-
PDF
- Split View
-
Views
-
Cite
Cite
Derek E. Wildman, Wei Wu, Morris Goodman, Lawrence I. Grossman, Episodic Positive Selection in Ape Cytochrome c Oxidase Subunit IV, Molecular Biology and Evolution, Volume 19, Issue 10, October 2002, Pages 1812–1815, https://doi.org/10.1093/oxfordjournals.molbev.a004005
Close - Share Icon Share
Periods of elevated rates of nonsynonymous nucleotide substitutions for electron transport chain (ETC) genes have occurred during the evolution of anthropoid primates (New World monkeys, Old World monkeys, apes, and humans) (Grossman et al. 2001 ). Given the vital role the ETC genes play in energy metabolism, and the coadaptive evolution apparently undergone by the holoenzymes of cytochrome bc1 (Complex III) and cytochrome c oxidase (Complex IV) in anthropoid primates (Andrews and Easteal 2000 ; Grossman et al. 2001 ; Schmidt et al. 2001 ), it is likely that positive selection rather than reduction of functional constraints is the cause of the elevated nonsynonymous substitution rates. ETC genes that have been identified as having undergone periods of positive selection in anthropoid primates are cytochromes b and c (CYB,CYC) and the Complex IV genes COX1,COX2, cytochrome c oxidase subunit IV ubiquitous isoform (COX4-1),1 and COX7AH (reviewed in Grossman et al. 2001 ). Lomax et al. (1992) first showed that the human COX4-1 gene had accumulated many more nonsynonymous substitutions than had its orthologues in mouse and cow, but they could not localize the specific ancestral primate lineage on which the rate increase occurred. In a phylogenetic study conducted after sequencing COX4-1 from a range of primates, Wu et al. (1997) provided evidence that positive selection for adaptive evolution of COX4-1 occurred in certain haplorhine primate lineages. Positive selection was especially pronounced in the descent of the stem catarrhines (the internode connecting anthropoids to catarrhines) and the stem hominids (the internode connecting Old World monkeys to apes and humans). In this lineage the nonsynonymous rate (Ka [nonsynonymous substitutions/nonsynonymous site]) was faster than the synonymous rate (Ks [synonymous substitutions/synonymous site]) but later, in the descent of the gorilla, chimpanzee, and human lineages, it was much slower, consistent with the established scenario (Goodman 1982 ) whereby natural selection first spreads advantageous gene changes (positive selection) and then preserves them (purifying selection). Because of the absence of a tarsier sequence in this data set, Wu et al. (1997) could not reconstruct an ancestral haplorhine (tarsier-anthropoid) sequence and thus determine if the period of positive selection began in the stem of the Anthropoidea. Here, we have added to the COX4-1 exon data set of Wu et al. (1997) the DNA sequence from Tarsius syrichta (accession number AH011618). Our phylogenetic analysis of the exon sequences confirms the earlier result of Wu et al. showing the rate increase to be an anthropoid phenomenon and extends those results by placing the main period of acceleration (Ka) on the hominid (ape-human) stem. We use the classification of Goodman et al. (1998) and Goodman (1999) , which places all living apes (including humans) in the family Hominidae. Traditional (e.g., precladistic) classification places all apes in the superfamily Hominoidea; however, the objective, time-based classification of Goodman includes apes and Old World monkeys in the same superfamily, which by the rules of nomenclature must be Cercopithecoidea. Under this scheme, the African hominids (humans, chimpanzees, and gorillas) are placed in the subtribe Hominina and are herein referred to as hominans.
We also used intron 3 (650 bp) to more accurately measure neutral evolution rather than using the approximately 60 synonymous sites of COX4-1 (Rooney and Zhang 1999 ). The COX4-1 intron 3 sequence data of Wu et al. (1997) and Wu (1999 , p. 161) consisted of anthropoid primate sequences and a strepsirrhine primate sequence, that of the potto (Perodicticus potto, accession number AF500218). Here we extend this intron data with the sequence from a second nonanthropoid primate, that of T. syrichta (accession number AF500221) and also from another anthropoid primate, the siamang gibbon (Hylobates syndactylus, accession number AF500220). In addition, three nonprimate mammal sequences were acquired (Bos taurus, AF500217; Rattus norvegicus, AF500219; and Mus musculus, AF500216).
Sequences were obtained from genomic DNA using standard automated methods. The primers used to amplify and sequence intron 3 are: COX4-1 5′1, AAGGAGAAGGCCGACTGGAGCAG; COX4-1 5′2, AGCCTTTCCAGGGATGAGAAAGTC; COX4-1 3′2, TCAGCGAAGCTCTCGTTAAACTG; and COX4-1 3′1, AAGAACATGGCCAGGCCCAC, for the outgroup taxa; COX4-1 5′3, AGAAGGCCGCCTGGAGCAG; COX4-1 3′3, AGAACATGGCACCGCCCAC for the gibbon sequence. Coding primers were previously described by Wu et al. (1997) . Sequences were aligned by eye or with T-COFFEE (Notredame, Higgins, and Heringa 2000) .
