Abstract
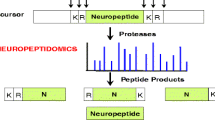
Neuropeptides serve many important roles in communication between cells and are an attractive target for drug discovery. Neuropeptides are produced from precursor proteins by selective cleavages at specific sites, and are then broken down by further cleavages. In general, the biosynthetic cleavages occur within the cell and the degradative cleavages occur postsecretion, although there are exceptions where intracellular processing leads to inactivation, or extracellular processing leads to activation of a particular neuropeptide. A relatively small number of peptidases are responsible for processing the majority of neuropeptides, both inside and outside of the cell. Thus, inhibition of any one enzyme will lead to a broad effect on several different neuropeptides and this makes it unlikely that such inhibitors would be useful therapeutics. However, studies with mutant animals that lack functional peptide-processing enzymes have facilitated the discovery of novel neuropeptides, many of which may be appropriate targets for therapeutics.
Similar content being viewed by others
References
Clynen E, De Loof A, Schoofs L. The use of peptidomics in endocrine research.Gen Comp Endocrinol. 2003;132:1–9.
Strand FL. Neuropeptides: general characteristics and neuropharmaceutical potential in treating CNS disordersProg Drug Res. 2003;61:1–37.
Tanaka H, Yoshida T, Miyamoto N, et al. Characterization of a family of endogenous neuropeptide ligands for the G protein-coupled receptors GPR7 and GPR8.Proc Natl Acad Sci USA. 2003;100:6251–6256.
Docherty K, Steiner DF. Post-translational proteolysis in polypeptide hormone biosynthesis.Annu Rev Physiol. 1982;44:625–638.
Lindberg I, Hutton JC. Peptide processing proteinases with selectivity for paired basic residues. In: Fricker LD, ed.Peptide Biosynthesis and Processing. Boca Raton, FL: CRC Press; 1991:141–174.
Devi L. Peptide processing at monobasic sites. In: Fricker LD, ed.Peptide Biosynthesis and Processing. Boca Raton, FL: CRC Press; 1991:175–198.
Zhou A, Webb G, Zhu X, Steiner DF. Proteolytic processing in the secretory pathway.J Biol Chem. 1999;274:20745–20748.
Seidah NG, Prat A. Precursor convertases in the secretory pathway, cytosol and extracellular milieu.Essays Biochem.2002;38:79–94.
Che F-Y, Yan L, Li H, Mzhavia N, Devi L, Fricker LD. Identification of peptides from brain and pituitary ofCpe fat/Cpefat mice.Proc Natl Acad Sci USA. 2001;98:9971–9976.
Sigafoos J, Chestnut WG, Merrill BM, Taylor LCE, Diliberto EJ, Viveros OH. Novel peptides from adrenomedullary chromaffin vesicles.J Anat. 1993;183:253–264.
Vilim FS, Aarnisalo AA, Nieminen M, et al. Gene for pain modulatory neuropeptide NPFF: induction in spinal cord by noxious stimuli.Mol Pharmacol. 1999;55:804–811.
Che F-Y, Fricker LD. Quantitation of neuropeptides inCpe fat/Cpefat mice using differential isotopic tags and mass spectrometry.Anal Chem. 2002;74:3190–3198.
Che F-Y, Fricker LD. Quantitative peptidomics of mouse pituitary: comparison of different stable isotopic tags.J Mass Spectrom. 2005;40:238–249.
Che F-Y, Biswas R, Fricker LD. Relative quantitation of peptides in wild type andCpefat/fat mouse pituitary using stable isotopic tags and mass spectrometry.J Mass Spectrom. 2005;40:227–237.
Pan H, Nanno D, Che FY, et al. Neuropeptide processing profile in mice lacking prohormone Convertase-1.Biochemistry. 2005;44:4939–4948.
Che FY, Yuan Q, Kalinina E, Fricker LD. Peptidomics of Cpefat/fat mouse hypothalamus: effect of food deprivation and exercise on peptide levels.J Biol Chem. 2005;280:4451–4461.
Smeekens SP, Steiner DF. Identification of a human insulinoma cDNA encoding a novel mammalian protein structurally related to the yeast dibasic processing protease Kex2.J Biol Chem. 1990;265:2997–3000.
