-
PDF
- Split View
-
Views
-
Cite
Cite
Toshihisa Kotake, Shinobu Takada, Kenji Nakahigashi, Masaaki Ohto, Koji Goto, Arabidopsis TERMINAL FLOWER 2 Gene Encodes a Heterochromatin Protein 1 Homolog and Represses both FLOWERING LOCUS T to Regulate Flowering Time and Several Floral Homeotic Genes, Plant and Cell Physiology, Volume 44, Issue 6, 15 June 2003, Pages 555–564, https://doi.org/10.1093/pcp/pcg091
Close - Share Icon Share
Abstract
Floral transition should be strictly regulated because it is one of the most critical developmental processes in plants. Arabidopsisterminal flower 2 (tfl2) mutants show an early-flowering phenotype that is relatively insensitive to photoperiod, as well as several other pleiotropic phenotypes. We found that the early flowering of tfl2 is caused mainly by ectopic expression of the FLOWERING LOCUS T (FT) gene, a floral pathway integrator. Molecular cloning of TFL2 showed that it encodes a protein with homology to heterochromatin protein 1 (HP1) of animals and Swi6 of fission yeast. TFL2 protein localizes in subnuclear foci and expression of the TFL2 gene complemented yeast swi6– mutants. These results suggested that TFL2 might function as an HP1 in Arabidopsis. Gene expression analyses using DNA microarrays, however, did not show an increase in the expression of heterochromatin genes in tfl2 mutants but instead showed the upregulation of the floral homeotic genes APETALA3, PISTILLATA, AGAMOUS and SEPALLATA3. The pleiotropic phenotype of the tfl2 mutant could reflect the fact that TFL2 represses the expression of multiple genes. Our results demonstrate that despite its homology to HP1, TFL2 is involved in the repression of specific euchromatin genes and not heterochromatin genes in Arabidopsis.
(Received April 24, 2003; Accepted May 12, 2003)
Introduction
In order to optimize reproduction, plants should flower at a preferable time. Many plants respond to environmental cues, such as day-length and temperature, to regulate flowering time. Phytohormones and age, two internal cues, also induce flowering. Molecular genetic studies using Arabidopsis have revealed that four major floral-promoting pathways — the photoperiod, vernalization, autonomous and gibberellin pathways — work in response to environmental and internal cues (Simpson et al. 1999, Reeves and Coupland 2000, Simpson and Dean 2002). These pathways form a network that integrates flowering signals into the regulation of several key genes termed ‘floral pathway integrators’. At present, three genes, LEAFY (LFY), FT and SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1/AGAMOUS-LIKE 20 (SOC1/AGL20), have been identified to function at this level (Weigel et al. 1992, Kardailsky et al. 1999, Kobayashi et al. 1999, Lee et al. 2000, Samach et al. 2000).
The photoperiod pathway is linked to the expression of CONSTANS (CO), which encodes a Zn-finger transcription factor (Putterill et al. 1995). CO directly upregulates the transcription of FT and SOC1/AGL20 genes (without de novo protein synthesis) to promote floral transition (Lee et al. 2000, Samach et al. 2000). The abundance of both CO and FT transcripts in the wild type is very low, which suggests that a small amount of their misexpression is sufficient for floral induction (Putterill et al. 1995, Simon et al. 1996, Kardailsky et al. 1999, Kobayashi et al. 1999). The unprogrammed expression of these genes must be therefore strictly repressed; however, a candidate repressor has not been identified as yet.
Most flower promoting genes, which activate the flowering pathways, have been identified by examining late-flowering mutants. By contrast, early-flowering mutants are useful to find genes that function as repressors of flower-promoting genes. Recent studies have revealed that some cloned genes whose mutants cause early flowering encode proteins with homology to polycomb group proteins or chromatin remodeling factors (Blazquez et al. 2001, Yoshida et al. 2001). These proteins are known to maintain genes in a silent state.
tfl2 mutations were originally identified as enhancers of the terminal flower 1 (tfl1) mutant phenotype (Larsson et al. 1998); however, single mutants of tfl2 show terminal flowers, as well as other pleiotropic phenotypes including early flowering, small plant size and curled leaf. This suggests that the TFL2 gene itself may regulate numerous developmental processes. We first focused on the early-flowering phenotype of tfl2, which is relatively insensitive to day length, because this phenotype suggests that TFL2 gene may function to repress target gene(s) involved in the photoperiod pathway.
We report here that FT is upregulated in the tfl2 mutant, whereas CO and other floral pathway integrators are not affected. We also report that the floral homeotic genes PISTILLATA (PI), APETALA3 (AP3), AGAMOUS (AG) and SEPALLTA3 (SEP3) are upregulated in the tfl2 mutant and thus may responsible for the pleiotropic phenotype of tfl2.
Results
Early-flowering phenotype of tfl2 is due to ectopic expression of FT
Arabidopsis is a long-day (LD) plant, therefore its flowering time is delayed in short-day (SD) conditions (Table 1). Compared with LD conditions, the numbers of rosette leaves at flowering in SD conditions increased by more than 40 leaves in wild-type plants, but by only about 5–6 leaves in tfl2 mutants. The difference between LD and SD was more obvious when the plants were grown on plates; that is, while the increase was 36.7 leaves in wild-type plants, it was less than 1.5 leaves in tfl2 mutants. For the flowering time and for other phenotypes, we did not find any difference in the severity among three different alleles of tfl2 (Table 1).
The day-length-insensitive early-flowering phenotype of tfl2 indicated that the TFL2 gene might function in the photoperiod pathway. Signals induced by LD are integrated into the activation of CO, which then directly activates FT and SOC1 (Samach et al. 2000). In order to investigate which gene in the photoperiod pathway is affected by TFL2 function, we compared the expression of CO, FT and SOC1 between the tfl2 mutant and the wild type background. We also measured the expression level of LFY. LFY, FT and SOC1 are called ‘floral pathway integrators’ and are assumed to be key regulators in promoting the floral transition (Blazquez and Weigel 2000, Samach et al. 2000, Suarez-Lopez et al. 2001; and reviewed in Araki 2001, Mouradov et al. 2002, Simpson and Dean 2002). We can deduce which pathway TFL2 is involved in from the genes that show altered expression in tfl2 mutants.
