-
PDF
- Split View
-
Views
-
Cite
Cite
W. Michael Caudle, Jason R. Richardson, Kristin C. Delea, Thomas S. Guillot, Minzheng Wang, Kurt D. Pennell, Gary W. Miller, Polychlorinated Biphenyl–Induced Reduction of Dopamine Transporter Expression as a Precursor to Parkinson's Disease–Associated Dopamine Toxicity, Toxicological Sciences, Volume 92, Issue 2, August 2006, Pages 490–499, https://doi.org/10.1093/toxsci/kfl018
Close - Share Icon Share
Abstract
Epidemiological and laboratory studies have suggested that exposure to polychlorinated biphenyls (PCBs) may be a risk factor for Parkinson's disease. The purpose of this study was to examine the potential mechanisms by which PCBs may disrupt normal functioning of the nigrostriatal dopamine (DA) system. We utilized an environmentally relevant exposure of PCBs (7.5 or 15 mg/kg/day Aroclor 1254:1260 for 30 days by oral gavage) to identify early signs of damage to the DA system. This dosing regimen, which resulted in PCB levels similar to those found in human brain samples, did not cause overt degeneration to the DA system as shown by a lack of change in striatal DA levels or tyrosine hydroxylase levels. However, we did observe a dramatic dose-dependent decrease in striatal dopamine transporter (DAT) levels. The observed reductions appear to be specific to the DAT populations located in the striatum, as no change was observed in other dopaminergic brain regions or to other neurotransmitter transporters present in the striatum. These data demonstrate that PCB tissue concentrations similar to those found in postmortem human brain specifically disrupt DA transport, which acts as a precursor to subsequent damage to the DA system. Furthermore, DAT imaging may be useful in evaluating alterations in brain function in human populations exposed to PCBs.
Epidemiological studies have established a role of the environment in the etiology of Parkinson's disease (PD) (Priyadarshi et al., 2000; Tanner and Goldman, 1996; Tanner and Langston, 1990; Tanner et al., 1999). Although specific environmental contaminants that contribute to the incidence of PD have yet to be identified, recent studies have implicated polychlorinated biphenyl (PCB) exposure as a possible risk factor. A recent mortality study of 17,000 workers occupationally exposed to PCBs revealed an increased incidence of PD in female subjects (Steenland et al., 2006). Additionally, studies by Corrigan et al. (1998, 2000) found an increased deposition of PCBs in the caudate nucleus and substantia nigra of brain tissue obtained postmortem from PD patients.
PCBs are members of a class of organic compounds known as halogenated aromatic hydrocarbons and are composed of biphenyl rings with varying degrees and positions of chlorine substitutions present on each ring. Because of their thermal stability, PCBs have been widely used as coolants and lubricants in transformers and capacitors, in hydraulic fluids, as well as in other electrical equipment (Erickson, 1986). Although the manufacturing and use of PCBs have been discontinued since 1977 in the United States, their physiochemical properties allow them to persist and bioaccumulate in the environment, increasing the risk for human exposure (Kamrin and Ringer, 1994; Safe, 1993).
PCB exposure is known to pose numerous health concerns (ATSDR, 2000). Indeed, several studies have shown them to be detrimental to the central nervous system, resulting in motor and cognitive deficits (reviewed by Faroon et al., 2001). A common finding has been a significant reduction of brain dopamine (DA) levels following exposure to PCBs. Several in vitro studies have demonstrated that individual congeners, congener mixtures, as well as extracts from PCB-contaminated Great Lakes fish produce a reduction in DA (Seegal et al., 1989, 1990, 1998; Shain et al., 1991). While the mechanism of DA reduction is unclear, it has been postulated, based on cell culture experiments, that decreased DA levels are a result of inhibition of tyrosine hydroxylase (TH) and L-aromatic acid decarboxylase (L-AADC) activity, two enzymes involved in the synthesis of DA (Angus and Contreras, 1996; Angus et al., 1997). Our laboratory and others have reported a significant decrease in striatal DA following high-dose, acute exposure of rodents to Aroclor 1016, 1254, or 1260 (Richardson and Miller, 2004; Seegal et al., 1986). In addition, Seegal and coworkers (1991a, 1994; Seegal, 2003) have reported decreases in DA in the striatum of nonhuman primates, as well as loss of dopaminergic cells in the substantia nigra, both of which are hallmark features of PD. While the mechanism for these reductions is not clear, disruption of DA handling and homeostasis may play a role.
Recent studies have shown that PCBs affect the dopamine transporters (DATs) important in the handling and packaging of DA. DA that has been released into the synapse following neurotransmission is removed through reuptake into the presynaptic terminal by the DAT (Giros and Caron, 1993). Inside the terminal, recycled and newly synthesized DA is transported into vesicles by the vesicular monoamine transporter 2 (VMAT2), where it is made available for release (Liu and Edwards, 1997). Several studies have determined the importance of DAT and VMAT2 in maintaining proper homeostasis of the DA system (Gainetdinov and Caron, 2003; Miller et al., 1999b). Genetic and pharmacological alteration of DAT or VMAT2 levels or function results in a decrease in total tissue DA (Fon et al., 1997; Fumagalli et al., 1999; Giros et al., 1996; Jones et al., 1998; Mooslehner et al., 2001; Sivam, 1995; Wang et al., 1997), suggesting that any chemical that affects DAT and VMAT2 function or expression may significantly impact DA homeostasis.
Previous in vitro studies have suggested that PCBs inhibit DA uptake in synaptosomal and vesicular preparations (Mariussen and Fonnum, 2001; Mariussen et al., 1999). Furthermore, Seegal et al. (2002) showed alterations in extracellular DA concentrations following daily exposure to Aroclor 1254, suggesting inhibition of the DAT by PCBs. Work from our laboratory has shown that high-dose exposure of mice to Aroclor 1016 or 1260 results in significant reductions in striatal DAT and VMAT2 protein levels (Richardson and Miller, 2004). To date, studies with PCBs have focused on loss of striatal DA or nigral DA neurons as the toxicological end points. The goal of this study was to identify the alterations in the DA system that precede the loss of striatal DA or DA neurons. Here, we report that an exposure paradigm which results in PCB brain concentrations similar to those found in postmortem human brains causes a dramatic reduction in DAT expression that precedes overt toxicity. These findings suggest that monitoring DAT expression through currently utilized imaging methods (Chou et al., 2004; Fernandez et al., 2001) may help identify PCB-induced brain alterations.