Seidah NG, Marcinkiewicz M, Benjannet S, et al. Cloning and primary sequence of a mouse candidate prohormone convertase PC1 homologous to PC2, furin, and Kex2: distinct chromosomal localization and messenger RNA distribution in brain and pituitary compared to PC2.Mol Endocrinol. 1991;5:111–122.
Smeekens SP, Avruch AS, LaMendola J, Cham SJ, Steiner DF. Identification of a cDNA encoding a second putative prohormone convertase related to PC2 in AtT-20 cells and islets of Langerhans.Proc Natl Acad Sci USA. 1991;88:340–344.
Schafer MKH, Day R, Cullinan WE, Chretien M, Seidah NG, Watson SJ. Gene expression of prohormone and proprotein convertases in the rat CNS: a comparative in situ hybridization analysis.J Neurosci. 1993;13:1258–1279.
Braks JAM, Martens GJM. 7B2 is a neuroendocrine chaperone that transiently interacts with prohormone convertase PC2 in the secretory pathway.Cell. 1994;78:263–273.
Fricker LD, McKinzie AA, Sun J, et al. Identification and characterization of proSAAS, a granin-like neuroendocrine peptide precursor that inhibits prohormone processing.J Neurosci. 2000;20:639–648.
Zhu X, Rouille Y, Lamango NS, Steiner DF, Lindberg I. Internal cleavage of the inhibitory 7B2 CT peptide by PC2: a potential mechanism for its inactivation.Proc Natl Acad Sci USA 1996;93:4919–4924.
Cameron A, Fortenberry Y, Lindberg I. The SAAS granin exhibits structural and functional homology to 7B2 and contains a highly potent hexapeptide inhibitor of PC1.FEBS Lett. 2000;473:135–138.
Furuta M, Yano H, Zhou A, et al. Defective prohormone processing and altered pancreatic islet morphology in mice lacking active SPC2.Proc Natl Acad Sci USA. 1997;94:6646–6651.
Furuta M, Zhou A, Webb G, et al. Severe defect in proglucag on processing in islet A-cells of prohormone convertase 2 null mice.J Biol Chem. 2001;276:27197–27202.
Zhu X, Orci L, Carroll R, Norrbom C, Ravazzola M, Steiner DF. Severe block in processing of proinsulin to insulin accompanied by elevation of des-64,65 proinsulin internediates in islets of mice lacking prohormone convertase 1/3.Proc Natl Acad Sci USA. 2002;99:10299–10304.
Zhou A, Bloomquist BT, Mains RE. The prohormone convertases PC1 and PC2 mediate distinct endoproteolytic cleavages in a strict temporal order during proopiomelanocortin biosynthetic processing.J Biol Chem. 1993;268:1763–1769.
Breslin MB, Lindberg I, Benjannet S, Mathis JP, Lazure C, Seidah NG. Differential processing of proenkephalin by prohormone convertases 1(3) and 2 and furin.J Biol Chem. 1993;268:27084–27093.
Zhou A, Mains RE. Endoproteolytic processing of proopiomelanocortin and prohormone convertases 1 and 2 in neuroendocrine cells overexpressing prohormone convertases 1 or 2J Biol Chem. 1994;269:17440–17447.
Fricker LD, Snyder SH. Enkephalin convertase: purification and characterization of a specific enkephalin-synthesizing carboxypeptidase localized to adrenal chromaffin granules.Proc Natl Acad Sci USA. 1982;79:3886–3890.
Fricker LD. Carboxypeptidase E.Annu Rev Physiol. 1988;50:309–321.
Aloy P, Companys V, Vendrell J, et al. The crystal structure of the inhibitor-complexed carboxypeptidase D domain II as a basis for the modelling of regulatory carboxypeptidases.J Biol Chem. 2001;276:16177–16184.
Nalamachu SR, Song L, Fricker LD. Regulation of carboxypeptidase E: effect of Ca2+ on enzyme activity and stability.J Biol Chem. 1994;269:11192–11195.
Fricker LD, Snyder SH. Purification and characterization of enkephalin convertase, an enkephalin-synthesizing carboxypeptidase.J Biol. Chem. 1983;258:10950–10955.
Smyth DG, Maruthainar K, Darby NJ, Fricker LD. C-terminal processing of neuropeptides: involvement of carboxypeptidase H.J Neurochem. 1989;53:489–493.
Chen H, Jawahar S, Qian Y, et al. A missense polymorphism in the human carboxypeptidase E gene alters its, enzymatic activity: possible implications in type 2 diabetes mellitus.Hum Mutat. 2001;18:120–131.
