-
PDF
- Split View
-
Views
-
Cite
Cite
Markus Geisler, Bibek Aryal, Martin di Donato, Pengchao Hao, A Critical View on ABC Transporters and Their Interacting Partners in Auxin Transport, Plant and Cell Physiology, Volume 58, Issue 10, October 2017, Pages 1601–1614, https://doi.org/10.1093/pcp/pcx104
Close - Share Icon Share
Abstract
Different subclasses of ATP-binding cassette (ABC) transporters have been implicated in the transport of native variants of the phytohormone auxin. Here, the putative, individual roles of key members belonging to the ABCB, ABCD and ABCG families, respectively, are highlighted and the knowledge of their assumed expression and transport routes is reviewed and compared with their mutant phenotypes. Protein–protein interactions between ABC transporters and regulatory components during auxin transport are summarized and their importance is critically discussed. There is a focus on the functional interaction between members of the ABCB family and the FKBP42, TWISTED DWARF1, acting as a chaperone during plasma membrane trafficking of ABCBs. Further, the mode and relevance of functional ABCB-PIN interactions is diagnostically re-evaluated. A new nomenclature describing precisely the most likely ABCB–PIN interaction scenarios is suggested. Finally, available tools for the detection and prediction of ABC transporter interactomes are summarized and the potential of future ABC transporter interactome maps is highlighted.
Introduction
Native auxins are a group of chemically related, mainly indolic compounds, which are ubiquitous key regulators of plant development and performance (Adamowski and Friml 2015, Grones and Friml 2015). The most abundant auxin, IAA, controls transcriptional regulation of multiple developmental processes mainly via activation of the TRANSPORT INHIBITOR RESPONSE 1(TIR1) family of auxin receptors (Grones and Friml 2015). However, post-transcriptional actions of IAA, such as actin cytoskeleton bundling and cell elongation, have also been shown to occur in a time frame that is too fast to be transcriptional and thus to occur independently of the auxin receptors (Schenck et al. 2010, Zhu and Geisler 2015, Geisler et al. 2016). To date, putative auxin-binding proteins that might integrate these effects have not yet been clearly assigned (Zhu and Geisler 2015).
IAA action as a developmental switch has been shown to be tightly connected to the presence of local auxin maxima and minima generated over tissues (Adamowski and Friml 2015). These are created, maintained and modulated by cell to cell transport of auxin (as reviewed in Adamowski and Friml 2015), a plant-specific process so far not verified for other plant hormones. [Beside auxin, transporters involved in long-distance transfer of ABA, strigolactones, cytokinins and, eventually, also jasmonic acid conjugates (Kretzschmar et al. 2011, Kretzschmar et al. 2012, Borghi et al. 2015, Zurcher et al. 2016, Li et al. 2017) have been characterized. However, currently it is still unclear if these indeed function in polar transport of the respective hormones and not simply in phloem/xylem (un)loading, which needs to be demonstrated.] This process, called polar auxin transport (PAT), is dependent on the action of members of at least three auxin transporter families: PIN (PIN-FORMED) proteins, AUX1/LAX (AUXIN1/LIKE AUXIN1) and ABCBs (ATP-binding cassette transporters of the B subfamily) (Kerr and Bennett 2007, Geisler et al. 2016). Despite the difficulties in demonstrating auxin transport for individual members, which is mainly caused by re-diffusion of protonated IAA (Geisler and Murphy 2006), now for most of the key members of all three families solid transport data also in respect of their substrate specificity have been provided that characterize them as bona fide auxin transporters (Geisler et al. 2014).
Beside IAA, the closely related native auxin, indole-3-butyric acid (IBA), is also discussed to be transported in a polar fashion and to be a substrate of different classes of ABC transporters (Strader and Bartel 2011). In the following, the roles of subfamilies and individual members of these in auxin transport, as well their interference with other interacting partners are summarized.
Roles of ABC Transporters in Auxin Transport
Discovery and features of ABCBs
ABCB19/MDR1/PGP19 was discovered as an induced gene responding to the anion channel blocker NPPB [5-nitro-2-(3-phenylpropyl amino)-benzoic acid] while searching for a chloride channel (Noh et al. 2001). However, auxin-related phenotypes, i.e. reduced and enhanced hypocotyl lengths under dim light, were reported before for ABCB1/PGP1 antisense and overexpressing lines, respectively (Sidler et al. 1998), although not yet linked to auxin transport defects. Like many ABC transporters, ABCB19 was also shown to be induced by auxin, and its loss-of-function mutants showed phenotypes usually found with auxin imbalance (Noh et al. 2001). Independently, ABCB19 together with ABCB1, the FKBP42, TWISTED DWARF1 (TWD1)/ULTRACURVATA2 (UCU2) and other proteins were isolated as 1-N-naphthylphthalamic acid (NPA)-binding proteins (Noh et al. 2001, Murphy et al. 2002, Geisler et al. 2003)—NPA being a non-competitive inhibitor of PAT (Katekar and Geissler 1980). PAT in abcb19 mutants was severely reduced, in both inflorescence stems and hypocotyls, and even more dramatically in abcb1 abcb19 (Noh et al. 2001). Their identity as auxin transporters was sustained by the finding that abcb1 abcb19 and twd1 showed overlapping dwarf phenotypes and similar drastic reductions in PAT (14% of the wild type; Geisler et al. 2003) that exceeded even those of pin1 (Bandyopadhyay et al. 2007, Blakeslee et al. 2007). Further proof of their catalytic activities was provided by demonstrating a gradual loss of specific auxin export from abcb1, abcb19 and abcb1 abcb19 Arabidopsis protoplasts and quantification of auxin export for ABCB1 after heterologous expression in the plasma membrane (PM) of baker’s yeast (Geisler et al. 2005). Demonstration of ABCB19-mediated auxin transport followed shortly after by employing the HeLa system (Bouchard et al. 2006). These initial transport data have since been verified independently by various groups using different expression systems (Blakeslee et al. 2007, Rojas-Pierce et al. 2007, Bailly et al. 2008, Kim et al. 2010).
It appears that a subgroup of ABCBs are primary active auxin pumps that are able to transport against steep auxin gradients by coupling their transport directly to ATP hydrolysis. This separates them from PINs and AUX1/LAXs, which are thought to act as secondary active auxin transporters that are dependent on electrochemical gradients (Grones and Friml 2015, Geisler et al. 2016). The ATP dependence of auxin transport has been demonstrated by ATP depletion, and pharmacologically by employing flavonols known to compete with ATP for binding sites on the nucleotide-binding folds of ABC transporters (Geisler et al. 2005). In contrast to their mammalian orthologs, that are known for their involvement in multidrug resistance (MDR) phenomena, plant ABCBs revealed a surprisingly high degree of substrate specificity toward only a few auxinic compounds [including IAA, 1-naphthaleneacetic acid 1-NAA) and 2.4-D] (Bouchard et al. 2006). Structurally related substances (such as benzoic acid or 2-NAA) or structurally distant classical mammalian ABCB substrates (such as rhodamin123, daunomycin and vinblastine) were not efficiently transported (Geisler et al. 2005, Bouchard et al. 2006). An in silico comparison of putative substrate-binding domains of Arabidopsis and mammalian ABCB1/PGP1 revealed an evolutionary shift toward charged amino acids in these domains apparently required for binding of the IAA anion (Bailly et al. 2011).