MATERIALS AND METHODS
Chemicals and Reagents
Aroclor 1254 (Lot 124-191-C) and 1260 (Lot 021-020) were purchased from Accustandard (New Haven, CT). Monoclonal anti-rat DAT antibodies, polyclonal anti-rabbit antibodies to GABA transporter 1 (GAT-1), excitatory amino acid transporter 1 (EAAT-1), excitatory amino acid transporter 2 (EAAT-2), TH, and VMAT2 were purchased from Chemicon (Temecula, CA). Monoclonal anti-mouse α-tubulin antibodies were purchased from Sigma (St Louis, MO), and monoclonal anti-mouse TH was purchased from Chemicon. Secondary antibodies conjugated to horseradish peroxidase were obtained from MP Biomedical, Inc. (antirat; Irvine, CA) and Bio Rad (anti-mouse and anti-rabbit; Hercules, CA). Secondary antibodies conjugated to biotin were purchased from Jackson Immunoresearch Labs (West Grove, PA). SuperSignal West Dura Extended duration substrate and stripping buffer were obtained from Pierce (Rockford, IL). Avidin-biotin complex (ABC) solution was purchased from Vector Labs (Burlingame, CA). 3,3′-Diaminobenzidine tetrachloride (DAB) was purchased from Sigma. Monoamine standards for DA, dihydroxyphenylacetic acid (DOPAC), and homovanillic acid (HVA) were purchased from Sigma. Mobile phase was obtained from ESA Inc (Chelmsford, MA). 3H-Dopamine (3H-DA), 3H-nisoxetine, 3H-paroxetine, and 3H-WIN 35,428 were purchased from Perkin-Elmer (Boston, MA). Cold tetrabenazine, nomifensine, fluoxetine, and desipramine were obtained from Sigma.
Animals and Treatment
Eight-week-old male C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were maintained on a 12:12 light/dark cycle with food and water available ad libitum. All procedures were conducted in accordance with the Guide for Care and Use of Laboratory Animals (National Institutes of Health) and approved by the Institutional Animal Care and Use Committee at Emory University.
Mice were orally gavaged with 30 μl of 0, 7.5, or 15 mg/kg of a 1:1 mixture of Aroclor 1254 and 1260 dissolved in corn oil vehicle daily for 3, 7, 14, or 30 days. These concentrations and exposure time points were based on previous studies performed in rats (Kodavanti et al., 1998; Seegal et al., 2002) in which no change in total DA levels was observed. Oral exposure was chosen since the most common route of human exposure to PCBs is through the ingestion of contaminated food (ATSDR, 2000). The mixture of Aroclor 1254 and 1260 was chosen because the congener profile determined in Great Lakes fish exhibited high homology to the 1:1 mixture of Aroclor 1254 and 1260 (Seegal et al., 1998) and represents an environmentally relevant mixture. Mice were sacrificed one day following the last exposure, and tissue was collected for analysis.
Gas Chromatography
Determination of PCBs in mouse brain tissue was performed following the extraction procedure described by Corrigan et al. (2000) and analytical method of Harju et al. (2003). It has previously been determined that PCBs distribute ubiquitously throughout the brain (Kodavanti et al., 1998); thus, we analyzed whole-brain samples lacking only the striatum, which were taken for neurochemical analysis. Approximately 200 mg of wet tissue was placed in an amber glass vial to which 5 ml of a 1:1 mixture of acetone and hexane, containing hexachlorobenzene as an internal standard, was added. The tissue was homogenized using a Tissue Tearer (Biospec Products, Bartlesville, OK, Model no. 985370) for approximately 3 min, placed in a sonication bath for 15 min, and mixed for an additional 2 min using a minivortex (VWR Scientific Products, Atlanta, GA). The supernatant was separated by centrifugation for 10 min at 2500 rpm and transferred to a 35-ml borosilicate glass centrifuge tube using a disposable glass Pasteur pipette. The extraction sequence was performed five times to yield a total extract volume of approximately 25 ml. The contents of each tube were reduced by heating at 50°C in an aluminum block, weighed to obtain the dry residue mass, and redissolved in 1 ml of 1:1 hexane:acetone. The reduced extract was transferred to a 25-ml, solid-phase extraction column containing 5 g of Florisil and 1 g of anhydrous sodium sulfate to remove lipids. The column was eluted with 5 ml of hexane five times to obtain a final volume of approximately 25 ml. The filtered extract was then reduced to 1 ml at 50°C and transferred to a glass autosampler vial, which was immediately sealed with a crimp cap and stored at 4°C.
The tissue extracts were analyzed for PCBs using a Hewlett Packard (HP) 6890N gas chromatograph (GC) equipped with a micro-electron capture detector. Congeners were separated using a DB-XLB capillary column (60 m length × 0.25 mm diameter, J&W Scientific, Mountain View, CA) operated at an initial oven temperature of 80°C, increased to 140°C (30°C/min), and subsequently increased to 230°C (3°C/min). Of the 125 PCB congeners present in the 1254:1260 mixture, the 66 most prominent, which accounted for 97% of the total mass, were resolved. Chromatograph peaks were identified by comparison of retention times with individual and mixed PCB standards (Accustandard) and verified by analysis of selected samples using a Varian Star 3400 GC equipped with a Saturn 2000 mass spectrometer. Individual PCB congener levels were computed from five-point calibration curves prepared in hexane over relevant concentration ranges. The method detection limit was approximately 0.02 μg/g, expressed per weight of wet tissue.
Western Blot Analysis
Western blots were used to quantify the amount of DAT, TH, VMAT2, and α-tubulin present in samples of striatal tissue from treated and control mice. Analysis was performed as previously described (Caudle et al., 2005). Briefly, striata samples were homogenized and samples subjected to polyacrylamide gel electrophoresis and electrophoretically transferred to polyvinylidene difluoride membranes. Nonspecific sites were blocked in 7.5% nonfat dry milk in Tris-buffered saline and then membranes incubated overnight in a monoclonal antibody to the N-terminus of DAT (1:5000) (Miller et al., 1997). DAT antibody binding was detected using a goat anti-rat horseradish peroxidase secondary antibody (1:10,000) and enhanced chemiluminescence. The luminescence signal was captured on an Alpha Innotech Fluorochem imaging system and stored as a digital image. Densitometric analysis was performed and calibrated to coblotted dilutional standards of pooled striata from all control samples. Membranes were stripped for 15 min at room temperature with Pierce Stripping Buffer and sequentially reprobed with GAT-1 (1:1000), EAAT-1 (1:50,000), EAAT-2 (1:50,000), α-tubulin (1:1000), TH (1:1000), and VMAT2 (1:1000) antibodies. α-Tubulin blots were used to ensure equal protein loading across samples.
Synaptosomal 3H-DA Uptake
DA uptake studies were performed as previously described (Elwan et al., 2006). Briefly, crude synaptosomes were prepared from fresh striatal tissue and incubated in assay buffer (4mM Tris, 6.25mM HEPES, 120mM NaCl, 5mM KCl, 1.2mM CaCl2, 1.2mM MgSO4, 0.6mM ascorbic acid, 5.5mM glucose, 10μM pargyline; pH 7.4) containing a saturating concentration of DA (1μM final concentration) and a tracer amount of 3H-DA (20nM). Uptake was allowed to proceed for 3 min at 37°C and then terminated by the addition of ice-cold buffer and rapid vacuum filtration over GF/B filter paper using a Brandel harvester. Filters were washed twice more with buffer, allowed to air dry, and placed in scintillation vials containing 8 ml of Econoscint (Fisher Scientific, Pittsburgh, PA) for scintillation counting. Uptake rates were calculated as specific uptake (total uptake − nonspecific uptake), with nonspecific uptake defined by the inclusion of 10μM nomifensine. Following determination of protein concentrations (Bradford, 1976), uptake rates were calculated as pmol/min/mg protein and expressed as percent change from control.