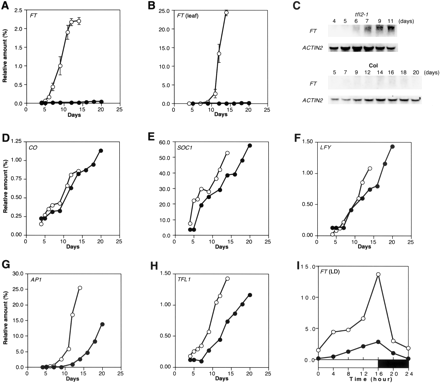
We measured the time course of expression of these genes after sowing by real-time quantitative RT-PCR. Of the genes measured, expression of FT was extensively upregulated in tfl2 mutants, whereas no obvious difference was found in the expression pattern of CO between tfl2 and wild type (Fig. 1). The abundance of FT RNA is very low in wild type (Kardailsky et al. 1999, Kobayashi et al. 1999); however, expression of FT started to increase in the tfl2-1 mutants as early as 5 d after sowing and reached a maximum level that was more than 100-fold higher than that of wild type (Fig. 1A, C). The accumulation of FT transcript was more obvious in the true leaves (1,500-fold higher than in wild type 14 d after sowing) (Fig. 1B). This accumulation of FT RNA was also observed in SD conditions, but its rate of increase was 1–2 d slower than in LD conditions (data not shown).
SOC1 expression in tfl2 reached the same levels observed in wild type at points 2–5 d earlier, whereas LFY expression in tfl2 was higher than in wild type after 10 d (Fig. 1E, F). These differences, however, were much smaller than the difference in FT expression; thus, TFL2 has very little effect on other pathways.
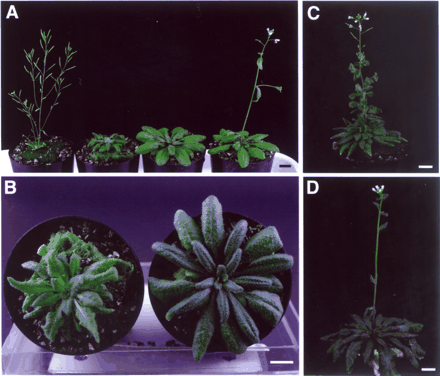
In order to confirm that the early-flowering phenotype of tfl2 is caused mainly by the ectopic expression of FT, we crossed tfl2-3 with ft-1 in the Col background (ft-1 introgressed into Col; see Materials and Methods) to make a tfl2-3;ft-1 double mutant. The early-flowering phenotype of tfl2 was suppressed in the tfl2-3;ft-1 double mutant (Table 1), suggesting that TFL2 functions upstream of FT in the flowering pathways. Other mutant phenotypes of tfl2 were not as suppressed as the flowering time; that is, the leaf shape of the double mutant showed an intermediate phenotype of tfl2 and ft, and the apical dominance was weaker than in ft mutants (see Fig. 5). Because the genetic loci of TFL2 and CO are so close (about 2 cM), we have not be able to obtain a tfl2;co double mutant as yet. However, we made a double mutant with gigantea (gi), another late-flowering mutant that is involved in the photoperiod pathway and is epistatic to co (Koornneef et al. 1998, Suarez-Lopez et al. 2001). The late-flowering phenotype of gi was rescued by a tfl2-3;gi double mutation (data not shown).
The CO gene, which encodes a Zn-finger transcription factor, is assumed to function directly upstream of FT (Putterill et al. 1995, Samach et al. 2000). Our experiments showed that the expression pattern of CO is not altered in tfl2 mutants and that the tfl2 mutation upregulates FT expression and suppresses the late-flowering phenotype of ft mutants. Taken together, these results suggest that, in the flowering pathway, TFL2 represses only the expression of FT and has very little effect on other flowering-associated genes. This would mean that TFL2 is involved in the photoperiod pathway as a repressor functioning upstream of FT and downstream of CO.
Because the expression of FT has been shown to fluctuate in time with the circadian oscillation of CO mRNA (Suarez-Lopez et al. 2001), we examined FT expression and found that FT mRNA shows a similar rhythm of expression in wild type and in tfl2 (Fig. 1I). This result suggests that expression of FT is induced by the same activator (most likely CO), regardless of repression by TFL2.
We also examined the expression levels of APETALA1 (AP1) and TERMINAL FLOWER 1 (TFL1), which function as meristem identity genes. These genes function downstream of floral pathway integrator genes and serve as references of the developmental stage and floral transition (Bradley et al. 1997, Hempel et al. 1997). An increase in the expression of these genes was observed 5–7 d earlier in tfl2-1 than in wild type (Fig. 1G, H). This was coincident with the reduction in the number of days taken to form visible flower buds between tfl2 and wild type (Larsson et al. 1998), which reflects the physical timing of the transition from the vegetative to reproductive phase.
TFL2 encodes an HP1/Swi6 homolog and may have similar function
In order to identify and characterize the TFL2 gene, we cloned it using a newly isolated T-DNA-tagged allele, tfl2-3. We mapped the insertion point of tfl2-3 close to the TFL2 locus, near the simple sequence length polymorphism marker nga106 on chromosome 5 (Larsson et al. 1998) and found that a 1519-bp region of a single gene was replaced by the T-DNA. We isolated the genomic DNA of tfl2-1: sequencing of this region showed that tf12-1 contains a point mutation (Q280 to a stop codon, Fig. 2A). In addition, we found that 1,302 bp 5′ upstream of the initiation codon and 6,216 bp 3′ downstream of the stop codon region of the TFL2 gene was deleted in tfl2-2. Finally, because a 5.7-kb NcoI fragment containing the single putative coding sequence complemented the tfl2-1 allele, we concluded that this gene was TFL2.
To characterize the TFL2 transcript, we carried out RT-PCR using TFL2-specific primers. TFL2 consists of six exons and encodes a protein of 445 amino acids with a chromo domain and a chromo shadow domain (Fig. 2B). This type of protein is unique in the whole of the Arabidopsis genome, as we found that TFL2 is identical to LIKE HETEROCHROMATIN PROTEIN 1 (LHP1), which was cloned recently using T-DNA tagging (Gaudin et al. 2001). Proteins with chromo and chromo shadow domains belong to the heterochromatin protein 1 (HP1) family of proteins and are found widely in animal genomes and in fission yeast (Fig. 2C) (Eissenberg and Elgin 2000).