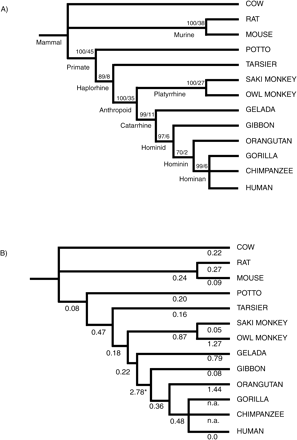
We inferred the gene tree for COX4-1 using both coding and noncoding data. The alignment of these data spans 1,361 characters, including gaps. Figure 1 shows the strict consensus of the two most parsimonious and the optimal maximum likelihood trees. The strict consensus MP tree has a length of 1,110 steps with a consistency index of 0.82 and a retention index of 0.72. The bootstrap values for 1,000 branch and bound replicates are shown. The likelihood score of −ln L = 6562.42 is based on 25 random addition sequence heuristic replicates under the HKY85 model with a transition-transversion ratio of 2:1.
The gene tree represented in figure 1 is congruent with the overall evidence on primate phylogeny (Goodman et al. 1998 ). But it failed to depict the sister grouping of the human and chimpanzee sequences, presumably because there is not enough variation in COX4-1 sequences to resolve the trichotomy among the three represented African hominan taxa (human, chimpanzee, and gorilla).
Wu et al. (1997) showed there was an increase in Ka during anthropoid primate evolution in descent between the ancestral node for all living anthropoids and that for all living apes including humans but could not determine whether the increase began in the anthropoid stem. We have now (fig. 1b ) more tightly localized the rate acceleration to the period on the hominid stem lineage between 25 and 18 mega annum (Ma). We estimated the numbers of nonsynonymous and synonymous substitutions using the method of Li (1993) as implemented in the program FENS (de Koning et al. 1998 ). Maximum likelihood analyses were also carried out using the program CodeML in PAML (Yang 2000) under the free ratio model (data not shown, available on request).
Using a parsimony-based approach, we have shown that the ACCTRAN Ka/Ks ratio on the hominid stem is 2.8 (fig. 1b ) and the DELTRAN value is 2.3; both values are significantly higher (P < 0.01) than the value of 1 that would be expected, given neutrality. All the methods show an increase in Ka/Ks at this link. The calculated average value of Ka/Ks (ACCTRAN) across all branches on the tree is 0.513. Therefore, the acceleration on the hominid stem lineage is five times that seen for the tree on average.
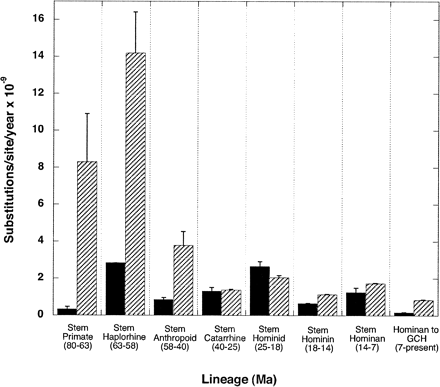
The observed elevated Ka/Ks value can be explained by either an increase in nonsynonymous rate, a decrease in synonymous rate, or some combination. In addition to synonymous sites, we used COX4-1 intron sequences as estimators of the neutral rate of nucleotide substitution for the gene. Using intron sequences instead of synonymous changes has a statistical advantage when studying small genes such as COX4-1. Because cytochrome c oxidase subunit IV protein (pCOX4-1) has fewer than 150 amino acids, there are fewer than 100 synonymous sites in the gene, leading to a relatively high standard error for distances between taxa and nodes. By contrast, intron 3 of COX4-1 has over 500 sites in all primate taxa. The distances between sequences are therefore less likely to be affected by statistical error. Consequently, we calculated the ratio Ka/Ki and Ks/Ki, where Ki is the number of intron changes/intron site. We note, however, that the small number of nonsynonymous sites (approximately 340) remains a source of statistical error not addressed by this approach.
As with Ka/Ks, the Ka/Ki value is significantly (P < 0.05) greater than 1 during the period between 25 and 18 Ma on the stem hominid lineage. However, the ACCTRAN value for Ka/Ki is less (1.34) than that for Ka/Ks (2.78) (fig. 2 ). If both the Ki and Ks values represent neutral evolution, then a Ks/Ki ratio of 1 is expected. Indeed, when Ks/Ki values are averaged over all branches on the tree in figure 1 , the average value is 1.005, supporting the idea that both introns and synonymous sites evolve at similar rates. The observed Ks/Ki ratio on this link is 0.48, but the ratio is not significantly less than the expected value of 1. Elevated rates of nonsynonymous substitution are often assumed to indicate positive selection (Li 1993 ; Messier and Stewart 1997 ; Wu et al. 1997 ; Grossman et al. 2001 ), and the observed high ratios for COX4-1 on the hominid stem lineage may also represent a period of positive selection. The argument that the changes observed conferred a selective advantage (rather than a lessening of functional constraints) is bolstered by the fact that the accelerated period of nonsynonymous change was followed by a period of low Ka/Ks and Ka/Ki ratios suggestive of purifying selection (fig. 1b ). Further, three of the four amino acid changes observed on the hominid stem lineage involve charge changes to the resulting amino acid, and are therefore “radical” amino acid replacements, suggesting some change in protein function (Zhang 2000 ). Indeed, these changes, along with the concurrent changes noted earlier to other members of the ETC, may have promoted the refinement of the aerobic metabolic pathways necessary for the increased oxygen requirements of the expanded anthropoid primate neocortex (Grossman et al. 2001 ).