Whereas ABCB1 and ABCB19 and all so far characterized PM-embedded PINs [long PINs (PIN1–PIN4 and PIN7) containing a large hydrophilic loop which is subject to multiple phosphorylation events regulating both PIN trafficking and activity (Adamowski and Friml 2015)] were shown to function as auxin exporters, members of the AUX1/LAX family are considered as permease-like auxin importers, most probably functioning as H+/IAA symporters (Kerr and Bennett 2007). Of special interest in this context is thus ABCB4 and its close homolog, ABCB21, for which, depending on the expression system, opposite import and export directionalities have been reported (Santelia et al. 2005, Terasaka et al. 2005, Cho et al. 2007, Kamimoto et al. 2012). Therefore, it was suggested that plant-specific factors might control transport directionalities (Cho et al. 2007). Recently, both ABCB isoforms were suggested to function as facultative IAA importers/exporters whose transport directionality is triggered by intracellular auxin concentrations (Kamimoto et al. 2012). This is an interesting phenomenon because the supposed transport bidirectionality as found for Arabidopsis ABCB4 and ABCB21, Oryza sativa ABCB14 (Xu et al. 2014) and Coptis japonica ABCB1/MDR1 (Shitan et al. 2003) seems to be limited to the plant kingdom. Therefore, identification of the molecular bases regulating transport directions—either the local auxin concentration itself or a second regulatory factor—both options being mutually non-exclusive—is of the highest priority.
ABCB expression and roles during polar auxin transport
In correlation with their identity as auxin pumps, ABCBs are mainly expressed in meristematic tissues (both root and shoot) with high auxin concentrations where they are thought to export IAA into the apoplast (Bailly et al. 2006). These roles are on one hand in agreement with Arabidopsis mutant phenotypes for abcb and abcb1 abcb19, showing epinastic leaves and dwarfism as expected for excess apical auxin, while the single abcb1 mutant has only a very subtle phenotype (Noh et al. 2001, Geisler et al. 2003). On the other hand, phenotypes are also in line with gradual reductions in auxin transport measured in a cellular and a polar fashion (Geisler et al. 2005), where the abcb1 abcb19 mutant showed a ca. 70% reduction in PAT (Blakeslee et al. 2007). These findings suggested a second function for ABCBs in maintaining long-range transport of auxin (see Fig. 2), which might either be connected to its primary role in clearing apoplastic auxin reflux or even form the basis of it. An involvement in long-distance auxin transport was recently confirmed by the analyses of dwarf phenotypes caused by ABCB1 loss of function in the agriculturally important crop mutants, maize zmabcb1/brachytic2, an ABCB ortholog of sorghum sbabcb1 dwarf3 (Multani et al. 2003). While ABCB21 functions have not been analyzed due to a lack of mutants, ABCB4 was shown to function primarily in the transport of auxin away from the root tip into the root elongation zone (Santelia et al. 2005, Cho et al. 2007, Lewis et al. 2007)
In comparison with PINs that, with a few exceptions, show concrete expression patterns, expression of ABCB1, ABCB4 and ABCB19 is more broad and overlapping. This is most obvious for ABCB1 and ABCB19 that show widely overlapping expression in the stele of roots, while ABCB1 (but not ABCB19) is additionally found in the epidermis and inner lateral root cap (Mravec et al. 2008). While ABCB19 expression is strongest in the pericycle and endodermis, ABCB4 is expressed from the root cap to the hair differentiation zone and was confined to the epidermis and outer cell layers of the columella and lateral root cap tissue (Cho et al. 2007). Common for all investigated ABCBs is that they are not found in the columella cells. This makes a direct involvement in lateral redirection of auxin streams during gravitropic responses (as shown for PIN3) unlikely.
Like most PINs, ABCBs also reveal a mix of polar and non-polar PM locations; however, in comparison with predominantly polarly localized PIN1 and PIN2, ABCB1, ABCB4 and ABCB19 clearly reveal a reduced polarity (Geisler and Murphy 2006). Therefore, their direct involvement—although demonstrated for Arabidopsis and crop plants—in polar or long-range auxin transport is not directly obvious. Three possible roles during PAT are currently discussed that are all mutually not exclusive: first, one possibility is that they function independently of PINs by pumping auxin out of the cell in meristematic tissues with high apoplastic auxin concentrations. A need for this option has been criticized by thermodynamic calculations arguing that apoplastic IAA concentrations would need to reach herbicidal (>100 µM) concentrations in order to require primary active export of nanomolar auxin (Spalding 2013). However, these calculations do not include the functions of cellular uptake. Therefore, we still believe that such steep transmembrane auxin gradients in the root tip are possible, especially in light of the fact that ABC transporters are often functioning in raft-like microdomains for which auxin concentrations might be very different from assumed cytoplasmic concentrations. A second, although not well supported option that will be discussed in detail later, is that ABCB–PIN pairs might act interactively (see Fig. 3b). A third scenario is based on the finding that all investigated ABCBs are apparently very weakly expressed in the lateral, outward-facing domain of the epidermis. The reason for lateral epidermal exclusion is unknown, but might suggest a role in canalizing auxin toward the auxin stream of the vasculature.
Correlation with ABCB mutant phenotypes
ABCB mutants have been mainly analyzed in terms of their root and hypocotyl bending capacities toward the gravitropic vector or the light, respectively (Noh et al. 2001, Noh et al. 2003, Bouchard et al. 2006, Lewis et al. 2007, Bailly et al. 2008, Ge et al. 2017). Results indicate that in the widest sense, ABCB1 and ABCB19 also have both a concerted function [in this review we propose a new nomenclature for functional transporter interactions (for details, see text and legends to Figs. 2 and 3) that is highlighted in italics] in root PAT, although they have been shown to function mainly in shoot-ward (basipetal) and root-ward (acropetal) directions (Geisler et al. 2003, Bouchard et al. 2006, Lewis et al. 2007), respectively (see Fig. 2). In line with subtle single loss-of-function phenotypes, single abcb1 and abcb19 mutant alleles so far tested show only minor root bending defects (Geisler et al. 2003, Bouchard et al. 2006, Lewis et al. 2007), but a lower rate of early root bending (Bouchard et al. 2006). [Conflicting hypergravitropic root bending reported initially for abcb19 (Noh et al. 2003) has subsequently been corrected by the same group (Lewis et al. 2007).] In light of strongly reduced auxin transport defects (around 30–80% depending on the tissue and method; Geisler et al. 2003, Bouchard et al. 2006, Lewis et al. 2007), these mild bending phenotypes point to a complementary action (see below and Fig. 3 for details) in abcb1 and abcb19 single mutants by ABCB19 and ABCB1 isoforms, respectively. This is sustained by drastic root bending (and dwarf) phenotypes for abcb1 abcb19 mutant roots (Noh et al. 2001, Geisler et al. 2003, Bouchard et al. 2006) that are the result of disruption of shoot-ward and root-ward auxin streams that apparently cannot be complemented by other ABCB isoforms or PIN proteins. Based on only very subtle root bending phenotypes for abcb19 and PIN mutants involved in this process (including PIN1, PIN4 and PIN7), root-ward auxin transport in the stele seems not to have a strong impact on the asymmetric auxin re-distribution causing the root to bend (Spalding 2013).
Further evidence for auxin transport defects in abcb1 abcb19 mutants is that their Columbia alleles display a non-fixed handed helical disorientation (‘twisting’) of epidermal layers (Wu et al. 2010, Wang et al. 2013). This is most likely to be the result of unequal elongation of cortical and epidermal layers most probably due to enhanced epidermal auxin concentrations (Wang et al. 2013) caused by a block of shoot-ward auxin transport. This phenotype is phenocopied and even stronger in the FKBP42/ TWISTED DWARF1 mutant (Wang et al. 2013), shown to be responsible for mislocation and subsequent degradation of ABCB1, ABCB19 and ABCB4 (Wu et al. 2010, Wang et al. 2013). Both epidermal twisting defects can be partially rescued by NPA, indicating a direct involvement of ABCBs, shown to be direct targets of NPA (Rojas-Pierce et al. 2007, Kim et al. 2010, Wang et al. 2013). A block of ABCB-mediated auxin transport by NPA in the abcb1 abcb19 and twd1 mutants, whose gene products are both a target of NPA (Rojas-Pierce et al. 2007, Nagashima et al. 2008), is at first counterintuitive, but might indicate that other ABCBs are complementing for ABCB1, ABCB4 and ABCB19.