Synaptosomal 3H-WIN 35,428 Binding
Determination of 3H-WIN 35,428 binding to DAT (Elwan et al., 2006) was performed essentially as described by Coffey and Reith (1994) with modifications to reduce the total volume to 200 μl, for assay in 96-well microtiter plates. Preliminary studies have indicated that the binding of 3H-WIN 35,428 to striatal synaptosomes is best fit to a one-site model determined by nonlinear curve-fitting techniques (GraphPad Prism 3.0) with a Kd of 6.58nM and a Bmax of 1.08 μmol/mg protein. Therefore, binding studies with crude striatal synaptosomes were conducted with a single concentration (10nM) of 3H-WIN 35,428 in 25mM sodium phosphate buffer (125mM NaCl, 5mM KCl, pH 7.4) for 1 h at 4°C in 96-well plates. Incubations were terminated by rapid vacuum filtration onto GF/B filter plates, and radioactivity was determined by liquid scintillation counting. Nonspecific binding was determined by the inclusion of 10μM nomifensine and specific binding calculated as the total binding (incubated without 10μM nomifensine) minus nonspecific binding (incubated with nomifensine). Following determination of protein concentrations (Bradford, 1976), binding to DAT was calculated as fmol/mg protein and expressed as percent change from control.
Immunohistochemistry
Immunohistochemical analysis of tissue was performed as previously described by Miller et al. (1999a). Briefly, animals were anesthetized and perfused transcardially with 4% paraformaldehyde. Brains were removed and serially sectioned at 25μM on a freezing microtome. Tissue was rinsed in 1× TBS and incubated in 3% H2O2 to remove endogenous peroxidases. Sections were blocked in 10% normal goat serum for 1 h at room temperature before being incubated overnight with monoclonal rat anti-DAT (1:750) or monoclonal mouse anti-TH (1:2000) primary antibodies. The following day, tissue was rinsed in 1× TBS and then incubated for 1 h in goat anti-rat (1:200) or goat anti-mouse (1:200) secondary antibody conjugated to biotin. Tissue was rinsed in 1× TBS and incubated for 1 h in ABC solution. Tissue was rinsed in 1× TBS and the final product visualized using DAB. Free-floating slices were mounted onto slides, serially dehydrated in ethanol, and coverslipped. Immunostained sections were analyzed using bright-field microscopy and images captured on a Leitz microscope (Leica, Wetzlar, Germany).
High Performance Liquid Chromotography-Electroechemical
High Performance Liquid Chromotography-Electroechemical (HPLC-EC) neurochemical analysis was performed as previously described by Richardson and Miller (2004). Briefly, dissected left striata were sonicated in 0.1M perchloric acid containing 347μM sodium bisulfite and 134μM EDTA. Homogenates were centrifuged at 15,000 × g for 10 min at 4°C, and the supernatant was removed and filtered through a 0.22-μm filter by centrifugation at 15,000 × g for 10 min. The supernatants were then analyzed for levels of DA, DOPAC, and HVA. Levels were measured using HPLC with an eight-channel coulometric electrode array (ESA Coularray). Quantification was made by reference to calibration curves made with individual monoamine standards.
TH Activity
TH activity was measured as described by Carlsson et al. (1972). Briefly, mice were injected ip with 100 mg/ml of the AADC inhibitor NSD 1015 and sacrificed 1 h later. Striata were removed bilaterally and prepared as described above for HPLC-EC. TH activity was determined by measuring the accumulation of L-DOPA (3,4-dihydroxy-L-phenylalanine).
3H-Nisoxetine and 3H-Paroxetine Binding
Determination of 3H-nisoxetine and 3H-paroxetine binding to the norepinephrine transporter (NET) and the serotonin transporter (SERT) in the frontal cortex and striatum was performed essentially as described by Tejani-Butt et al. (1990) and Mellerup et al. (1983), respectively, with modifications to reduce the total volume to 200 μl for the assay in 96-well microtiter plates. Briefly, samples from the frontal cortex of control and treated animals were incubated with a single concentration of 3H-nisoxetine (5nM) for 3–4 h at 4°C or a single concentration of 3H-paroxetine (10nM) for 1 h at 25°C in 96-well plates. Incubations were terminated by rapid vacuum filtration onto GF/B filter plates, and radioactivity was determined by liquid scintillation counting. Nonspecific binding was determined by the inclusion of 1μM desipramine (3H-nisoxetine binding) or 1nM fluoxetine (3H-paroxetine binding) and specific binding calculated as total binding (incubated without desipramine or fluoxetine) minus nonspecific binding (incubated with desipramine or fluoxetine). Determination of 3H-paroxetine binding in the striatum was performed as described for the frontal cortex. Binding to SERT and NET was calculated as fmol/mg protein and reported as raw values.
Dopamine Transporter mRNA
RNA isolation and cDNA synthesis.
RNA was isolated from ventral mesencephalon from control and treated animals using the Qiagen RNEasy Lipid Tissue Mini Kit (Valencia, CA) according to instructions by the manufacturer. RNA concentration was determined by standard spectrophotometric analysis and 1 μg of total RNA used for cDNA synthesis with the Applied Biosystems High Capacity cDNA Archive kit (Bedford, MA) according to the manufacturer's protocol.
Quantitative reverse transcriptase PCR (real-time PCR).
Primers for mouse DAT (forward [5′–3′] atcaacccaccgcagacaccagt and reverse [5′–3′] ggcatcccggcaataaccat) were designed using the Primer Select software program (Lasergene) and synthesized by MWG Biotech, Inc (High Point, NC). Real-time PCR was performed using the ABI PRISM 7000 Sequence Detection System (Applied Biosystems) to determine mRNA expression in ventral mesencephalon. Reactions were performed in a total volume of 25 μl using SyBr Green Master Mix reagent (Applied Biosystems); 2 μl of cDNA per sample was used as template for the reaction with 10μM forward and reverse primers. Both target and 18S amplifications were performed in duplicate. Thermal cycling conditions included 2 min at 50°C and 10 min at 95°C followed by 40 cycles of 95°C for 15 s and 1 min at the appropriate annealing temperature for each primer set. To normalize the amount of total mRNA present in each reaction, levels of the 18S ribosomal subunit were monitored in parallel samples. Results are expressed as relative levels of mRNA, referred to as control samples (the calibrator), chosen to represent 1× expression of the gene. The amount of target (treated sample), normalized to an endogenous reference (18S) and relative to the calibrator (control sample), was defined by the Ct method as described by Livak and Schmittgen (2001). All primer sets yielded a single PCR product of expected size by agarose gel electrophoresis, and specificity was routinely monitored by checking product melting curves (dissociation curves) in each reaction well.
Statistical Analysis
All statistical analysis was performed on raw data for each treatment group by one-way ANOVA. Post hoc analysis was performed using Student-Newman-Keuls test. Statistical significance is reported at the p < 0.05 level.