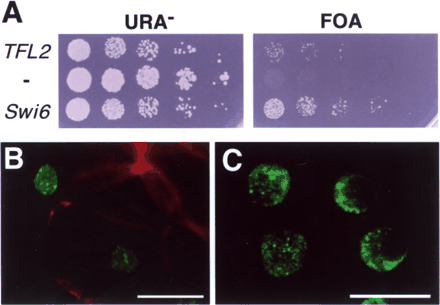
In order to examine whether TFL2 can function as a chromatin-associated gene silencing factor, we introduced it into an swi6– mutant of fission yeast (Fig. 3A). A yeast strain carrying an swi6-115 mutation (Allshire et al. 1995) could be rescued by either Swi6 or TFL2, suggesting that TFL2 can repress gene expression in the heterochromatin of fission yeast, although the complementation level of TFL2 was weaker than that of Swi6.
In transgenic Arabidopsis carrying a TFL2:GFP fusion gene expressed either from the CaMV 35S promoter (35S::TFL2:GFP) or in a genomic context (gTFL2:GFP), which complements tfl2 mutation, we observed that TFL2 protein was localized in foci within the nuclei (Fig. 3B). This protein localization is similar to previous reports (Gaudin et al. 2001), and we are currently investigating whether this localization is coincident with the heterochromatin region.
TFL2 is expressed in the proliferating cells of meristems
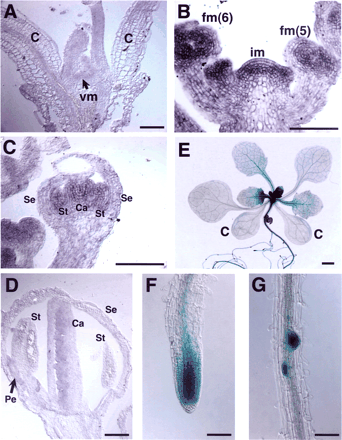
RNA in situ hybridization experiments showed that TFL2 transcripts accumulated in proliferating cells in the meristematic tissues of vegetative, inflorescence, and floral organs (Fig. 4A–D). In vegetative tissue, TFL2 RNA was detected in the meristem and young leaf tissue, but not in the matured cotyledons (Fig. 4A). TFL2 expression was detected continuously in the meristems throughout the vegetative, inflorescence and floral phases. Until flower stage 4 [floral stages are according to Smyth et al. (1990)] TFL2 was expressed uniformly in the floral bud (Fig. 4B), but by flower stage 5/6 TFL2 expression had decreased in the sepals (Fig. 4B, C). The accumulation of TFL2 RNA was reduced during the maturation of stamens and carpels, and at flower stage 9 TFL2 RNA was observed in developing ovules and petals, where cells were proliferating actively (Fig. 4D).
To observe TFL2 expression pattern in the whole plant and in tissues that are not easily examined by RNA in situ hybridization, we constructed a gTFL2:GUS gene containing a 5.7-kb TFL2 genomic fragment with a uidA gene insertion instead of the stop codon of TFL2. This gTFL2:GUS gene was introduced into tfl2-1 plants, which resulted in complementation of the mutant phenotype in these transgenic plants. The GUS staining pattern in the shoot meristems reproduced the results of in situ experiments (data not shown). In addition, we found that gTFL2:GUS was expressed in the young leaves, the root apical meristem and pericycle, and the emerging lateral roots (Fig. 4E–G). In the developing leaves, gTFL2:GUS was expressed in the petiole and petiole side of leaf blade, where cells were proliferating, but this expression disappeared in the mature leaves and GUS staining remained in the vascular tissue (Fig. 4E). The expression of TFL2 in the root meristem, together with the observation that the root growth rate of tfl2 is lower than that of the wild type (unpublished results), suggests that TFL2 functions in the root although morphological lesion of the tfl2 root has not been well observed.
Microarray analysis revealed that some floral homeotic genes are upregulated in the tfl2 mutant
Several aspects of the tfl2 mutant phenotype and the fact that TFL2 is an HP1 homolog suggested that TFL2 might repress additional genes to FT. In order to identify such target genes, we compared the genome-wide gene expression profile of tfl2 and wild type. We carried out the microarray analysis using an Affymetrix GeneChip containing about 8200 Arabidopsis genes (first version). The expression profile of the tfl2;ft double mutant plant was also examined to discriminate genes that were induced by the misexpression of FT or by the early progression of flowering as a result of FT misexpression. We compared the expression level of genes between Col and tfl2, and between Col and tfl2;ft at 6 d after sowing (manuscript detailing the analysis is in preparation).
Of the flowering-associated genes contained on the GeneChip, only the expression of FT was increased in tfl2, consistent with our previous findings. However, the MADS-box floral organ identity genes PISTILLATA (PI), APETALA3 (AP3), AGAMOUS (AG) and SEPALLATA3 (SEP3) were found to be upregulated in both tfl2 and tfl2;ft. The differential expression of these genes was confirmed by real time quantitative RT-PCR (Table 2), and their upregulation in leaves was also observed (data not shown). The ectopic expression of these genes may be related to the curled leaf phenotype of tlf2, because constitutive expression of SEP3, AG and both PI and AP3 is known to lead to a curled leaf morphology (Mizukami and Ma 1992, Krizek and Meyerowitz 1996, Honma and Goto 2000). Homeotic changes of flower, however, are not observed in tfl2 mutants.
Notably, we did not detect by microarray analysis an increase in the expression of genes located in the region defined as the ‘heterochromatic knob’ (The Cold Spring Harbor Laboratory 2000, Gendrel et al. 2002) in the tfl2 mutant (manuscript in preparation). These results suggest that despite its structural homology to HP1, TFL2 mainly functions as a repressor of both specific euchromatin genes such as FT and several floral homeotic genes in Arabidopsis.
Discussion
Our findings show that TFL2 functions as a negative regulator of flowering by repressing the expression of FT – one of the floral pathway integrators; however, TFL2 does not affect the expression of other integrators, such as LFY and SOC1/AGL20. The relatively day-length insensitive early-flowering phenotype of tfl2 is mainly due to the overexpression of FT during the non-inductive developmental stages. It is consistent with the day-length insensitive early flowering phenotype of FT overexpressing plants (Kardailsky et al. 1999, Kobayashi et al. 1999). FT is directly upregulated by the transcription factor CO, which is induced by LD photoperiod signals (Samach et al. 2000). Our results from both RT-PCR and microarray analysis did not show significant upregulation of CO in the tfl2 mutants.