For abcb4 mutants, reduced (Terasaka et al. 2005) and enhanced root bending data (Lewis et al. 2007) have been reported, although not using identical mutant alleles. A high-resolution analysis indicated that both abcb4‐1 and abcb4‐2 alleles bent earlier and at a faster rate than the wild type (Lewis et al. 2007). In agreement with reduced shoot-ward transport rates (40% reduction; Terasaka et al. 2005, Lewis et al. 2007), monitoring DR5:GFP (green fluorescent protein) auxin reporter gene expression during bending revealed that in abcb4 mutants shoot-ward auxin progression was slowed down, resulting in faster bending (Lewis et al. 2007). These findings are overall in line with the proposed uptake directionality for ABCB4 during shoot-ward PAT. As mentioned above, abcb21 mutants have not yet been analyzed; however, recently, rice ABCB14 was shown to function as an auxin importer in analogy to ABCB4 and ABCB21 (Xu et al. 2014).
Interestingly, abcb19 and abcb1,19 hypocotyls were shown to exhibit greatly impaired root-ward auxin transport (Lewis et al. 2007, Nagashima et al. 2008) but also enhanced shoot phototropism and gravitropism (Noh et al. 2003). These results strongly suggest that ABCB19 contributes to the suppression of the differential growth of hypocotyls, which is partially shared by ABCB1 (Nagashima et al. 2008). Follow-up work indicated that activation of the blue light receptor kinase, phot1, inhibits ABCB19 activity at the illuminated side by means of protein phosphorylation (Christie et al. 2011; see below for details). This block would enhance auxin at the shaded side, which is enhanced by concerted action (for a definition, see Figs. 2 and 3) with PIN3 that was shown to be redistributed to this lateral subdomain (Ding et al. 2011). Whether ABCB19 and PIN3 also act interactively by means of physical interaction has not yet been investigated.
Although these examples clearly illustrate concerted and complementary modes of action during auxin transport, overall developmental phenotypes for ABCB and PIN mutants are distinct. Mutations in PIN1 and combined mutations in other PIN genes with PIN1 result in organogenesis defects, which was interpreted as a proof of concept that PINs provide directional auxin flow essential for organogenesis (Benkova et al. 2003, Blilou et al. 2005). However, recently a strong mutant allele of ABCB19, abcb19-5, was identified, which showed stem–cauline leaf and stem–pedicel fusion defects due to increased auxin levels at the organ boundary region in the inflorescence apex (Zhao et al. 2013). Recently, ABCB1 was shown to act as a major player during auxin-controlled anther development, while ABCB19 plays only a minor role here (Cecchetti et al. 2015). For abcb1 abcb19 mutant combinations, significant apical shifts in root hair polarity were reported (Zhu et al. 2016) that were previously overlooked. In light of these findings, the separation into polarly expressed PINs controlling development by organizing an auxin reflux system and non-polar ABCBs contributing to long-range transport that does not play a role in plant development obviously needs some readjustment.
Arabidopsis contains 21 full-size ABCB genes (Kang et al. 2011), but so far only for four isoforms, ABCB1, ABCB4, ABCB19 and ABCB21, have solid auxin transport data been provided. As substrate specificities cannot easily be deduced from sequence (Geisler and Murphy 2006, Bailly et al. 2011), it is currently unclear how many of the 17 remaining ABCBs function as auxin transporters. However, a recent publication suggested that there are eventually more to come: ABCB14 and ABCB15, both apparently involved in stem lignification, promote auxin transport since inflorescence stems in both mutants showed a reduction in PAT (Kaneda et al. 2011) (see Fig. 1). However, ABCB14 was previously identified as a malate importer implicated in stomatal regulation (Lee et al. 2008), indicating eventually that altered auxin transport in abcb14 might be caused indirectly.
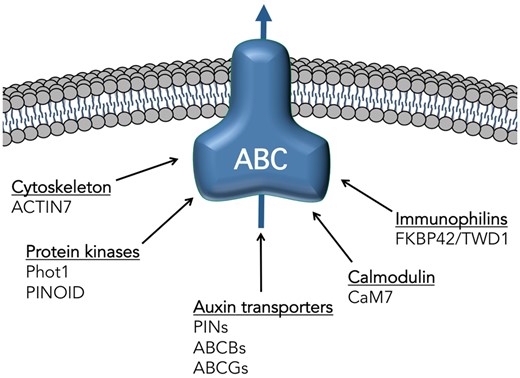
Known and suspected interacting partners of ABC transporters involved in auxin transport. Known subclasses (underlined) and key members shown to interact physically with ABC transporters involved in transport of the native auxins, IAA or IBA. References can be found in the text.
Novel roles for ABC transporters in IBA transport?
IBA, long considered as a synthetic auxin, is a closely related native auxin and can be converted to IAA in a peroxisome-dependent reaction (Zolman et al. 2008). Currently it is unclear if IBA acts as an auxin by itself or if it is a precursor of IAA and thus acts via IAA (Ludwig-Muller 2007, Strader and Bartel 2008, Schlicht et al. 2013). Moreover, the existence of IBA in the model plant Arabidopsis has been questioned (Novak et al. 2012), although this might simply be a question of extraction and detection methods.
However, for full IBA responsiveness, the ABC transporter PEROXISOMAL ABC TRANSPORTER1 [PXA1/ABCD1/PED3; also known as COMATOSE (CTS)] seems to be essential (Zolman et al. 2001, Footitt et al. 2002, Hooks et al. 2007). Mutant alleles for this gene, ped3 (peroxisome-defective 3), were isolated in a screen for mutants resistant to 2,4-D (Hayashi et al. 1998), an auxin analog, but only mapped later (Zolman et al. 2001). The cts mutant of Arabidopsis was isolated in a forward genetic screen to identify lines that possessed reduced germination potential and found to encode an ortholog of human ABCD1 (ALDP; adrenoleukodystrophy protein) known to transport long-chain and very-long-chain fatty acyl-CoA, respectively (Morita and Imanaka 2012). A mutation in the same gene was isolated from a screen to identify mutants resistant to IBA (Zolman et al. 2000). Moreover, cts mutants contain low residual levels of jasmonic acid and are male fertile, which has led to the suggestion that ABCD1 might also transport jasmonic acid precursors (Theodoulou et al. 2005). Because clear-cut transport data have not been provided yet, the nature of the ABCD1 substrate(s) is unclear but it is thought to import fatty acid and/or acyl-CoAs, acetate, jasmonic acid precursors and IBA into peroxisomes.
Barley has, like yeast and humans, two ABCD1 homologs, designated HvABCD1 and HvABCD2, which play roles in the metabolism of the auxins, 2,4-D and IBA, but also in jasmonate biosynthesis and determination of grain size (Mendiondo et al. 2014).
Currently it is unclear if IBA is indeed transported in a polar fashion. Two independent studies support this concept (Rashotte et al. 2003. Liu et al. 2012); however, only one study verified the identity of the transported radiotracer as IBA (Liu et al. 2012). In contrast, Ruzicka et al. (2010) came to the conclusion that most of the applied IBA was converted to IAA.