RESULTS
We chose a paradigm in which eight-week-old male C57BL/6J mice were exposed to a dose of a 1:1 mixture of Aroclor 1254:1260 by oral gavage at concentrations of 0, 7.5, or 15 mg/kg for 3, 7, 14, or 30 days and examined the effect on the nigrostriatal DA system. This dosing paradigm is lower than the lowest levels previously shown to decrease DA and results in tissue concentrations of PCBs similar to those found in postmortem human brain (DeWailly et al., 1999). Administration of Aroclor 1254:1260 daily for the indicated times resulted in no overt signs of toxicity, including no significant changes in body or brain weight, and no change in appearance or gross behavior (data not shown).
Analysis of PCB Congener Levels in the Brain of Mice Exposed to Aroclor 1254:1260 for 30 Days
Studies have determined that the PCB congeners 118, 138, 153, and 180 accumulate in the brain of exposed animals to a greater degree than other congeners (Kodavanti et al., 1998). Thus, using GC, we determined the concentrations of these congeners in the brains of mice treated with 7.5 or 15 mg/kg of Aroclor 1254:1260 for 30 days (Table 1). Upon analysis of all congeners present in the brain, we found PCB congeners 95, 118, 138, 153, 170, and 180 to be at higher concentrations at both the 7.5- and 15-mg/kg concentrations.
Mice Exposed to Aroclor 1254:1260 at Concentrations of 0, 7.5, or 15 mg/kg for 30 Days Show an Increase in Total Brain Concentration of PCBs as well as PCB Congeners. Data Are Expressed as Mean ± SEM (n = 3–5). Estimated Total PCB Levels Based on the Sum of 44 Congeners, Representing Approximately 91% of the Injected Mass
. | Mean PCB congener levels (μg/g wet weight) . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | 95 . | 118 . | 138 . | 153 . | 170 . | 180 . | Estimated Total PCB . | ||||||
| Control | 0.15 ± 0.34 | 0.06 ± 0.02 | 0.47 ± 0.08 | 0.49 ± 0.15 | 0.34 ± 0.06 | 1.12 ± 0.15 | 6.18 ± 0.87 | ||||||
| 7.5 mg/kg | 2.13 ± 0.56 | 1.19 ± 0.22 | 6.12 ± 1.43 | 7.42 ± 1.81 | 3.53 ± 0.89 | 10.40 ± 2.54 | 67.58 ± 14.37 | ||||||
| 15 mg/kg | 3.02 ± 0.91 | 1.94 ± 0.44 | 9.26 ± 2.12 | 7.45 ± 2.10 | 6.22 ± 1.66 | 15.55 ± 3.45 | 113.07 ± 16.63 | ||||||
. | Mean PCB congener levels (μg/g wet weight) . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | 95 . | 118 . | 138 . | 153 . | 170 . | 180 . | Estimated Total PCB . | ||||||
| Control | 0.15 ± 0.34 | 0.06 ± 0.02 | 0.47 ± 0.08 | 0.49 ± 0.15 | 0.34 ± 0.06 | 1.12 ± 0.15 | 6.18 ± 0.87 | ||||||
| 7.5 mg/kg | 2.13 ± 0.56 | 1.19 ± 0.22 | 6.12 ± 1.43 | 7.42 ± 1.81 | 3.53 ± 0.89 | 10.40 ± 2.54 | 67.58 ± 14.37 | ||||||
| 15 mg/kg | 3.02 ± 0.91 | 1.94 ± 0.44 | 9.26 ± 2.12 | 7.45 ± 2.10 | 6.22 ± 1.66 | 15.55 ± 3.45 | 113.07 ± 16.63 | ||||||
Mice Exposed to Aroclor 1254:1260 at Concentrations of 0, 7.5, or 15 mg/kg for 30 Days Show an Increase in Total Brain Concentration of PCBs as well as PCB Congeners. Data Are Expressed as Mean ± SEM (n = 3–5). Estimated Total PCB Levels Based on the Sum of 44 Congeners, Representing Approximately 91% of the Injected Mass
. | Mean PCB congener levels (μg/g wet weight) . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | 95 . | 118 . | 138 . | 153 . | 170 . | 180 . | Estimated Total PCB . | ||||||
| Control | 0.15 ± 0.34 | 0.06 ± 0.02 | 0.47 ± 0.08 | 0.49 ± 0.15 | 0.34 ± 0.06 | 1.12 ± 0.15 | 6.18 ± 0.87 | ||||||
| 7.5 mg/kg | 2.13 ± 0.56 | 1.19 ± 0.22 | 6.12 ± 1.43 | 7.42 ± 1.81 | 3.53 ± 0.89 | 10.40 ± 2.54 | 67.58 ± 14.37 | ||||||
| 15 mg/kg | 3.02 ± 0.91 | 1.94 ± 0.44 | 9.26 ± 2.12 | 7.45 ± 2.10 | 6.22 ± 1.66 | 15.55 ± 3.45 | 113.07 ± 16.63 | ||||||
. | Mean PCB congener levels (μg/g wet weight) . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | 95 . | 118 . | 138 . | 153 . | 170 . | 180 . | Estimated Total PCB . | ||||||
| Control | 0.15 ± 0.34 | 0.06 ± 0.02 | 0.47 ± 0.08 | 0.49 ± 0.15 | 0.34 ± 0.06 | 1.12 ± 0.15 | 6.18 ± 0.87 | ||||||
| 7.5 mg/kg | 2.13 ± 0.56 | 1.19 ± 0.22 | 6.12 ± 1.43 | 7.42 ± 1.81 | 3.53 ± 0.89 | 10.40 ± 2.54 | 67.58 ± 14.37 | ||||||
| 15 mg/kg | 3.02 ± 0.91 | 1.94 ± 0.44 | 9.26 ± 2.12 | 7.45 ± 2.10 | 6.22 ± 1.66 | 15.55 ± 3.45 | 113.07 ± 16.63 | ||||||
Effects of Subchronic Aroclor 1254:1260 Exposure on Striatal DA and Metabolite Levels, TH Synthesis, and TH Protein Expression
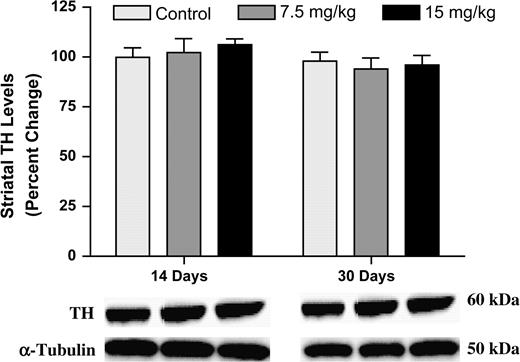
We examined the effects of a subchronic exposure to Aroclor 1254:1260 on DA and its metabolite levels in the striatum, as well as changes to DA synthesis. As shown in Table 2, no changes were seen in striatal DA, DOPAC, or HVA levels. Previous groups have suggested inhibition of DA synthesis rates following exposure to PCBs as a cause for reduction in total DA levels (Angus et al., 1997; Chishti et al., 1996). Thus, we measured DA synthesis to ensure that the lack of change in striatal DA levels we observed were not due to a compensatory increase in DA production. As seen with striatal DA, no change in DA synthesis was observed (control: 30 ± 4.2 pg L-DOPA/min-mg, 7.5 mg/kg: 32 ± 3.4 pg L-DOPA/min/mg, and 15 mg/kg: 33 ± 1.7 pg L-DOPA/min/mg). Finally, no change in TH protein levels was measured by immunoblotting or immunochemical techniques (Figs. 1 and 3B).