To our knowledge, there are no other known activators of FT; furthermore, we did not observe a flowering-associated gene whose expression level was significantly affected in the tfl2 mutant in any of our experiments, including microarray analysis. Gaudin et al. (2001) observed the weak upregulation of CO in lhp1-1 (a tfl2 allele) at an early vegetative stage. In our microarray results, the expression of CO was higher in tfl2 than in wild type at 6 d after sowing. However, this upregulation was not observed at other time points or in the RT-PCR experiments, and the increase in FT was much greater than this. With the genetic results showing that the early-flowering phenotype of tfl2 is suppressed by an ft mutation, we can conclude that TFL2 specifically represses FT in the flowering pathway.
The existence of both an activator (CO) and a repressor (TFL2) suggests that expression of FT is regulated in a reciprocal manner. Ectopic expression of FT induces early flowering irrespective of environmental cues and the expression of FT is very low in wild-type plants, even in the inductive phase (Kardailsky et al. 1999, Kobayashi et al. 1999) (Fig. 1A–C). These observations show that even a small misexpression of FT is sufficient for floral induction, and thus FT must be strictly maintained in a silent state during the non-inductive phase. The known function of HP1, with which TFL2 shares homology, fits this kind of regulation, therefore TFL2 might act as an HP1-like repressor in Arabidopsis. It is plausible that not only the activator but also the repressor is required for the precise regulation of flowering, because this is the most crucial process of plant development. FT is also repressed during the non-inductive phase by the EARLY BOLTING IN SHORTDAYS (EBS) gene (Gomez-Mena et al. 2001), showing that FT expression is doubly repressed. Recent studies have revealed that genes encoding proteins with homology to factors that function to regulate chromatin structure (chromatin remodeling factors, polycomb proteins and so on) participate in flowering pathways by repressing flower-promoting genes (Blazquez et al. 2001, Gendall et al. 2001, Yoshida et al. 2001; and reviewed in Sung et al. 2003). TFL2 represents such a protein, and these findings suggest that early-flowering mutants provide a good tool with which to identify repressors that are important in the flowering pathway.
The factor HP1/Swi6 maintains genes in a transcriptionally inactive state by remodeling the chromatin structure in the heterochromatin region. From its sequence homology to HP1, its subnuclear localization and its ability to complement swi6 mutation, TFL2 may function as an HP1 that modifies chromatin structure to maintain genes in a silent state. By microarray analysis, however, we did not detect an upregulation of heterochromatin genes in the tfl2 mutant. By contrast, euchromatin genes such as FT, PI, AP3, AG and SEP3 were significantly activated in the tfl2 mutant. Recent studies have shown that euchromatic genes are also silenced through HP1 (Firestein et al. 2000, Hwang et al. 2001, Nielsen et al. 2001; and reviewed in Li et al. 2002). In mammals, there are three known genes in the HP1 family (α, β, γ), and HP1γ is mainly involved in euchromatin gene repression. In the Arabidopsis genome, the TFL2 gene is the only HP1-like gene (that is, it contains both chromo and chromo shadow domains). Our analyses revealed the aspects of euchromatin gene repression, but TFL2 is still expected to function like HP1α and β, the relationship of TFL2 and heterochromatin gene silencing remains to be solved. Because the tfl2 mutation is not lethal even in the null allele (tfl2-2), unlike in Drosophila, which also has single HP1 gene, tfl2 mutants will provide a good system in which to investigate the function of HP1-like proteins. In plants, mutants of chromatin factors show pleiotropic phenotypes, whereas in animals defects in these homologous genes are lethal (Wagner 2003). This suggests that there may be distinct mechanisms of chromatin regulation in the plant and animal kingdoms.
The pleiotropic phenotypes of tfl2 mutants suggest that TFL2 function is required for the regulation of multiple genes, and indeed we found that several floral homeotic genes are upregulated in the tfl2 mutant. We found that PI, AP3, AG and SEP3 genes are under the repression of TFL2, whereas the expression of AP1 is not greatly affected by TFL2. Misexpression of these genes in the leaves may cause the curled leaf phenotype seen in tfl2 mutants. The curlyleaf (clf) mutant shows a curled leaf phenotype and ectopic expression of AG and AP3 is observed in the leaves of these plants (Goodrich et al. 1997). clf mutants also show homeotic changes in the flower that are not observed in tfl2 mutants; this difference in the flowers is due to differences in the pattern and/or amount of ectopic expression of these genes in tfl2 and clf. Ectopic expression of AG causes early flowering (Mizukami and Ma 1997), but the contribution of AG to early flowering in tfl2 is low because the tfl2;ft double mutant shows a late-flowering phenotype.
The molecular mechanism by which TFL2 represses euchromatic genes (FT, PI, AP3, AG and SEP3) remains unclear. A recent study has shown that CHROMOMATHYLASE 3 (CMT3) interacts with TFL2 (LHP1) and thus DNA methylation and histone H3 methylation is linked by TFL2 (Jackson et al. 2002). We compared nucleotide methylation in the promoter regions of FT, AP3 and AG between tfl2 and the wild type. The tfl2 mutants showed a reduction in methylation in the FT promoter region, but most cytosines in CpNpG – the region that is methylated by CMT3 – are unmethylated in the wild type. The methylation of the AP3 and AG promoters is very low in the wild type, and we could not find a significant difference between the wild type and tfl2 (unpublished results).
The other question that should be addressed in future studies is how the ubiquitously expressed TFL2 gene can repress target genes in the inactivated phase and derepress in the activated phase of the plant. For example, FT is activated after the floral transition, and floral homeotic genes are upregulated in the floral organs. At these stages and in these organs, TFL2 is actively transcribed but target genes are derepressed in response to the activators. The gene activation mechanism underlying gene silencing mediated by HP1 is widely unknown. Regulation of FT, through activation by CO and repression by TFL2, would provide a good model with which to reveal these molecular mechanisms.
Conclusions
TFL2 gene is a unique gene in the Arabidopsis genome that encodes a protein with extensive homology to HP1. We found here that TFL2 represses the flowering pathway integrator FT, as well as the floral homeotic genes PI, AP3, AG and SEP3. In contrast to the clear repression of these euchromatin genes, the expression of heterochromatin genes is not released from silencing in the tfl2 mutant. This suggests that members of the HP1 family of proteins may have different roles in plants and animals. The day-length-insensitive early-flowering phenotype of tfl2 is due to ectopic expression of FT at an early developmental stage. The existence of both a transcriptional activator and a transcriptional repressor means that expression of FT is regulated by a reciprocal mechanism to control flowering time precisely.