However, members of all three major IAA transporter families have tested negative for IBA transport (Swarup et al. 2008, Ruzicka et al. 2010). Independent IAA and IBA transport catalysts are also supported by the finding that neither NPA nor the competitive auxin inhibitor, TIBA (2,3,5-triiodobenzoic acid), blocks IBA transport (Rashotte et al. 2003, Liu et al. 2012). Genetic approaches in Arabidopsis support the idea that IBA efflux from root cells is catalyzed by at least two members of the PLEIOTROPIC DRUG RESISTANCE (PDR) subclade of the ABCG family of ABC transporters (Borghi et al. 2015): loss of ABCG36/PDR8/PEN3 (Strader and Bartel 2009, Lu et al. 2015) and ABCG37/PDR9/PIS1 (Strader et al. 2008, Ruzicka et al. 2010) increases sensitivity to IBA, but not IAA. The IBA hypersensitivity phenotypes of mutants defective in these transporters suggest that IBA is a common substrate effluxed by both ABCG36 and ABCG37, but clear transport data have so far only been provided for ABCG37 (Ruzicka et al. 2010). However, it should be mentioned that, although ABCGs obviously do not transport IAA (Ruzicka et al. 2010), both ABCG36 and ABCG37 obviously have a wider but clearly delimited specificity for structurally unrelated substrates typical for PDR-type ABC transporters (Kang et al. 2010, Borghi et al. 2015). For ABCG36, these include auxinic compounds (such as IBA and 2.4-D; Ito and Gray 2006), heavy metals (such as Cd, Fe and Pb; Kim et al. 2007) and not yet clearly identified indole glucosinolates (Stein et al. 2006, Lu et al. 2015). ABCG37 has been suggested to transport—beside auxinic compounds such as IBA, 2.4-D and NPA (Ruzicka et al. 2010)—phenolic compounds including coumarin (Fourcroy et al. 2014, Fourcroy et al. 2016, Ziegler et al. 2017).
Currently it is unclear if ABCG36/37 indeed function in polar IBA transport. If so, in light of their predominant expression in lateral epidermal PM domains, a putative role could be that they function in reducing proposed basipetal (shoot-ward) polar IBA streams that would be provided by as yet unidentified IBA transporters.
In summary, it appears that we are only at the start of identifying the individual roles of ABC transporters from different subclasses in auxin transport and have only a very limited insight into their individual roles during this complex process. In light of reported complementary and/or concerted action of some isoforms, two obvious strategies will in the future allow dissection of individual roles for ABC transporters in auxin transport: first, the identities of all isoforms of individual ABC transporters subclasses must be tested for auxin transport capacities, which can be perceived by transport studies or mutant analyses. Secondly, complementary action of some isoforms can be tested by the creation of knock-out combinations or cluster-wise down-regulation by artificial microRNAs.
Importance of Protein–Protein Interactions During Auxin Transport
Transport proteins, especially primary active ones, rarely act alone as their functions need to be tightly regulated (Geisler 2014). This regulatory event usually employs transient or stable protein–protein interactions (PPIs), which are defined as the physical contact between two or more protein molecules. These physical associations between chains or subdomains are provided by electrostatic forces (mainly hydrophobic effects) and regulate virtually all cellular processes.
For transporters, an involvement of PPIs in a quick and economic regulation of transporter activity, trafficking and stability has been demonstrated (reviewed in Geisler 2014, Geisler et al. 2016). In the following, we will limit our discussion mainly to the relevance of only a few described PPIs of single ABCB transporters involved in auxin transport. For auxin transporters of the ABCG and ABCD classes, the information on functional interactions by PPIs is very limited.
Immunophilins function as ABC transporter chaperones
Immunophilins form a superfamily of evolutionarily unrelated proteins originally discovered to bind clinically relevant immunosuppressant drugs (Geisler et al. 2016). FKBPs (FK506-binding proteins) bind the macrolides, FK506 and rapamycin, while cyclophilins bind the cyclic peptide cyclosporin A (CsA). A common feature is their [cis-trans-petidyl prolyl isomerase (PPIase) or rotamase] activity, which is inhibited by immunosuppressant drugs (Romano et al. 2005, Geisler and Bailly 2007, Gollan et al. 2012, Geisler et al. 2016). In addition, some immunophilins were shown to harbor an intrinsic chaperone activity that is independent of their PPIase activity and unaffected by immunosuppressants (Geisler et al. 2016). Larger, multidomain immunophilins own a co-chaperone activity that seems to depend on functional interaction with HSP90 at the tetratricopeptide (TPR) domain (Iki et al. 2012). PPIase and chaperone activities might be involved separately or co-operatively in assisting protein folding (Geisler et al. 2016).
Both FKBP and cyclophilin families seem to be expanded in plants with 23 and 29 members, respectively, in Arabidopsis which was interpreted as an adaptation to a sessile lifestyle providing, for example stress resistance and developmental plasticity (Geisler and Bailly 2007, Geisler et al. 2016).
Among plant FKBPs, the FKBP42, TWD1/UCU2, was shown in detail to regulate both ABCB transport activity and ABCB transporter presence in the PM (Wu et al. 2010, Henrichs et al. 2012, Wang et al. 2013). ABCB1 was initially identified in yeast two-hybrid (Y2H) screens for TWD1-interacting proteins (Geisler et al. 2003). Using co-immunoprecipitation and BRET (bioluminescence resonance energy transfer) assays in plant and yeast systems, the TWD1–ABCB1 interaction was strengthened (Wang et al. 2013).
Heterologous co-expression supported a regulatory impact on ABCB1 and ABCB19 transport activity (Bouchard et al. 2006, Bailly et al. 2008, Henrichs et al. 2012) as was found for yeast FKBP12 on mouse ABCB3/MDR3 when co-expressed in yeast (Hemenway and Heitman 1996). Initially, and in agreement with a functional interaction on the PM, the regulatory action by TWD1 was thought to take place there and to employ a refolding of the ABCB1 C-terminus, shown to provide interaction (Geisler et al. 2003). Refolding was thought to be catalyzed by the TWD1 PPIase activity and to activate ABCB1 activity by causing a conformational change that might enhance access of either ATP or the substrate (Geisler 2014). However, all attempts to demonstrate clearly such a PPIase activity either by classical calorimetric or nuclear magnetic resonance (NMR) assays failed (Kamphausen et al. 2002; M. di Donato, A. Bailly, D. Carnevale and M. Geisler, unpublished). Also, no immunosuppressant drug binding could be detected at TWD1, which usually correlates with a loss of PPIase activity. A structural reason was provided by the finding that the TWD1 FKBD retained only three out of 11 conserved key residues in the hydrophobic FK506-binding pocket (Geisler and Bailly 2007). However, Kamphausen et al. (2001) provided evidence for an aggregation/holdase activity by TWD1, which is a PPIase-independent mode of chaperone action that may be responsible for ABCB stability/regulation. This activity is usually dependent on the interaction with other chaperones provided by a TPR domain, suggesting that TWD1 action may be mediated by a larger chaperone complex.
Interestingly, yeast co-expression with TWD1 up-regulated ABCB1- and ABCB19-mediated IAA but not 1-NAA export, which suggests that TWD1 acts as a specificity filter (Bailly et al. 2014). This finding was supported by the fact that IAA but not 1-NAA export was reduced in twd1 but not in abcb1 abcb19 mutants. Although the overall concept that ABCB substrate specificity is determined by an immunophilin awaits verification in a mammalian system, it might be of major clinical importance because it implies that multidrug resistance is caused by ABCB–FKBP imbalance during ABCB overexpression in tumor cells.
It has been clear for some time that TWD1 functions primarily as an ABCB chaperone during endoplasmic reticulum (ER) to PM trafficking, which is based on the findings that ABCB1, ABCB4 and ABCB19 are mislocalized on the ER and degraded in twd1 (Wu et al. 2010, Wang et al. 2013). This chaperone function on ABCB transporters seems to be conserved as the mammalian ortholog, FKBP38, was described to promote ER to PM delivery of ABCC7/CFTR (cystic fibrosis transmembrane conductance regulator), a chloride channel whose loss of function is responsible for the genetic disease mucoviscidosis (Banasavadi-Siddegowda et al. 2011, Aryal et al. 2015). As for TWD1, for FKBP38–CFTR regulation an involvement of PPIase activity was discussed but never clearly demonstrated.