Immunoblotting for striatal TH showed no change in protein levels following 14 or 30 days of exposure to Aroclor 1254:1260. Columns represent the percent change from control. Data represent the mean ± SEM (four to five mice per treatment group). Light gray column = control, gray column = 7.5 mg/kg group, and black column = 15 mg/kg group.
Striatal DA, DOPAC, and HVA Levels Following 30 Days of Exposure to 0, 7.5, or 15 mg/kg of Aroclor 1254:1260. Data Represents Raw Values (ng/mg tissue) ± SEM (four to five animals per treatment group)
. | DA . | DOPAC . | HVA . |
|---|---|---|---|
| Control | 21.33 ± 0.77 | 0.96 ± 0.06 | 1.30 ± 0.07 |
| 7.5 mg/kg | 21.76 ± 1.21 | 0.87 ± 0.12 | 1.18 ± 0.13 |
| 15 mg/kg | 20.61 ± 1.05 | 0.97 ± 0.06 | 1.29 ± 0.05 |
. | DA . | DOPAC . | HVA . |
|---|---|---|---|
| Control | 21.33 ± 0.77 | 0.96 ± 0.06 | 1.30 ± 0.07 |
| 7.5 mg/kg | 21.76 ± 1.21 | 0.87 ± 0.12 | 1.18 ± 0.13 |
| 15 mg/kg | 20.61 ± 1.05 | 0.97 ± 0.06 | 1.29 ± 0.05 |
Striatal DA, DOPAC, and HVA Levels Following 30 Days of Exposure to 0, 7.5, or 15 mg/kg of Aroclor 1254:1260. Data Represents Raw Values (ng/mg tissue) ± SEM (four to five animals per treatment group)
. | DA . | DOPAC . | HVA . |
|---|---|---|---|
| Control | 21.33 ± 0.77 | 0.96 ± 0.06 | 1.30 ± 0.07 |
| 7.5 mg/kg | 21.76 ± 1.21 | 0.87 ± 0.12 | 1.18 ± 0.13 |
| 15 mg/kg | 20.61 ± 1.05 | 0.97 ± 0.06 | 1.29 ± 0.05 |
. | DA . | DOPAC . | HVA . |
|---|---|---|---|
| Control | 21.33 ± 0.77 | 0.96 ± 0.06 | 1.30 ± 0.07 |
| 7.5 mg/kg | 21.76 ± 1.21 | 0.87 ± 0.12 | 1.18 ± 0.13 |
| 15 mg/kg | 20.61 ± 1.05 | 0.97 ± 0.06 | 1.29 ± 0.05 |
Effects of Subchronic Aroclor 1254:1260 Exposure on Nigrostriatal DAT Levels and Function
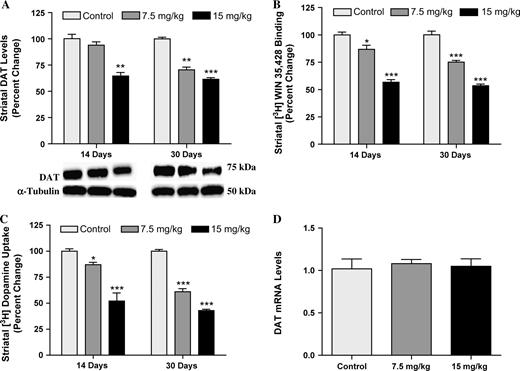
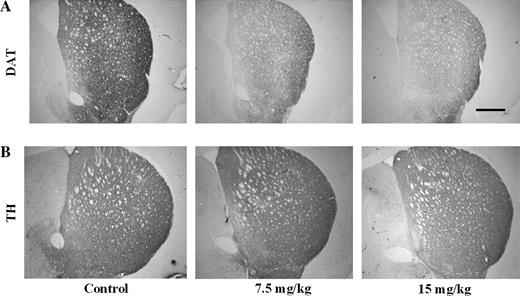
To assess the impact of PCB exposure on the DA system, we looked at the DAT, the phenotypic marker of DA neurons. To determine the earliest time point in which we would detect changes in the DAT in the striatum, we exposed animals to 0, 7.5, or 15 mg/kg of Aroclor 1254:1260 for 3, 7, 14, or 30 days. Animals exposed for 3 or 7 days did not show any changes in DAT levels, as measured by immunoblotting, 3H-WIN 35,428 binding, and 3H-DA uptake (data not shown). However, animals exposed to Aroclor 1254:1260 for 14 days exhibited a 10% reduction in DAT at the 7.5-mg/kg dose and a 35% reduction in DAT at the 15-mg/kg dose (Fig. 2A). In addition, these decreases in DAT were confirmed by 3H-WIN 35,428 binding, which demonstrated a 15 and 35% reduction in DAT at each respective dose (Fig. 2B). Finally, to examine the effects of DAT reduction on the function of the transporter, we performed 3H-DA uptake and observed a 15 and 50% reduction in DA uptake at the 7.5- and 15-mg/kg doses, respectively (Fig. 2C). Similar to the results observed in the 14-day exposure group, we observed a 30 and 40% decrease in DAT levels by immunoblotting following 30 days of exposure to 7.5 and 15 mg/kg, respectively (Fig. 2A). 3H-WIN 35,428 binding showed similar levels of DAT reductions in the striatum (25 and 50%, respectively, for the 7.5- and 15-mg/kg doses) (Fig. 2B). Using 3H-DA uptake, we observed a 40% decrease in DA transport through the DAT with the 7.5-mg/kg dose of Aroclor 1254:1260 and a 60% reduction in DA transport in the 15-mg/kg dose (Fig. 2C). Finally, immunohistochemical analysis revealed a significant reduction in DAT in the 7.5- and 15-mg/kg groups (Fig. 3A).