Materials and Methods
Plant materials and growth conditions
We isolated the mutant allele tfl2-3 in a Col background by T-DNA tagging. Crosses with tfl2-1 (obtained from the Arabidopsis Biological Resource Center) showed that tfl2-3 is allelic to tfl2-1 (Larsson et al. 1998).
We used the Col ecotype for genetic and gene expression analyses unless otherwise indicated. Plant crossing was carried out by manual cross-pollination. For introgression into the Col ecotype, we crossed ft-1 (originally in Ler) with Col six times, and its self-fertilized progeny that showed an ft phenotype was obtained. To identify the tfl2-3 mutation, we confirmed the presence of T-DNA by PCR.
For genetic analyses, plants were grown in soil at 22°C under LL (constant light, about 50 µmol s–1 m–2), LD (16-h light, about 50 µmol s–1 m–2/8-h dark), or SD (10-h light, about 80 µmol s–1 m–2/14-h dark) conditions. For gene-expression analyses, plants were grown on 0.8% agar plates containing MS salts and 2% sugar at 22°C under LD or SD conditions.
Plant transformation was carried out by Agrobacterium-mediated vacuum transformation.
Real-time quantitative PCR
Samples were collected each day after sowing at dawn in LD conditions. We used the aerial parts of seedlings without leaves (leaves were cut off at petioles) or rosette leaves. Tissue was ground in liquid nitrogen, and RNA was extracted by Isogen (Nippon gene) according to the manufacturer’s instructions. Total RNA was resuspended in DNase I buffer and treated with RNase-free DNase I (Gibco-BRL). For the synthesis of cDNA, 2 µg of total RNA was primed with the dT12–18 primer (Pharmacia) and reverse-transcribed with SuperscriptII (Gibco-BRL). cDNA was resuspended in 50 µl of water and 2.5 µl aliquots were analyzed quantitatively for the expression of each gene by a fluorogenic 5′-nuclease PCR assay (Holland et al. 1991). Gene-specific PCR products were measured continuously by means of an ABI PRISM 7700 Sequence Detection System (Applied Biosystems) over 40 cycles. Absolute quantification of the initial cDNA was done by a dilution series of cloned cDNA. Each PCR assay was done twice. Specific primers and probes were designed by the PrimerExpress program (Applied Biosystems) so that they would not detect homologous genes and had the following sequences: for FT (forward, 5′-CAACCCTCACCTCCGAGAATAT-3′; reverse, 5′-TGCCAAAGGTTGTTCCAGTTGT-3′; probe, 5′-TCCATTGGTTGGTGACTGATATCCCTGC-3′): for CO (forward, 5′-AACGACATAGGTAGTGGAGAGAACAAC-3′; reverse, 5′-GCAGAATCTGCATGGCAATACA-3′; probe, 5′-ACGACCCTGTGACACATGCCGGT-3′; these sequence data were kindly provided by H. Onouchi and G. Coupland); for TFL2 (forward, 5′-GCATCTGTATCAGACAATGTCCAG-3′; reverse, 5′-TGTCCACCAATGCTTCCTTCC-3′; probe, 5′-TCAGACCTCAGCGCTAAAAAGGTCACCAAC-3′): for TFL1 (forward, 5′-CCTGCACTGGATCGTTACAAAC-3′; reverse, 5′-CATAGCTCACCACCTCTTTGCC-3′; probe, 5′-TTCCCGGCACAACAGATGCTACGTT-3′): for LFY (forward, 5′-TTAAAGAACGCGGTGAGAACG-3′; reverse, 5′-AGCGATGTTCACAAGTGGCTT-3′): for SOC1 (forward, 5′-CAACAGATTGAGCAACAGCTTGAG-3′; reverse, 5′-AGCTTCTCGTTTTCTGCAGCTAG-3′): for AP1 (forward, 5′-GCAAGCAATGAGCCCTAAAGAG-3′; reverse, 5′-AGTGCGGATGTGCTTAAGAGC-3′; probe, 5′-TTCAGAATCTGGAGCAGCAGCAGCTTGACAC-3′): for PI (forward, 5′-AAAATCTGATGGCTGTCGAGC-3′; reverse, 5′-CCATCTGGTGGTCTCGGACT-3′; probe, 5′-CGCCATTGAACATGGCCTCGACA-3′): for AP3 (forward, 5′-GAGTGTTTGGACGAGCTTGACA-3′; reverse, 5′-CGCGAACGAGTTTGAAAGTGTT-3′; probe, 5′-TCATCCTCAAGACGACGCAGCTCCT-3′): for SEP3 (forward, 5′-GCATGCTTCGGACACTGGA-3′; reverse, 5′-GGCCTCTCTTGAAGGCACATT-3′; probe, 5′-AGGTACCAAAAGTGTAACTATGGAGCACCAGAACC-3′): for AG (forward, 5′-CAACCGTTTGATTCACGGAA-3′; reverse, 5′-GGCGGATGAGTAATGGTGATTG-3′; probe, 5′-TATTTCCAAGTCGCGGCATTGCAAC-3′). ACTIN2 was used for normalization (Feys et al. 2001): (forward, 5′-GCTGAGAGATTCAGATGCCCA-3′; reverse, 5′-GTGGATTCCAGCAGCTTCCAT-3′; probe, 5′-AAGTCTTGTTCCAGCCCTCGTTTGTGG-3′).
Northern blot analysis
Total RNA (10 µg) was subjected to electrophoresis and transferred to nylon membranes. We detected the expression of FT and ACTIN2 by gene-specific probes using the DIG luminescent detection kit (Roche).
Construct of TFL2 fusion genes
We constructed the gTFL2:GUS chimeric gene by inserting the GUS coding sequence into the 3′ end of a TFL2 genomic fragment (the TFL2 stop codon was replaced with the GUS initiation codon). This chimeric gene contains the promoter (2,946 bp), 3′ region (695 bp) and introns, and it complements the tfl2 mutant. 35S::TFL2:GFP was constructed by fusing the GFP (S65T) ORF in-frame to the 3′ terminus of the TFL2 cDNA via a BamHI linker in the pCGN transformation vector. gTFL2:GFP was made in a similar manner to gTFL2:GUS, but TFL2 and GFP were fused via a poly-glycine linker.