A functional difference between both modules lies in the fact that interaction with ABCBs is provided by the FKBP domain, while CFTR seems to employ FKBP and TPR domains (Banasavadi-Siddegowda et al. 2011). The TWD1 TPR domain was shown to interact functionally with vacuolar transporters, ABCC1 and ABCC2 (Geisler et al. 2004), indicating that interacting domains might encode specificity of ABC transporter subclass interaction.
An interesting, although not well understood, aspect of the proposed chaperone function for TWD1 is that by means of fluorescence reporter gene fusion, TWD1 was shown to reside, beside on the PM, mainly in the ER, while ABCBs have been limited to the PM in the wild type (Wu et al. 2010, Wang et al. 2013). In contrast, by immunolocalizations using TWD1-specific antisera, TWD1 was shown to be limited to lateral, outward-facing, PM domains of epidermal cells, where it co-localizes with small amounts of ABCB1 found there (Wang et al. 2013). The discrepancies between both methods are unclear; however, functional interaction on lateral PM subdomains was interpreted as a way to boost lateral ABCB1-mediated auxin export. The latter is supported by enhanced responsiveness of IAA-induced hypocotyl elongation caused by overexpression of TWD1 (Wang et al. 2013, Bailly et al. 2014).
Another fascinating facet of this interaction is that TWD1/FKBP42, like FKBP38, contains a C-terminal in-plane membrane (IPM) anchor that docks TWD1 to the membranes (Geisler et al. 2003). This anchor is not strictly required for functionality because expression of a C-terminal deleted version still complemented the twisted dwarf1 syndrome in all aspects (Bailly et al. 2014). Surprisingly, deletion of the membrane anchor led to hypermorphic growth caused by enhanced cell elongation, again supporting the concept of enhanced auxin export into the apoplast. This is currently interpreted as a way to dampen the polar auxin stream (Bailly et al. 2014).
This proposed chaperone function on ABCBs, which was shown not to affect PINs, might be interconnected with a second regulatory role for TWD1 on the actin cytoskeleton. Recently, TWD1 was shown to interact physically with the actin isoform, ACTIN7, and to interfere dually with actin bundling and dynamics: TWD1 promotes actin filament turnover and blocks actin de-bundling; however, both actions are most probably perceived via as yet unknown actin-binding proteins (Zhu et al. 2016). As in twd1, in actin7 too, ABCB1, ABCB4 and ABCB19—but additionally also PIN1 and PIN2—were mislocalized, although on vacuolar and early endosomal compartments. This, on the one hand, suggests overlapping action, but, on the other hand, that ACTIN7 and TWD1 control the same trafficking events although on different levels (Zhu et al. 2016).
Using BRET and NPA chromatography, micromolar concentrations of NPA, or its functional analog, BUM [2-(4-diethylamino-2-hydroxybenzoyl)benzoic acid] were shown to disrupt TWD1–ABCB1 interactions (Geisler et al. 2003, Bailly et al. 2008, Kim et al. 2010, Wang et al. 2013). This phenomenon is in agreement with findings that both ABCBs and TWD1 were shown to bind NPA and that ABCB-mediated auxin transport is inhibited by NPA and BUM (Rojas-Pierce et al. 2007, Kim et al. 2010, Zhu et al. 2016). As proposed binding sites were mapped to the C-terminal nucleotide binding fold (NBD2) of ABCB1 and the TWD1 FKBD, respectively (Kim et al. 2010, Zhu et al. 2016), both previously shown to provide PPIs (Geisler et al. 2003), these findings suggest that NPA/BUM interfere with the ABCB–TWD1 interface where they might compete for the interacting partner.
Interestingly, flavonols, a class of phenolic compounds that also block PAT, were even more effective in disrupting ABCB–TWD1 interaction, with quercetin being the most effective (Bailly et al. 2008). However, quercetin does not bind to TWD1, while it still binds and inhibits plant and mammalian ABCBs (Conseil et al. 1998, Geisler et al. 2005, Zhu et al. 2016), which might suggest that binding of flavonols to ABCBs is sufficient to disrupt interaction. Taken together, it seems likely that non-competitive auxin transport inhibitors (such as NPA, BUM or flavonols) inhibit auxin transport by blocking the chaperone effect of TWD1 on ABCBs via disruption of ABCB–TWD1 interaction. This model is supported by the finding that micromolar concentrations of NPA reduce the presence of ABCBs on the PM (Kubes et al. 2012).
The involvement of ACTIN7 in ABCB chaperoning by TWD1 was indirectly supported by the finding that TWD1 mediates NPA action on the actin cytoskeleton (Zhu et al. 2016). While the impact of NPA on actin de-bundling was TWD1 independent, TWD1 was shown to mediate directly the inhibitory effect of NPA on actin filament turnover.
Recently the cyclophilin DIAGEOTROPICA (DGT/CypA) was characterized in regulation of PIN sorting, while ABCBs were not affected (Ivanchenko et al. 2015, Geisler et al. 2016). While TWD1 was shown to increase ABCB functionality, the opposite was shown for DGT. Interestingly, both PIN1 and ABCB1 co-expression increased the presence of DGT on the PM, suggesting an interaction with both transporter classes (Geisler et al. 2016). In summary, it appears that members of both FKBP and cyclophilin classes of immunophilins serve as chaperones during secretion of auxin transporters by means of PPI. However, it remains open whether these actions employ PPIase activities and, if so, if the roles of PPIase and chaperone activities are interconnected.
ABCB transport activity is regulated by AGC protein kinases
Two recent studies support the concept that members of the plant-specific AGC protein kinase family, orthologs of mammalian protein kinases A, C and G, regulate ABCBs involved in auxin transport (Aryal et al. 2015): first, the AGC4 kinase, PHOTROPIN1 (phot1) was shown to interact with the NBDs of ABCB19 (but not with ABCB1). Co-expression in HeLa cells reduced ABCB19 activity, which was further accelerated by blue light irradiation. ABCB19 phosphorylation by phot1 was shown in vitro but not yet in vivo (Christie et al. 2011). Secondly, the AGC3 kinase, PINOID (PID), was identified and characterized as a TWD1 interactor by co-immunoprecipitation/liquid chromatography–mass spectrometry (LC-MS) and BRET analyses (Henrichs et al. 2012). In contrast to ABCB19–phot1 interaction, PID was shown to phosphorylate ABCB1 at S634 in the regulatory linker domain that had a promoting impact on ABCB1 transport activity (Henrichs et al. 2012). The finding that ABCB1 transport activity was inhibited when co-expressed with PID in the presence of TWD1 was at first interpreted as there being a second, TWD1-specific phosphorylation site (Henrichs et al. 2012). However, another option might be that in the presence of TWD1, ABCB1 phosphorylation of S634 might simply be reduced, which is currently under investigation.
Currently it is unclear if ABCB phosphorylation by AGC kinases strictly requires TWD1. ABCB1–PID interaction has not yet been tested in both the presence and absence of TWD1. Co-immunoprecipitation data revealed that blue light reduced ABCB19–TWD1 interaction, which is dependent on phot1 (Christie et al. 2011). These data together support a model in which ABCB phosphorylation by AGC kinases, such as phot1 or PID, alters the chaperoning role of TWD1 on ABCB PM trafficking by reducing ABCB–TWD1 interaction (Aryal et al. 2015). A similar mode of action has been suggested for CFTR/ABCC7 secretion that is dependent both on interaction with FKBP38 and phosphorylation of the regulatory R domain, the equivalent of the ABCB linker, by protein kinase A. In summary, it seems as if an interconnected, regulatory module consisting of protein phosphorylation and PPI is conserved across kingdoms.