Mice exposed to Aroclor 1254:1260 at concentrations of 0, 7.5, or 15 mg/kg for 14 or 30 days show a dose-dependent reduction in DAT protein level and function in the striatum. (A) Immunoblotting determination of DAT reductions. (B) 3H-WIN 35,428 binding measurement of DAT decreases. Mice treated for 14 days with Aroclor 1254:1260. Control: 807 ± 20.8, 7.5 mg/kg: 701 ± 29.8, and 15 mg/kg: 458 ± 19.4. Mice treated for 30 days with Aroclor 1254:1260. Control: 810 ± 29.8, 7.5 mg/kg: 669 ± 13.5, and 15 mg/kg: 476 ± 13.2. (C) 3H-DA uptake shows reduction of DA uptake by DAT. Mice treated for 14 days with Aroclor 1254:1260. Control: 230 ± 5.2, 7.5 mg/kg: 200 ± 5.3, and 15 mg/kg: 120 ± 17.8. Mice treated for 30 days with Aroclor 1254:1260. Control: 240 ± 4.7, 7.5 mg/kg: 182 ± 9.0, and 15 mg/kg: 127 ± 3.9. (D) DAT mRNA in the ventral mesencephalon of animals exposed to 0, 7.5, or 15 mg/kg of Aroclor 1254:1260 for 30 days. Columns represent the percent change from control. Data represent mean ± SEM (four to five mice per treatment group). Light gray column = control, gray = 7.5 mg/kg group, and black column = 15 mg/kg group. ***Values for treated animals that are significantly different from controls (p < 0.001). **Values for animals that are significantly different from controls (p < 0.01). *Values for animals that are significantly different from controls (p < 0.05).
Immunohistochemical analysis of striatal DAT and TH following a 30-day exposure to 0, 7.5, or 15 mg/kg of Aroclor 1254:1260. Analysis performed on three to four animals per treatment group. Representative sections are shown; scale bar, 300 μm.
To determine whether the reduction in DAT was the result of reduced mRNA synthesis at the cell body, we performed PCR for DAT on samples isolated from the midbrain of animals exposed to 7.5 or 15 mg/kg of Aroclor 1254:1260 for 30 days. Results from these experiments show no change in DAT mRNA at either concentration (Fig. 2D).
Effects of Subchronic Aroclor 1254:1260 Exposure on DAT Levels in the Frontal Cortex, Hypothalamus, and Midbrain
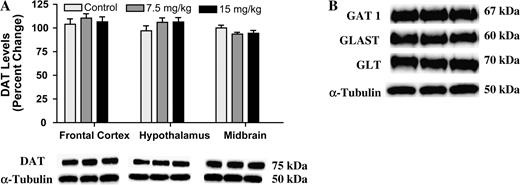
To determine whether the changes in DAT were specific to the striatum, we measured DAT levels in other DA-containing regions of the brain, specifically the frontal cortex, hypothalamus, and midbrain. In contrast to the reductions observed in the striatum, exposure to Aroclor 1254:1260 did not alter DAT levels in the frontal cortex, hypothalamus, or the midbrain (Fig. 4A). In addition to a dense dopaminergic population of neurons, the striatum is composed of several other neurotransmitter populations (GABA; Augood et al., 1995; glutamate; Lehre et al., 1995; serotonin; Boja et al., 1992). We also examined possible changes to other neurotransmitter transporters present in the striatum to determine whether or not the changes were specific to the DA population. Thus, by immunoblotting we were able to determine that exposure to Aroclor 1254:1260 did not alter GAT-1 or two glutamate transporters, EAAT-2 (excitatory amino acid transporter 2) and EAAT-1 (excitatory amino acid transporter 1) in the striatum (Fig. 4B). In addition, through radioligand binding, we also found no change in the SERT in the striatum and frontal cortex or the NET in the frontal cortex (Table 3).
(A) Immunoblotting for DAT in the frontal cortex, hypothalamus, and midbrain of mice treated with 15 mg/kg of Aroclor 1254:1260 for 30 days. Columns represent the percent change from control. Light gray column = control, gray column = 7.5 mg/kg group, and black column = 15 mg/kg group. (B) Immunoblotting for GAT-1, GLAST, and GLT in the striatum following 30 days of exposure to 15 mg/kg of Aroclor 1254:1260 showed no change in protein levels. Data represent the mean ± SEM (four to five mice per treatment group).
SERT and NET Binding in the Frontal Cortex and Striatum of Mice Exposed to Aroclor 1254:1260 for 30 Days. Data Represent the Mean (fmol/mg protein) ± SEM (four to five mice per treatment group)
. | 3H-Nisoxetine and 3H-paroxetine binding in striatum and frontal cortex . | . | . | ||
|---|---|---|---|---|---|
. | 3H-nisoxetine (cortex) . | 3H-paroxetine (cortex) . | 3H-paroxetine (striatum) . | ||
| Control | 98 ± 13 | 380 ± 17 | 360 ± 21 | ||
| 7.5 mg/kg | 100 ± 10 | 386 ± 26 | 359 ± 13 | ||
| 15 mg/kg | 104 ± 11 | 367 ± 20 | 339 ± 45 | ||
. | 3H-Nisoxetine and 3H-paroxetine binding in striatum and frontal cortex . | . | . | ||
|---|---|---|---|---|---|
. | 3H-nisoxetine (cortex) . | 3H-paroxetine (cortex) . | 3H-paroxetine (striatum) . | ||
| Control | 98 ± 13 | 380 ± 17 | 360 ± 21 | ||
| 7.5 mg/kg | 100 ± 10 | 386 ± 26 | 359 ± 13 | ||
| 15 mg/kg | 104 ± 11 | 367 ± 20 | 339 ± 45 | ||
SERT and NET Binding in the Frontal Cortex and Striatum of Mice Exposed to Aroclor 1254:1260 for 30 Days. Data Represent the Mean (fmol/mg protein) ± SEM (four to five mice per treatment group)
. | 3H-Nisoxetine and 3H-paroxetine binding in striatum and frontal cortex . | . | . | ||
|---|---|---|---|---|---|
. | 3H-nisoxetine (cortex) . | 3H-paroxetine (cortex) . | 3H-paroxetine (striatum) . | ||
| Control | 98 ± 13 | 380 ± 17 | 360 ± 21 | ||
| 7.5 mg/kg | 100 ± 10 | 386 ± 26 | 359 ± 13 | ||
| 15 mg/kg | 104 ± 11 | 367 ± 20 | 339 ± 45 | ||
. | 3H-Nisoxetine and 3H-paroxetine binding in striatum and frontal cortex . | . | . | ||
|---|---|---|---|---|---|
. | 3H-nisoxetine (cortex) . | 3H-paroxetine (cortex) . | 3H-paroxetine (striatum) . | ||
| Control | 98 ± 13 | 380 ± 17 | 360 ± 21 | ||
| 7.5 mg/kg | 100 ± 10 | 386 ± 26 | 359 ± 13 | ||
| 15 mg/kg | 104 ± 11 | 367 ± 20 | 339 ± 45 | ||
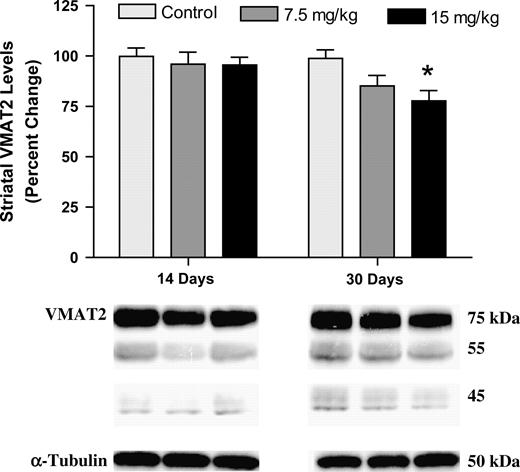
Effects of Subchronic Aroclor 1254:1260 Exposure on Striatal VMAT2 Levels
Newly synthesized DA, as well as DA that has been retrieved from the synapse by DAT, is sequestered into neurotransmitter vesicles by VMAT2 and packaged for release. This action highlights VMAT2 as an important player in DA handling in the cytoplasm. Exposure to Aroclor 1254:1260 for 30 days produced a dose-dependent reduction in VMAT2 levels in the striatum, with a significant reduction of 20% seen with the 15-mg/kg concentration (Fig. 5).