Subcellular localization
The roots of transgenic Arabidopsis carrying TFL2:GFP genes were observed by confocal laser scanning microscopy (Bio-Rad Radiance 2000). To visualize GFP, an Argon ion laser (488 nm) was used for excitation in combination with a band-pass filter (HQ 515/530). Cell walls were stained by propidium iodide and visualized by the same excitation with a long-pass filter (E600LP).
In situ hybridization and GUS staining
The methods for in situ hybridization (Takada et al. 2001) and GUS staining (Honma and Goto 2000) have been described. For the TFL2 probe, we used as a template a 993-bp region (HindIII–EcoRI fragment) of the TFL2 cDNA that excluded the chromo domain.
Acknowledgments
We are grateful to K. Oda for technical advice, and to N. Kuroda, K. Nagae and T. Kainou for technical assistance. R. Allshire and Y. Murakami generously provided the yeast strains and Swi6 gene used in this study. This work is supported in part by Grant-in Aid for Scientific Research on Priority Areas of MEXT (grant #14036232).
These authors contributed equally to this work.
Present address: Faculty of Science, Saitama University, Saitama, 338-8570 Japan
Present address: Section of Plant Biology, Division of Biological Sciences, University of California, Davis, CA 95616, U.S.A.
Corresponding author: E-mail, kgoto@v004.vaio.ne.jp; Fax, +81-866-56-9454.
Fig. 1 Expression of FT and flowering genes in wild type and tfl2 grown in LD. (A–C) Quantification of FT mRNA in aerial parts without leaves (A) and in leaves (B). Data are the mean ± SEM of three independent experiments. For wild type, error bars lie within circles. (C) Northern blot analysis of FT RNA over time from all aerial parts; ACTIN2 was used for normalization. Each lane contains 10 µg of total RNA. (D–H) Quantification of CO (D), SOC1 (E), LFY (F), AP1 (G) and TFL1 (H) mRNA in aerial parts without leaves. Typical results representing three independent experiments are shown. (I) Expression rhythm of FT mRNA from aerial parts without leaves during a LD day at 7 d (tfl2-1) and 9 d (wild type) after sowing. Shown is a representative result of two independent experiments. Open and filled bars represent light and dark periods, respectively. Dawn is designated as 0 h. Amounts are shown relative to ACTIN2. Wild type, filled circles; tfl2-1, open circles. Days are relative to sowing, which is designated as day 0.
Fig. 2 Genomic and protein structures of TFL2. (A) Genomic organization of the TFL2 gene in the 5.7-kb region which complements tfl2 mutants. Six exons are shown as a box and the mutation sites of tfl2-1 and tfl2-3 are indicated. LB, left border of T-DNA; RB, right border of T-DNA. (B) Putative amino acid sequence deduced from TFL2 cDNA (DDBJ/GenBank accession number AB073490). The ED-rich region (hatched line), chromo domain (box) and chromo shadow domain (solid line) are indicated. (C) Alignment of chromo and chromo shadow domains of TFL2 and HP1-like proteins from rice (OsHP1, AC074354–1), tomato (LeHP1, AF428244–1), carrot (DcHP1, D83719), fruitfly (DmHP1), human (HsHP1α) and fission yeast (Swi6). Identical and conserved amino residues are indicated by bold and light shadows, respectively.
Fig. 3 Complementation experiments in yeast and subnuclear localization of TFL2. (A) TFL2 complements the swi6– mutant in fission yeast. Yeast strain FY711 (otr1R(SphI)::ura4+; swi6-115) (Gaudin et al. 2001) was transformed with TFL2, Swi6 or an pREP1 vector (–). Precultured cells (4×103) and their serial dilutions (1 : 5) were spotted onto URA– and FOA plates and incubated at 30°C for 6 d. (B–C) Confocal microscopy images of root meristem cells of transgenic Arabidopsis carrying 35S::TFL2:GFP (B) and gTFL2:GFP (C). GFP (green) signals are observed in only the nuclei and the localization pattern is very similar in both transgenic plants. Cell walls appear in red. Five and four optical sections were projected, respectively. Scale bars, 0.01 mm.
Fig. 4 Expression pattern of TFL2 in the plant tissue. (A–D) In situ hybridization to TFL2 mRNA in the vegetative meristem (A), the inflorescence meristem and young floral buds (B), and the flower of stage 7 (C) and stage 9 (D). (E–G) GUS staining patterns of transgenic plants carrying the gTFL2:GUS gene. A 12-day-old plant (E), root apical meristem (F) and emerging lateral roots (G) are shown. c, cotyledons; vm, vegetative meristem; im, inflorescence meristem; fm, floral meristem, stages are shown in parentheses; Se, sepal; Pe, petal; St, stamen; Ca, carpel. Scale bars, 1 mm (E); 0.1 mm (A–D, F, G).
Fig. 5 Phenotype of the tfl2-3;ft-1 double mutant. (A) From left to right, 36-day-old tfl2-3, tfl2-3;ft-1, ft-1 and WT (Col) plants. (B) Top view of 42-day-old tfl2-3;ft-1 (left) and ft-1 (right) plants. (C, D) Two-month-old tfl2-3;ft-1 (C) and ft-1 (D) plants. Scale bars, 1 cm.