Do primary and secondary active auxin transporters functionally interact with each other?
Interactive action between individual auxin transporters is not well understood but has been previously suggested for specific ABCB–PIN pairs (Blakeslee et al. 2007). The original motivation to investigate these was that heterologously expressed ABCBs, but especially PIN proteins, were able to function independently as auxin transport catalysts but were less specific compared with their in vivo environment, suggesting the lack of plant-specific factors. Y2H and co-immunoprecipitation analyses provided evidence for physical interaction of selected ABCB1 and ABCB19 and PIN1 and PIN2 pairs (Blakeslee et al. 2007, Rojas-Pierce et al. 2007, Kim et al. 2010). In analogy to ABCB–TWD1 interaction, ABCB–PIN interaction is also provided by the C-terminal ends of ABCBs interacting with hydrophilic loops of PINs (Blakeslee et al. 2007). Co-expressed PIN1–ABCB1 and PIN1–ABCB19 combinations showed increased export rates and substrate specificity compared with single systems, while PIN2 only had an effect on substrate specificity. PINs co-expressed with ABCB4 had an influence on transport directionality, although proof of physical PIN–ABCB4 interactions is still awaited (Bandyopadhyay et al. 2007, Blakeslee et al. 2007). Functional PIN–ABCB interactions were supported by subcellular co-localization studies indicating limited, overlapping ABCB1 expression with PIN1 in stelar tissues and with PIN2 signal in cortical and epidermal cells (Blakeslee et al. 2007). Finally, genetic analyses were employed to dissect auxin transport mechanisms; however, mutant phenotypes were found to be complex: abcb19 pin1 shoot phenotypes and agravitropism for abcb19 pin2 supported additive mechanisms (Blakeslee et al. 2007). Interestingly, unlike in pin1 or pin1 abcb19 plants, in pin1 abcb1 abcb19 triple mutants floral development was restored presumably as a result of ectopic auxin accumulation in the shoot apical meristem (Blakeslee et al. 2007). On the other hand, pin1 abcb1 abcb19 triple (but not pin1 or abcb1 abcb19) mutants were found to have strong defects in cotyledon development during embryogenesis, indicating synergistic action during embryogenesis (Mravec et al. 2008).
In summary, these data suggest that PINs and ABCBs characterize co-ordinated, independent auxin transport mechanisms but also support the possibility of interactivity between single members of the ABCB and PIN families functioning as synergistic or antagonistic transport complexes in auxin transport. In these tissue-specific pairings, PINs were thought to add a vectorial dimension to ABCB-mediated transport. This concept, in a physiological context, makes sense for described pairs (such as ABCB19 and PIN1 or ABCB1/ABCB4 and PIN2) in that concerted actions in the same transport directionality have been shown. However, the functional relevance for some suggested interactive pairs (such as ABCB19–PIN2), in that no concerted action or co-location has so far been shown, might be questioned. Moreover, based on the low degree of co-locations for described ABCB–PIN pairs, it is more likely that the interactivity of these complexes is limited to some polar PM subdomains, where ABCBs and PINs would physically interact (see below for details).
An interesting result of the original study was that co-expressed PIN1–ABCB1 and PIN1–ABCB19 combinations showed a higher degree of NPA sensitivity (Blakeslee et al. 2007). This outcome might be helpful to understand the discrepancy between the finding that growth on NPA/BUM results on the one hand in pin-formed inflorescences but that PINs investigated thus far neither bind NPA/BUM nor are inhibited by either inhibitor in heterologous systems (Rojas-Pierce et al. 2007, Kim et al. 2010, Zhu et al. 2016). In this context it might also be helpful to recall that based on biochemical work the regulatory NPA-binding protein was predicted to be distinct from the transport catalyst (Muday and Murphy 2002).
Proposed ABCB–PIN interaction on the one hand as well as NPA/BUM insensitivity of PIN-mediated transport leads to the next interesting but not yet solved question: what in this proposed interaction works as a transport catalyst and what solely (or additionally) as a transport regulator? Transport studies for ABCBs and PINs employing heterologous—especially non-plant—systems argue for independent action as transporters (Kerr and Bennett 2007); however, they do not strictly exclude functional interaction with endogenous ABCB/PIN-like orthologs, respectively. However, PINs, with the exception of PIN1 [PIN1 transport activity was shown to depend on protein phosphorylation by members of the AGC family of protein kinases (Zourelidou et al. 2014)], have so far been proven to be active in baker’s yeast and oocytes (Blakeslee et al. 2007, Kim et al. 2010, Zourelidou et al. 2014) not containing ABCBs, which makes a strong point for PINs as independent export catalysts that do not depend on ABCBs. The inverse has not been shown; however, it is important to recall that ABCBs are an old invention already existing in green algae, while PINs coincided with the appearance of land plants (Galvan-Ampudia and Offringa 2007). This makes a strict requirement of PINs for ABCB transport activity unlikely. On the other hand, it is well known that ABC transporters can, besides functioning as transporters and channels, also act as regulators of other secondary active transport systems, including channels (Spalding 2013, Aryal et al. 2015). A prominent example for such a functional interaction is the sulfonylurea receptor (SUR; ABCC8/9) that associates with the potassium channel proteins Kir6.1 or Kir6.2 to form the ATP-sensitive potassium channel (Principalli et al. 2015). Within the channel complex, SUR serves as a regulatory subunit, which fine-tunes channel gating. Another, although distinct, example is functional interaction between the chloride channel, ABCC7/CFTR, and isoforms of the anion exchanger family, SLC26 (SOLUTE CARRIER26) that exchange chloride against bicarbonate (El Khouri and Toure 2014, Aryal et al. 2015).
ABCBs and PINs act independently as auxin transport catalysts (Fig. 3) as is supported by single, heterologous expression experiments and independent evolution. However, ABCBs and PINs in some cell files show overlapping, concerted action (such as ABCB19/PIN1, ABCB1/PIN2, ABCB4/PIN2 or ABCB19/PIN3; see Fig. 2), which does not require PPIs. Concerted action, however, does not essentially have to employ the same transport directionalities and thus might result in a reduced net auxin stream. Such a case is easy to imagine in cell files that express exporters (such as PIN2) and putative importers/exporters (such as ABCB4) on the same PM subdomain.
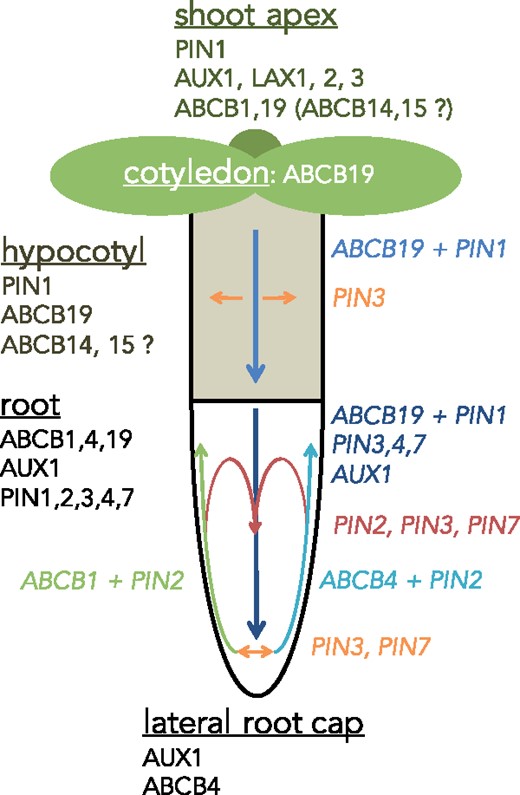
Main expression of ABCBs and their contribution to PAT in Arabidopsis seedlings. Main expression of ABCBs shown to be involved in auxin transport in the indicated organs (underlined). Their contribution to PAT and concerted action (italics) with PIN-type auxin exporters is indicated by arrows. Note that concerted actions of ABCB1 + PIN2 and ABCB4 + PIN2, respectively, are symmetric under vertical growth conditions. Further, note that ABCB1 and ABCB19 can complement each other for basipetal and acropetal transport defects in abcb19 and abcb1 mutant backgrounds, respectively, although their dominant directionalities in basipetal (shoot-ward) and acropetal (root-ward) PAT are opposite. Note that expression and routes of AUX1/LAX transporters are also indicated, although no functional interaction with ABC transporters has yet been described. Figure content is partially based on Cho and Cho (2012).