Immunoblotting of striatal VMAT2. Immunoblotting for VMAT2 showed no change in protein levels after 14 days of exposure. Conversely, a significant reduction in VMAT2 was observed in the 15-mg/kg treatment group after 30 days of exposure (asterisk indicates p < 0.01). Data represent the mean ± SEM (four to five mice per treatment group). Light gray column = control, gray column = 7.5 mg/kg group, and black column = 15 mg/kg group.
DISCUSSION
Given the potential link between PD and PCB exposure (Corrigan et al., 1998, 2000; Steenland et al., 2006), we decided to examine the neurochemical events that precede the loss of striatal DA following PCB exposure. In this study we have empirically demonstrated that an exposure paradigm that results in tissue concentrations of PCBs similar to those found in postmortem human brain shows a dose-dependent reduction in DAT and VMAT2 levels in the striatum, leaving striatal DA and TH levels unchanged. Changes to the DAT appear to be specific to the dopaminergic population in the striatum, as no changes to DAT were recorded in other dopaminergic brain regions or in other neurotransmitter transporters in the striatum. These changes demonstrate a marked disruption of nigrostriatal DA function in the absence of overt toxicity and may represent an early event in PCB-induced DA neuron degeneration.
PCBs consist of approximately 209 congeners that differ in the position and number of chlorines present on the phenyl rings. The dopaminergic reductions observed following PCB exposure appear to be due to only a limited number of PCB congeners that have been shown to accumulate in the brain (Seegal et al., 1991a, 1994). These congeners are typically highly chlorinated and ortho substituted. Specifically, it has been determined that congeners 138, 153, and 180 accumulate in the highest concentrations in the striatum of nonhuman primates and rats following exposure to Aroclor 1254 and 1260 (Seegal et al., 1991a,b). In addition, analyses of PCB congeners present in the plasma and adipose tissue of workers occupationally exposed to PCBs have determined PCB 118, 138, 153, and 180 to be present in higher concentrations than other congeners (Wolff et al., 1982). In our study, exposure of mice to 1:1 mixture of Aroclor 1254:1260 resulted in a greater accumulation of PCB 95, 118, 138, 153, 170, and 180 than other congeners found in these mixtures. Similar to our study, Kodavanti et al. (1998) found the more highly chlorinated congeners predominantly collected in the brain, specifically PCB 138 and 153, following a 30-day exposure to 25 mg/kg of Aroclor 1254. Furthermore, the concentrations of the congeners found in our study are in the same range as those observed in postmortem samples collected from human autopsies in Greenland (DeWailly et al., 1999). Although the levels of PCBs that were detected in the mice were higher than those found in the postmortem human samples (Corrigan et al., 1998, 2000; DeWailly et al., 1999), these measurements were taken directly after exposure as opposed to decades after peak exposure in the human population. This suggests that the exposure paradigm used in this study results in a deposition of PCBs in the mouse brain similar to that observed in the human population.
While we were able to detect significant levels of PCB deposition in the brain, we did not observe any reductions of total tissue DA in the striatum or reductions in striatal TH protein levels. While several studies have reported reductions in striatal DA levels following PCB exposure, these studies have generally employed an exposure paradigm that utilized higher concentrations of PCBs or have used chronic dosing paradigms that resulted in a much higher cumulative dose (Richardson and Miller, 2004; Seegal et al., 1991a,b, 1994). The dopaminergic reductions observed in these studies were primarily seen in the substantia nigra, caudate nucleus, and putamen, regions most susceptible to DA loss in PD (Miller et al., 1997). Interestingly, changes to other neurotransmitters were not observed in brain regions expressing serotonergic, glutamatergic, noradrenergic, or GABAergic innervation (Seegal et al., 1990, 1991a). Our data are in agreement with others, who have used a similar dosing paradigm in rats and mice and found neuronal dysfunction in the absence of DA loss (Kodavanti et al., 1998; Seegal et al., 2002). These results are especially striking given the fact that the dosing levels used in our study are lower than those used in previously reported studies. Finally, TH is considered to be a reliable marker of DA neuron terminal integrity and terminal loss. Given the unchanged striatal TH protein levels with the dosing paradigm observed in this study, the alterations to the nigrostriatal DA system were likely not due to neuronal toxicity. Interestingly, we have previously demonstrated an intermediate state of dopaminergic neuron toxicity following a single 500-mg/kg exposure of Aroclor 1260 to mice (Richardson and Miller, 2004). With the single, high-dose exposure, we not only observed a reduction in DAT and VMAT2, similar to the present study, but also observed a decrease in striatal DA. However, no change was seen in TH protein expression. Furthermore, although not as robust as the reductions observed in DAT protein expression, the decrease in VMAT2 protein expression following 30 days of exposure to 15 mg/kg of Aroclor 1254:1260 may serve as an initial sign of DA terminal degeneration. Thus, the reduction in DAT observed in our study may represent a precursory event leading to deficits in DA levels and further damage to the dopaminergic system.
By utilizing a more sensitive end point (DAT), we were able to detect changes in the DA system at a cumulative dose lower than that previously reported (Kodavanti et al., 1998; Seegal, 2003; Seegal et al., 1991a, 1994, 2002). Disruption of the DAT has been shown to lead to a reduction of tissue DA levels, as well as increase the amount of time DA remains in the synapse (Giros et al., 1996; Jones et al., 1998). Thus, the DAT plays an important role in regulating DA homeostasis. Recent studies have utilized single-photon emission tomography to further define the progression of dopaminergic neuronal loss in PD. Utilization of these techniques has consistently shown the ability to measure changes in striatal DAT before the appearance of parkinsonian symptoms in humans, as well as animal models of PD (Chalon et al., 1999; Chou et al., 2004; Fernandez et al., 2001; Prunier et al., 2003). The implications of this concept are substantial given that it defines a period in which DA depletion progresses without symptoms.