Numbers of leaves at flowering in tfl2 and ft
| Soil | Plate | ||||||||||||
| LD | SD | LD | SD | ||||||||||
| Rosette leaves | Cauline leaves | n | Rosette leaves | Cauline leaves | n | Rosette leaves | n | Rosette leaves | n | ||||
| WT (Col) | 11.8±0.27 | 3.1±0.20 | 20 | 52.0±1.9 | 7.7±0.25 | 20 | 7.6±0.11 | 38 | 44.3±3.9 | 7 | |||
| tfl2-1 | 7.1±0.13 | 2.8±0.09 | 20 | 12.1±0.20 | 3.5±0.11 | 20 | 6.7±0.18 | 36 | 7.0±0.16 | 37 | |||
| tfl2-2 | 7.6±0.18 | 2.7±0.11 | 20 | 12.3±0.16 | 3.7±0.11 | 20 | 7.1±0.15 | 30 | 6.4±0.16 | 39 | |||
| tfl2-3 | 7.4±0.13 | 2.5±0.11 | 20 | 13.5±0.20 | 3.9±0.07 | 20 | 6.4±0.11 | 30 | 7.9±0.16 | 39 | |||
| ft | 43.9±1.8 | 6.3±0.28 | 10 | ||||||||||
| tfl2-3;ft | 42.1±2.3 | 6.6±0.42 | 7 | ||||||||||
| Soil | Plate | ||||||||||||
| LD | SD | LD | SD | ||||||||||
| Rosette leaves | Cauline leaves | n | Rosette leaves | Cauline leaves | n | Rosette leaves | n | Rosette leaves | n | ||||
| WT (Col) | 11.8±0.27 | 3.1±0.20 | 20 | 52.0±1.9 | 7.7±0.25 | 20 | 7.6±0.11 | 38 | 44.3±3.9 | 7 | |||
| tfl2-1 | 7.1±0.13 | 2.8±0.09 | 20 | 12.1±0.20 | 3.5±0.11 | 20 | 6.7±0.18 | 36 | 7.0±0.16 | 37 | |||
| tfl2-2 | 7.6±0.18 | 2.7±0.11 | 20 | 12.3±0.16 | 3.7±0.11 | 20 | 7.1±0.15 | 30 | 6.4±0.16 | 39 | |||
| tfl2-3 | 7.4±0.13 | 2.5±0.11 | 20 | 13.5±0.20 | 3.9±0.07 | 20 | 6.4±0.11 | 30 | 7.9±0.16 | 39 | |||
| ft | 43.9±1.8 | 6.3±0.28 | 10 | ||||||||||
| tfl2-3;ft | 42.1±2.3 | 6.6±0.42 | 7 | ||||||||||
Flowering time is indicated as mean ± SEM of leaves.
Numbers of leaves at flowering in tfl2 and ft
| Soil | Plate | ||||||||||||
| LD | SD | LD | SD | ||||||||||
| Rosette leaves | Cauline leaves | n | Rosette leaves | Cauline leaves | n | Rosette leaves | n | Rosette leaves | n | ||||
| WT (Col) | 11.8±0.27 | 3.1±0.20 | 20 | 52.0±1.9 | 7.7±0.25 | 20 | 7.6±0.11 | 38 | 44.3±3.9 | 7 | |||
| tfl2-1 | 7.1±0.13 | 2.8±0.09 | 20 | 12.1±0.20 | 3.5±0.11 | 20 | 6.7±0.18 | 36 | 7.0±0.16 | 37 | |||
| tfl2-2 | 7.6±0.18 | 2.7±0.11 | 20 | 12.3±0.16 | 3.7±0.11 | 20 | 7.1±0.15 | 30 | 6.4±0.16 | 39 | |||
| tfl2-3 | 7.4±0.13 | 2.5±0.11 | 20 | 13.5±0.20 | 3.9±0.07 | 20 | 6.4±0.11 | 30 | 7.9±0.16 | 39 | |||
| ft | 43.9±1.8 | 6.3±0.28 | 10 | ||||||||||
| tfl2-3;ft | 42.1±2.3 | 6.6±0.42 | 7 | ||||||||||
| Soil | Plate | ||||||||||||
| LD | SD | LD | SD | ||||||||||
| Rosette leaves | Cauline leaves | n | Rosette leaves | Cauline leaves | n | Rosette leaves | n | Rosette leaves | n | ||||
| WT (Col) | 11.8±0.27 | 3.1±0.20 | 20 | 52.0±1.9 | 7.7±0.25 | 20 | 7.6±0.11 | 38 | 44.3±3.9 | 7 | |||
| tfl2-1 | 7.1±0.13 | 2.8±0.09 | 20 | 12.1±0.20 | 3.5±0.11 | 20 | 6.7±0.18 | 36 | 7.0±0.16 | 37 | |||
| tfl2-2 | 7.6±0.18 | 2.7±0.11 | 20 | 12.3±0.16 | 3.7±0.11 | 20 | 7.1±0.15 | 30 | 6.4±0.16 | 39 | |||
| tfl2-3 | 7.4±0.13 | 2.5±0.11 | 20 | 13.5±0.20 | 3.9±0.07 | 20 | 6.4±0.11 | 30 | 7.9±0.16 | 39 | |||
| ft | 43.9±1.8 | 6.3±0.28 | 10 | ||||||||||
| tfl2-3;ft | 42.1±2.3 | 6.6±0.42 | 7 | ||||||||||
Flowering time is indicated as mean ± SEM of leaves.
Expression of floral homeotic genes in wild type, tfl2, and tfl2;ft
| Col | tfl2-2 | tfl2-3 | tfl2-3;ft-1 | |
| AP1 | 0.057±0.025 | 0.223±0.084 (3.9) | 0.089±0.020 (1.6) | 0.132±0.027 (2.3) |
| PI | 0.031±0.010 | 0.827±0.248 (26.6) | 0.361±0.068 (11.6) | 0.629±0.091 (20.3) |
| AP3 | 0.015±0.009 | 0.668±0.144 (45.3) | 0.176±0.013 (11.9) | 0.227±0.051 (15.1) |
| AG | 0.024±0.007 | 1.375±0.132 (56.3) | 0.966±0.099 (39.6) | 1.059±0.363 (43.4) |
| SEP3 | 0.209±0.066 | 8.502±2.571 (40.6) | 3.659±2.320 (17.5) | 3.499±1.293 (16.7) |
| Col | tfl2-2 | tfl2-3 | tfl2-3;ft-1 | |
| AP1 | 0.057±0.025 | 0.223±0.084 (3.9) | 0.089±0.020 (1.6) | 0.132±0.027 (2.3) |
| PI | 0.031±0.010 | 0.827±0.248 (26.6) | 0.361±0.068 (11.6) | 0.629±0.091 (20.3) |
| AP3 | 0.015±0.009 | 0.668±0.144 (45.3) | 0.176±0.013 (11.9) | 0.227±0.051 (15.1) |
| AG | 0.024±0.007 | 1.375±0.132 (56.3) | 0.966±0.099 (39.6) | 1.059±0.363 (43.4) |
| SEP3 | 0.209±0.066 | 8.502±2.571 (40.6) | 3.659±2.320 (17.5) | 3.499±1.293 (16.7) |
mRNA expression of floral homeotic genes in the aerial parts of 6-day-old plants. Data are shown as relative to ACTIN2 mRNA and the mean ± SEM of four (Col), three (tfl2-2 and tfl2-3;ft-1), and two (tfl2-3) independent experiments. In parentheses, fold activations against Col are indicated.