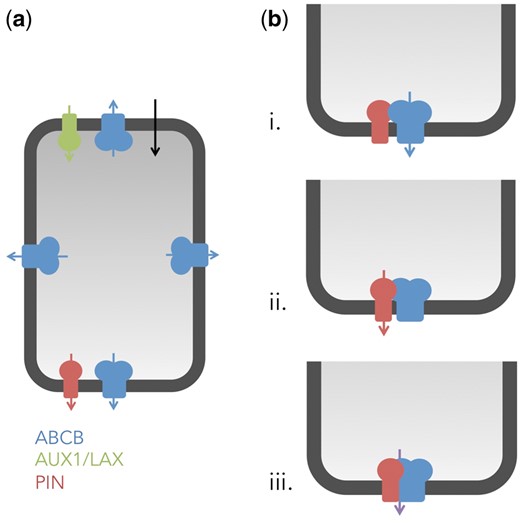
Speculative models on independent and interactive actions of ABCB and PIN proteins during polar auxin transport. (a) In an independent action, members of the ABCB, PIN or AUX1/LAX families function as independent auxin transport catalysts that do not interact physically. Note that independent action does not exclude a concerted action of ABCB and PIN exporters as depicted in Fig. 2. We would like to define concerted action, as shared, overlapping function of two (or more) proteins (such as ABCB1 and PIN2, ABCB4 and PIN2 or ABCB19 and PIN3), which, however, does not require physical interaction. (b) In an interactive action, the activity of ABCBs and PINs depends on functional interaction with each other. Interactive action can be divided into co-operative or mutual cases. In a co-operative interactive action, one component acts as the transport catalyst, while the other works as the regulator (indicated by arrows). Currently it is not known if PINs can act as regulators of ABCBs (i.) or the inverse (ii.), resulting in an interaction that can have a synergistic (activating) or antagonistic (inhibitory) effect on transport. In a mutual interactive interaction (iii.), none of the ABCBs or PINs can act as transport catalysts independently but require strictly functional interaction that forms a transport–competent complex.
Moreover, one might imagine a complementary mode of action, where ABCB (or PIN) isoforms take over roles of another ABCB (or PIN) isoform in the respective mutant background, as for example shown for ABCB1 and ABCB19. The strong abcb1 abcb19 phenotype would thus be the sum of individual roles of ABCB1 and ABCB19 in basipetal and acropetal PAT and the inability for functional complementation.
- 2.
For local, polar PM subdomains, we propose an interactive ABCB–PIN action that does strictly require physical interaction. In a co-operative interactive action of ABCB–PIN pairs, either ABCBs or PINs act as regulators of export catalysts, respectively. A physical contact would result in synergistic or antagonistic net auxin transport (Fig. 3b i., ii.).
In an unorthodox, alternative scenario, transport-incompetent ABCBs and PINs would require a mutual interactive mode in order to form a novel, transport-competent auxin efflux complex (Fig. 3b iii.). However, this scenario is unlikely because it is not supported by heterologous expression and co-localization data and does not correlate well with known auxin routes.
In summary it appears that our understanding on the here proposed concerted and/or interactive actions and how they can be integrated into widely non-overlapping phenotypes between PIN and ABCB mutants is very limited. The only way that will allow safe determination of individual functions of putative transporters as auxin export catalysts and their interplay is a functional, single and pairwise reconstitution of ABCBs and PINs in a cell-free system.
Other relevant ABC interactions during auxin transport
Several other PPIs with ABC transporters involved in auxin transport have been reported recently (see Fig. 1 for a summary); however, the impact of most of these on transporter activity or membrane presence and its relevance for auxin transport is only poorly understood.
The N-terminus of the putative IBA transporter, ABCG36/PEN3, was shown to co-localize with and to bind to the calmodulin isoform, CaM7, in a calcium-dependent manner (Campe et al. 2016). Interestingly, as in abcg36, in cam7 mutants non-host resistance is also compromised (Campe et al. 2016), indicating that CaM7 might have a negative impact on ABCG36-mediated export of defense compounds in this process.
As mentioned already above, several lines of evidence support the idea that trafficking of ABCB transporters involved in auxin transport is directly or indirectly dependent on interaction with ACTIN7 (Zhu et al. 2016). Very recently it was shown that ACTIN7 is also involved in lateral distribution of ABCG36/PEN3/PDR8 and the aquaporin, NIP5;1 (Mao et al. 2016), suggesting a generic role during PM secretion of polar (basal/apical and lateral) and non-polar PM proteins (Geisler 2016). ACTIN7 was isolated as an interactor of TWD1, although in an indirect interaction most probably involving actin-binding proteins (Zhu et al. 2016). However, ACTIN7 was also pulled down in co-immunoprecipitations using ABCB1 (Zhu et al. 2016) and ABCG36 (B. Aryal and M. Geisler, unpublished) as bait, suggesting that the actin cytoskeleton might also directly interfere with auxin transporters itself.
The polarly localized SHADE AVOIDANCE4 (SAV4) protein was recently shown to be required for proper auxin distribution in Arabidopsis hypocotyls (Ge et al. 2017). SAV4 exhibits a dual localization at the PM and the nucleus. SAV4 contains a putative ARM repeat- and a TPR-like domain, both of which are often involved in PPIs. Interestingly, SAV4 physically interacts with ABCB1 and inhibits ABCB1 transport activity upon co-expression in tobacco (Ge et al. 2017). Overall, this regulatory mechanism resembles roughly those of the immunophilins, TWD1 and DGT; interacting domains and mechanisms here are not completely clear.
Toward an Identification of ABC Transporter Interactomes During Auxin Transport
Transporters do not act independently but in a network of complex molecular interactions. This is supported by the first, partial Arabidopsis interactome (Arabidopsis Interactome Mapping Consortium 2011) revealing a strong enrichment of a few network communities, in that, not surprisingly, transmembrane transport and vesicle trafficking hubs tightly interact.
In the past, mainly GAL4-based Y2H systems and affinity purification followed by mass spectrometry (AP-MS) were used to detect PPIs. Both methods were adapted for high-throughput approaches, and allowed feeding of literature curator PPI databases, such as MINT, DIP Intact, BioGRID, MPIDB and MatrixDb. Unfortunately, the overlap between these databases was soon found to be low due to different standards of curation, organisms and underlying methods (Mosca et al. 2013). To overcome this problem, the International Molecular Exchange (IMEx; http://imex.sourceforge.net/) consortium was formed aiming to enable the exchange of data and to avoid duplication of the curation efforts.
However, due to their hydrophobicity and need for detergent solubilization, transporter interactions are usually under-represented in PPI databases (Geisler 2014). These limitations have been partially overcome by the invention of membrane-based Y2H systems (such as the split ubiquitin Y2H system) allowing detection of interactions between membrane proteins and by the use of milder solubilization techniques. Still, several groups in the (ABC) transporter field have launched individual small-scale PPI projects that focused on a limited subset of (ABC) transporters (Paumi et al. 2007, Paumi et al. 2009) or interacting signaling components (Lalonde et al. 2010). Paumi et al. (2007) reported the identification of six potential partners of the yeast ABC transporter of the ABCC subclass, YCF1 (Yeast Cadmium Factor1). In a follow-up report, the same consortium provided the first yeast ABC interactome by analyzing 19 of all 22 yeast ABC transporters (Snider et al. 2013). An unexpected outcome was the large number of functionally diverse interactors. Another interesting observation was the tendency of full-size ABC transporters to interact with one another and with secondary active transporters. In another interesting report, by comparison of the CFTR and CFTR508 interactome, key interactors involved in rescue of channel function were identified (Pankow et al. 2015).