In conclusion, exposure of mice to short-term, low-levels of Aroclor 1254:1260 results in a congener profile and concentration similar to those found in human brain samples. These similar concentrations result in inhibition of the DAT and VMAT2 (Mariussen and Fonnum, 2001; Mariussen et al., 1999; our unpublished observations). We propose that the prolonged inhibition of VMAT2 and DAT by PCBs leads to altered DA compartmentalization (Miller et al., 1999b) and thereby increased oxidative damage (Hastings et al., 1996; Lee and Opanashuk, 2004). This is followed by downregulation of DAT, and to a lesser extent, VMAT2. Together, these changes decrease the recycling of DA, which over time should result in reduced striatal DA content. The prolonged disruption of DA homeostasis is likely to increase the vulnerability of the DA neuron and ultimately result in a higher degree of cell death. These data provide a plausible mechanism by which PCBs damage the nigrostriatal DA system and lead to an increased incidence of PD. Finally, monitoring of DAT expression in the striatum could serve as a biomarker of PCB exposure, but it should be noted that interpreting such a reduction as a sign of DA terminal loss is not warranted.
This work was supported by National Institutes of Health grants U54 ES012068 as part of the Collaborative Centers for Parkinson's Disease Environmental Research (G.W.M.), F32ES013457 (J.R.R.), and an EPA STAR Fellowship (T.S.G.).
References
Agency for Toxic Substances and Disease Registry (ATSDR). (
Angus, W. G., and Contreras, M. L. (
Angus, W. G., Mousa, M. A., Vargas, V. M., Quensen, J. F., Boyd, S. A., and Contreras, M. L. (
Augood, S. J., Herbison, A. E., and Emson, P. C. (
Boja, J. W., Mitchell, W. M., Patel, A., Kopajtic, T. A., Carroll, F. I., Lewin, A. H., Abraham, P., and Kuhar, M. J. (
Bradford, M. M. (
Carlsson, A., Davis, J. N., Kehr, W., Lindqvist, M., and Atack, C. V. (
Caudle, W. M., Richardson, J. R., Wang, M., and Miller, G. W. (
Chalon, S., Emond, P., Bodard, S., Vilar, M.-P., Thiercelin, C., Besnard, J.-C., and Guilloteau, D. (
Chishti, M. A., Fisher, J. P., and Seegal, R. F. (
Chou, K. L., Hurtig, H. I., Stern, M. B., Colcher, A., Ravina, B., Newberg, A., Mozley, P. D., and Siderowf, A. (
Coffey, L. L., and Reith, M. E. (
Corrigan, F. M., Murray, L., Wyatt, C. L., and Shore, R. F. (
Corrigan, F. M., Wienburg, C. L., Shore, R. F., Daniel, S. E., and Mann, D. (
DeWailly, E., Mulvad, G., Pedersen, H. S., Ayotte, P., Demers, A., Weber, J. P., and Hansen, J. C. (
Elwan, M. A., Richardson, J. R., Guillot, T. S., Caudle, W. M., and Miller, G. W. (
Faroon, O. M., Jones, D., and de Rosa, C. (
Fernandez, H. H., Friedman, J. H., Fischman, A. J., Noto, R. B., and Lannon, M. C. (
Fon, E. A., Pothos, E. N., Sun, B. C., Killeen, N., Sulzer, D., and Edwards, R. H. (
Fumagalli, F., Gainetdinov, R. R., Wang, Y. M., Valenzano, K. J., Miller, G. W., and Caron, M. G. (
Gainetdinov, R. R., and Caron, M. G. (
Giros, B., and Caron, M. G. (
Giros, B., Jaber, M., Jones, S. R., Wightman, R. M., and Caron, M. G. (
Harju, M., Berman, A., Olsson, M., Roos, A., and Haglund, P. (
Hastings, T. G., Lewis, D. A., and Zigmond, M. J. (
Jones, S. R., Gainetdinov, R. R., Jaber, M., Giros, B., Wightman, R. M., and Caron, M. G. (
Kamrin, M. A., and Ringer, R. K. (
Kodavanti, P. R., Ward, T. R., Derr-Yellin, E. C., Mundy, W. R., Casey, A. C., Bush, B., and Tilson, H. A. (
Lee, D. W., and Opanashuk, L. A. (
Lehre, K. P., Levy, L. M., Ottersen, O. P., Storm-Mathisen, J., and Danbolt, N. C. (
Liu, Y., and Edwards, R. H. (
Livak, K. J., and Schmittgen, T. D. (
Mariussen, E., and Fonnum, F. (
Mariussen, E., Morch Andersen, J., and Fonnum, F. (
Mellerup, E. T., Plenge, P., and Engelstoft, M. (
Miller, G. W., Erickson, J. D., Perez, J. T., Penland, S. N., Mash, D. C., Rye, D. B., and Levey, A. I. (
Miller, G. W., Gainetdinov, R. R., Levey, A. I., and Caron, M. G. (
Miller, G. W., Staley, J. K., Heilman, C. J., Perez, J. T., Mash, D. C., Rye, D. B., and Levey, A. I. (
Mooslehner, K. A., Chan, P. M., Xu, W., Liu, L., Smadja, C., Humby, T., Allen, N. D., Wilkinson, L. S., and Emson, P. C. (
Priyadarshi, A., Khuder, S. A., Schaub, E. A., and Shrivastava, S. (
Prunier, C., Bezaed, E., Montharu, J., Mantzarides, M., Besnard, J.-C., Baulieu, J.-L., Gross, C., Guilloteau, D., and Chalon, S. (
Richardson, J. R., and Miller, G. W. (
Safe, S. (
Seegal, R. F., Brosch, K. O., and Bush, B. (
Seegal, R. F., Brosch, K. O., Bush, B., Ritz, M., and Shain, W. (
Seegal, R. F., Brosch, K. O., and Shain, W. (
Seegal, R. F., Bush, B., and Brosch, K. O. (
Seegal, R. F., Bush, B., and Brosch, K. O. (
Seegal, R. F., Bush, B., and Brosch, K. O. (
Seegal, R. F., Okoniewski, R. J., Brosch, K. O., and Bemis, J. C. (
Seegal, R. F., Pappas, B. A., and Park, G. A. S. (
Shain, W., Bush, B., and Seegal, R. (
Sivam, S. P. (
Steenland, K., Hein, M. J., Cassinelli, R. T., II, Prince, M. M., Nilsen, M. B., Whelan, E. A., Waters, M. A., Ruder, A. M., and Schorr, T. M. (
Tanner, C. W., and Goldman, S. M. (
Tanner, C. W., and Langston, J. W. (
Tanner, C. W., Ottman, R., Goldman, S. M., Ellenberg, J., Chan, P., Mayeux, R., and Langston, J. W. (
Tejani-Butt, S. M., Brunswick, D. J., and Frazer, A. (
Wang, Y. M., Gainetdinov, R. R., Fumagalli, F., Xu, F., Jones, S. R., Bock, C. B., Miller, G. W., Wightman, R. M., and Caron, M. G. (
Author notes
*Center for Neurodegenerative Disease, School of Medicine and †Department of Environmental and Occupational Health, Rollins School of Public Health, Emory University, Atlanta, Georgia 30322-3090; ‡Environmental and Occupational Health Sciences Institute and Department of Environmental and Occupational Medicine, University of Medicine and Dentistry-New Jersey/Robert Wood Johnson Medical School, Piscataway, New Jersey 08854; and §School of Civil and Environmental Engineering, Georgia Institute of Technology, Atlanta, Georgia 30332




Comments