In summary, COX4-1 underwent a period of accelerated evolution between 25 and 18 Ma on the stem hominid lineage. Additionally, the gene tree for COX4-1 is congruent with the overall evidence on primate phylogeny. Finally, both intron and synonymous sites agree with other evidence for a hominid slowdown (Goodman 1985 ; Bailey et al. 1991 ), in which the rate of noncoding substitution has slowed throughout anthropoid primate evolution, especially in hominids (fig. 2 ).
Rodney Honeycutt, Reviewing Editor
Abbreviations: COX4-1, cytochrome c oxidase subunit IV ubiquitous isoform; pCOX4-1, cytochrome c oxidase subunit IV protein; ETC, electron transport chain; Ma, Mega annum; Ka, nonsynonymous rate; Ks, synonymous rate; Ki, intron substitutions/intron site.
Keywords: COX4-1 cytochrome c oxidase primates primate phylogeny
Address for correspondence and reprints: Lawrence I. Grossman, Center for Molecular Medicine and Genetics, Wayne State University School of Medicine, 540 East Canfield Avenue, Detroit, Michigan 48201. l.grossman@wayne.edu .
COX subunit IV was thought to be encoded by a single-copy gene at the time of our initial investigations (Lomax et al. 1992 ; Wu et al. 1997 ). This is now known to be incorrect because of the discovery of tissue-specific isoforms (Hüttemann, Kadenbach, and Grossman 2001) . The gene we are currently studying is now designated COX4-1.
Fig. 1.—Phylogenetic tree inferred from the complete COX4-1 data set. (a) This tree is the strict consensus of the two most parsimonious trees (the African ape branching pattern could not be resolved) and is congruent with the maximum likelihood tree. Stem lineages are indicated, and bootstrap values/decay index (Bremer support) scores are also shown. (b) Ka/Ks ratios on the internal and terminal branches of the phylogenetic tree (only ACCTRAN values are shown). *, a value is significantly greater than both the mean Ka/Ks value and the generally accepted Ka/Ks value of 1 (taken to indicate neutrality); n.a., an undefined value due to no observed synonymous substitution
Fig. 2.—Rates of change for COX4-1 sequences. The rate of evolution of COX4-1 intron 3 sites (hatched bars) has slowed during the evolution of primate stem lineages, whereas no clear pattern emerges for nonsynonymous sites (filled bars). Synonymous rates show no trend, probably because of the small number of synonymous sites (not shown). This rate slowdown trend is general, with prosimians showing a higher substitution rate than anthropoids. Additionally, the rate increase (for all nucleotides) on the stem haplorhine branch may be an artifact of our chosen divergence date of 58 Ma. If tarsiers diverged from the anthropoids more recently than 58 Ma, a lesser rate increase would be seen. Finally, the elevated Ka value compared with Ki is apparent between 25 and 18 Ma on the stem hominid lineage. Values represented are the average of ACCTRAN and DELTRAN reconstructions on each stem lineage. The limit bars show half the range to the actual ACCTRAN or DELTRAN values. Stem lineages are given in figure 1a . GCH, average value for gorilla, chimpanzee, and human
We thank Maik Hüttemann for providing rat and mouse DNA and Tim Schmidt for useful advice and comments. The suggestions of two anonymous reviewers on an earlier version of this manuscript were very helpful. This study was supported by NSF grants MCB-9816923 and BCS-9910679.
References
Andrews T. D., S. Easteal,
Bailey W. J., D. H. Fitch, D. A. Tagle, J. Czelusniak, J. L. Slightom, M. Goodman,
de Koning A. P. J., W. Messier, C. B. Stewart,
Goodman M., C. A. Porter, J. Czelusniak, S. L. Page, H. Schneider, J. Shoshani, G. Gunnell, C. P. Groves,
Grossman L. I., T. R. Schmidt, D. E. Wildman, M. Goodman,
Hüttemann M., B. Kadenbach, L. I. Grossman,
Li W. H.,
Lomax M. I., D. Hewett-Emmett, T. L. Yang, L. I. Grossman,
Notredame C., D. G. Higgins, J. Heringa,
Rooney A. P., J. Zhang,
Schmidt T. R., W. Wu, M. Goodman, L. I. Grossman,
Wu W.,
Wu W., M. Goodman, M. I. Lomax, L. I. Grossman,
Yang Z.,