The maintenance of experimental PPI databases by literature curation is expensive, often error-prone and can even sometimes be biased (Mosca et al. 2013). Therefore, computational methods have been created in order to make the next step from experiments to predictions. Computational methods to predict PPIs can employ either high-throughput experimental data or 3D structural information. However, the methods based on the prediction of the 3D structure of protein complexes (= docking) have serious limitations, as discussed in detail in Mosca et al. (2013).
A number of new methods have been designed to overcome these limitations. Particularly exciting is a new concept that is based on ‘co-evolution’, which refers to co-ordinated changes that occur in proteins to maintain or to regulate protein folding, stability, function and interactions. Applying this algorithm to a subset of ABC transporter complexes resulted in accurate prediction (Bitbol et al. 2016). Finally, there are hybrid methods that integrate various methods, such as low-resolution structural modeling, with other functional data, such as co-expression, and functional and evolutionary similarities.
Unfortunately, all these tools have not yet been used to uncover interactomes of individual auxin transporters or transporter families, either of the ABCB, ABCG or ABCD families. This is surprising as the required lines are now available and techniques are affordable. Interactomes for individual ABC transporters involved in auxin transport under controlled standard conditions could be easily generated from different organs, tissues or even individual cell types generated by cell sorting of marker lines. These high-confidence interaction maps could provide us with detailed spatio-temporal information on ABC transporter activity, regulation and trafficking during auxin transport at high resolution. ABC interactomes could obviously also be compared with those of different model plants, with other Arabidopsis ecotypes, with developmental stages in a given model plant or with mutants or plants grown under special conditions, such as altered exposure to gravity, light etc.
Conclusions and Outlook
Members of three classes of ABC transporter subfamilies, ABCB, ABCD and ABCG, have been implicated in the transport of native auxins. A critical re-evaluation of the current literature suggests that we have only started to identify the individual catalytic components of auxin transport. While peroxisomal import of IBA by ABCD1/PXA1/PED3/CTS still awaits demonstration, independent reports have indicated that members of the ABCG families might catalyze the transport of IBA (Strader and Bartel 2011). In contrast, members of the ABCB family have been widely accepted as IAA transporters (Geisler and Murphy 2006, Bailly et al. 2011, Cho and Cho 2012, Spalding 2013) and, despite their mixed polarity, ABCBs seem to be directly involved in the polar distribution of IAA. In contrast, the roles of ABCGs in polar IBA transport have not yet been established, also because IBA transport measurements are hindered by conversion to IAA. Also it is unknown how many of the 21 ABCB and 12 full-size (PDR-type) ABCG isoforms are actually functioning as auxin transporters.
The individual roles of the three key members of the ABCB subfamily, ABCB1, ABCB4 and ABCB19, show a sufficient correlation with their expression patterns on one hand and with their reported mutant phenotypes on the other (Cho and Cho 2012). However, at the moment, the evolutionary advantage of having primary active (ABCBs) and secondary active IAA export systems (PINs) is unclear. The fact that widely non-polarly expressed ABCBs are ancient auxin transporters, while PINs appeared with the first land plants (Galvan-Ampudia and Offringa 2007) argues for their involvement in a more advanced plant architecture and performance apparently requiring a faster establishment of auxin gradients as can be only provided by polarly expressed secondary active transporters that usually show a higher turnover number. Connected to this, we have only a glimpse of the relevance of described concerted actions during PAT, which have been described for single ABCB–PIN pairs (see Fig. 2). We have even less understanding of the mechanism and significance of proposed interactive ABCB–PIN pairs, thought to take place at local polar PM subdomains by PPI (see Fig. 3). Although, based on heterologous expression, it is likely that both members of the ABCB and PIN families can act independently as auxin transport catalysts, a strict co-operative or mutual functionality cannot be excluded (see Fig. 3).
Beside ABCB–PIN interactions, ABCB-mediated auxin transport has been shown to be regulated by physical interactions with regulatory components such as kinases and immunophilins (Aryal et al. 2015, Geisler et al. 2016). These PPIs apparently allow for a quick and economic regulation of ABCBs and were shown to have an impact on transport activity, trafficking and stability. Current data support a model in which ABCB phosphorylation by AGC kinases, such as phot1 or PID, might have an impact on the chaperoning role of TWD1 on ABCB PM trafficking by interfering with ABCB–TWD1 interaction. A similar mode of action has been suggested for CFTR/ABCC7 secretion that is dependent on both interaction with FKBP38 and phosphorylation by protein kinase A (Aryal et al. 2015). Although CFTR belongs to a different ABC transporter subclass, this regulatory module consisting of protein phosphorylation and PPI seems to be conserved.
Unlike for yeast or mammalian ABC transporters, a systematic, genome-wide interactome of any auxin transporter has not yet been assembled for any plant organism. This is a pity because a positive regulatory impact on transport activity or location by a so-called ‘auxin integrator’ (Zhu and Geisler 2015) that is thought to act via PPI (Zhu and Geisler 2015) would be perfectly in agreement with the proposed positive feedback loop between auxin flux and the cell’s auxin transport capacity, originally referred to as the ‘canalization concept’ (Sachs 1969; Stoma et al. 2008). Although auxins at micromolar concentrations were shown to promote their own transport by blocking PIN endocytosis (Paciorek et al. 2005), the mechanisms and regulatory components (such as the proposed ‘flux sensor’; Merks et al. 2007) by which auxin feeds back on auxin transport have not been discovered (Zhu and Geisler 2015).
High-confidence interaction maps for individual ABC transporters involved in auxin transport are therefore highly desirable because they could provide us with detailed spatio-temporal information on ABC transporter activity, regulation and trafficking during auxin transport at high resolution. This information will provide a greater insight into interplay between transporters and regulatory components during plant development.
Funding
Work in the Geisler Lab is currently funded by the Swiss National Funds [project 31003A_165877/1] and the European Space Association [CORA-GBF project LIRAT].
Abbreviations
- ABC
ATP-binding cassette
- ABCB
ABC transporter B subfamily
- ABCD
ABC transporter D subfamily
- ABCG
ABC transporter G subfamily
- ALDP
adrenoleukodystrophy protein
- AUX1
Auxin-resistant1
- BRET
bioluminescence resonance energy transfer
- BUM
2-(4-diethylamino-2-hydroxybenzoyl)benzoic acid
- CFTR
cystic fibrosis transmembrane conductance regulator
- DGT
DIAGEOTROPICA
- ER
endoplasmic reticulum
- FKBP
FK506-binding protein
- IBA
indole-3-butyric acid
- IAA
indole-3-acetic acid
- LAX
LIKE AUX1
- MDR
multidrug resistance
- NAA
naphthaleneacetic acid
- NPA
1-N-naphthylphthalamic acid
- PAT
polar auxin transport
- PDR
pleitropic drug resistance
- PID
PINOID
- PIN
PIN-FORMED
- PM
plasma membrane
- PPI
protein–protein interaction
- PPIase
cis-trans-petidyl prolyl isomerase
- TPR
tetratricopeptide
- TWD1
TWISTED DWARF1
- UCU2
Ultracurvata2
- Y2H
yeast two-hybrid
Acknowledgments
The authors would like to dedicate this review to Enrico Martinoia on the occasion of his forthcoming retirement.
Disclosures
The authors have no conflicts of interest to declare.