolution of partial reproductive isolation, and thus to speciation, in the Galician Littorina saxatilis hybrid zone. In summary, this hybrid zone seems, in addition to the stickleback case (Nagel & Schluter, 1998; McKinnon et al., 2004; Boughman, Rundle & Schluter, 2005) one of the best known examples linking incomplete speciation with adaptation processes.
Although the Galician L. saxatilis system has been extensively studied by different research groups during the last 10 years; there are still many deficiencies in our knowledge. For example, it will be necessary to accomplish extensive breeding and crossing in the laboratory to characterize the genetic architecture of the traits involved in adaptation or speciation. In addition, it will be necessary to investigate the traits at a metabolic scale, as well as to characterize the proteomic differences between ecotypes, in order to understand in detail how natural selection is modelling the process of adaptation. As knowledge of this system grows, so too will the opportunity for experimental manipulation, as has occurred in other model organisms like Drosophila. I hope that the next 10 years will witness a methodological and conceptual revolution in the study of this species.
ACKNOWLEDGEMENTS
This review is in debt to all people from my lab (and other labs) who in one way or another have contributed and are still contributing to the study of these two ecotypes, and in particular to the technicians N. Santamaría and P. Alvariño. P. Conde-Padín, J. Galindo and H. Quesada have allowed me the use of their unpublished results. I thank also E. Boulding, H. Quesada, D.G. Reid, E. Rolán (senior) and two anonymous referees for useful comments on the manuscript. E. Rolán (senior) also helped with the formatting of figures. This works has been partially funded by Ministerio de Educación y Ciencia (CGL2004-03920 BOS), Xunta de Galicia and University of Vigo.