Expression of floral homeotic genes in wild type, tfl2, and tfl2;ft
| Col | tfl2-2 | tfl2-3 | tfl2-3;ft-1 | |
| AP1 | 0.057±0.025 | 0.223±0.084 (3.9) | 0.089±0.020 (1.6) | 0.132±0.027 (2.3) |
| PI | 0.031±0.010 | 0.827±0.248 (26.6) | 0.361±0.068 (11.6) | 0.629±0.091 (20.3) |
| AP3 | 0.015±0.009 | 0.668±0.144 (45.3) | 0.176±0.013 (11.9) | 0.227±0.051 (15.1) |
| AG | 0.024±0.007 | 1.375±0.132 (56.3) | 0.966±0.099 (39.6) | 1.059±0.363 (43.4) |
| SEP3 | 0.209±0.066 | 8.502±2.571 (40.6) | 3.659±2.320 (17.5) | 3.499±1.293 (16.7) |
| Col | tfl2-2 | tfl2-3 | tfl2-3;ft-1 | |
| AP1 | 0.057±0.025 | 0.223±0.084 (3.9) | 0.089±0.020 (1.6) | 0.132±0.027 (2.3) |
| PI | 0.031±0.010 | 0.827±0.248 (26.6) | 0.361±0.068 (11.6) | 0.629±0.091 (20.3) |
| AP3 | 0.015±0.009 | 0.668±0.144 (45.3) | 0.176±0.013 (11.9) | 0.227±0.051 (15.1) |
| AG | 0.024±0.007 | 1.375±0.132 (56.3) | 0.966±0.099 (39.6) | 1.059±0.363 (43.4) |
| SEP3 | 0.209±0.066 | 8.502±2.571 (40.6) | 3.659±2.320 (17.5) | 3.499±1.293 (16.7) |
mRNA expression of floral homeotic genes in the aerial parts of 6-day-old plants. Data are shown as relative to ACTIN2 mRNA and the mean ± SEM of four (Col), three (tfl2-2 and tfl2-3;ft-1), and two (tfl2-3) independent experiments. In parentheses, fold activations against Col are indicated.
The nucleotide sequence for TFL2 cDNA reported in this paper has been submitted to the DDBJ/GenBank under the accession number AB073490.
References
Allshire, R.C., Nimmo, E.R., Ekwall, K., Javerzat, J.P. and Cranston, G. (
Blazquez, M., Koornneef, M. and Putterill, J. (
Blazquez, M.A. and Weigel, D. (
Bradley, D., Ratcliffe, O., Vincent, C., Carpenter, R. and Coen, E. (
Cold Spring Harbor Laboratory, The, W.U.G.S.C. and PE Biosystems Arabidopsis Sequencing Consortium (
Eissenberg, J.C. and Elgin, S.C. (
Feys, B.J., Moisan, L.J., Newman, M.A. and Parker, J.E. (
Firestein, R., Cui, X., Huie, P. and Cleary, M.L. (
Gaudin, V., Libault, M., Pouteau, S., Juul, T., Zhao, G., Lefebvre, D. and Grandjean, O. (
Gendall, A.R., Levy, Y.Y., Wilson, A. and Dean, C. (
Gendrel, A.V., Lippman, Z., Yordan, C., Colot, V. and Martienssen, R.A. (
Gomez-Mena, C., Pineiro, M., Franco-Zorrilla, J.M., Salinas, J., Coupland, G. and Martinez-Zapater, J.M. (
Goodrich, J., Puangsomlee, P., Martin, M., Long, D., Meyerowitz, E.M. and Coupland, G. (
Hempel, F.D., Weigel, D., Mandel, M.A., Ditta, G., Zambryski, P.C., Feldman, L.J. and Yanofsky, M.F. (
Holland, P.M., Abramson, R.D., Watson, R. and Gelfand, D.H. (
Honma, T. and Goto, K. (
Hwang, K.K., Eissenberg, J.C. and Worman, H.J. (
Jackson, J.P., Lindroth, A.M., Cao, X. and Jacobsen, S.E. (
Kardailsky, I., Shukla, V.K., Ahn, J.H., Dagenais, N., Christensen, S.K., Nguyen, J.T., Chory, J., Harrison, M.J. and Weigel, D. (
Kobayashi, Y., Kaya, H., Goto, K., Iwabuchi, M. and Araki, T. (
Koornneef, M., Alonso-Blanco, C., Peeters, A.J. and Soppe, W. (
Krizek, B.A. and Meyerowitz, E.M. (
Larsson, A.S., Landberg, K. and Meeks-Wagner, D.R. (
Lee, H., Suh, S.S., Park, E., Cho, E., Ahn, J.H., Kim, S.G., Lee, J.S., Kwon, Y.M. and Lee, I. (
Li, Y., Kirschmann, D.A. and Wallrath, L.L. (
Mizukami, Y. and Ma, H. (
Mizukami, Y. and Ma, H. (
Mouradov, A., Cremer, F. and Coupland, G. (
Nielsen, S.J., Schneider, R., Bauer, U.M., Bannister, A.J., Morrison, A., O’Carroll, D., Firestein, R., Cleary, M., Jenuwein, T., Herrera, R.E. and Kouzarides, T. (
Putterill, J., Robson, F., Lee, K., Simon, R. and Coupland, G. (
Reeves, P.H. and Coupland, G. (
Samach, A., Onouchi, H., Gold, S.E., Ditta, G.S., Schwarz-Sommer, Z., Yanofsky, M.F. and Coupland, G. (
Simon, R., Igeno, M.I. and Coupland, G. (
Simpson, G.G. and Dean, C. (
Simpson, G.G., Gendall, A.R. and Dean, C. (
Smyth, D.R., Bowman, J.L. and Meyerowitz, E.M. (
Suarez-Lopez, P., Wheatley, K., Robson, F., Onouchi, H., Valverde, F. and Coupland, G. (
Sung, Z.R., Chen, L., Moon, Y.H. and Lertpiriyapong, K. (
Takada, S., Hibara, K., Ishida, T. and Tasaka, M. (
Weigel, D., Alvarez, J., Smyth, D.R., Yanofsky, M.F. and Meyerowitz, E.M. (