Naggert JK, Fricker LD, Varlamov O, et al. Hyperproinsulinemia in obesefat/fat mice associated with a point mutation in the carboxypeptidase E gene and reduced carboxypeptidase E activity in the pancreatic islets.Nat Genet. 1995;10:135–142.
Varlamov O, Leiter EH, Fricker LD. Induced and spontaneous mutations at Ser202 of carboxypeptidase E: effect on enzyme expression, activity, and intracellular routing.J Biol Chem. 1996;271:13981–13986.
Fricket LD, Berman YL, Leiter EH, Devi LA. Carboxypeptidase E activity is deficient in mice with the fat mutation: effect on peptide processing.J Biol Chem. 1996;271:30619–30624.
Rovere C, Viale A, Nahon J, Kitabgi P. Impaired processing of brain proneurotensin and promelanin-concentrating hormone in obesefat/fat mice.Endocrinology. 1996;137:2954–2958.
Udupi V, Gomez P, Song L, et al. Effect of carboxypeptidase E deficiency on progastrin processing and gastrin mRNA expression in mice with the fat mutation.Endocrinology. 1997;138:1959–1963.
Cain BM, Wang W, Beinfeld MC. Cholecystokinin (CCK) levels are greatly reduced in the brains but not the duodenums ofCpe fat/Cpefat mice: a regional difference in the involvement of carboxypeptidase E (Cpe) in pro-CCK processing.Endocrinology. 1997;138:4034–4037.
Song L, Fricker LD. Purification and characterization of carboxypeptidase D, a novel carboxypeptidase E-like enzyme, from bovine pituitary.J Biol Chem. 1995;270:25007–25013.
Varlamov O, Fricker LD. Intracellular trafficking of metallocarboxypeptidase D in AtT-20 cells: localization to the trans-Golgi network and recycling from the cell surface.J Cell Sci. 1998;111:877–885.
Varlamov O, Eng FJ, Novikova EG, Fricker LD. Localization of metallocarboxypeptidase D in AtT-20 cells: potential role in prohormone processing.J Biol Chem. 1999;274:14759–14767.
Eipper BA, Milgram SL, Husten EJ, Yun HY, Mains RE. Peptidylglycine alpha-amidating monooxygenase: a multifunctional protein with catalytic, processing, and routing domains.Protein Sci. 1993;2:489–497.
Ouafik LH, Stoffers DA, Campbell TA, et al. The multifunctional peptidylglycine alpha-amidating monooxygenase gene: exon/intron organization of catalytic, processing, and routing domains.Mol Endo crinol. 1992;6:1571–1584.
Prigge ST, Mains RE, Eipper BA, Amzel LM. New insights into copper monooxygenase and peptide amidation: structure, mechanism, and function.Cell Mol Life Sci. 2000;57:1236–1259.
Kolhekar AS, Mains RE, Eipper BA. Peptidylglycine alpha-amidating monooxygenase: an ascorbate-requiring enzyme.Meth Enzymol. 1997;279:34–43.
Bradbury AF, Smyth DG. Modification of the N-and C-termini of bioactive peptides: amidation and acetylation. In: Fricker LD, ed.Peptide Biosynthesis and Processing. Boca Raton, FL: CRC Press; 1991:231–250.
Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach.Nature. 1999;402:656–660.
Lee RWH, Huttner WB. Tyrosine O-sulfated proteins of PC1 pheochromocytoma cells and their sulfation by tyrosylprotein sulfotransferase.J Biol Chem. 1983;258:11326–11332.
Bennett HPJ. Glycosylation, phosphorylation, and sulfation of peptide hormones and their precursors. In: Fricker LD, ed.Peptide Biosynthesis and Processing. Boca Raton, FL: CRC Press; 1991:111–140.
Svensson M, Skold K, Svenningsson P, Andren PE. Peptidomicsbased discovery of novel neuropeptides.J Proteome Res. 2003;2:213–219.
Turner AJ. Exploring the structure and function of zinc metallopeptidases: old enzymes and new discoveries.Biochem Soc Trans. 2003;31:723–727.
Albiston AL, Ye S, Chai SY. Membrane bound members of the M1 family: more than aminopeptidases.Protein Pept Lett. 2004;11:491–500.
Kim SI, Grum-Tokars V, Swanson TA, et al. Novel roles of neuropeptide processing enzymes: EC3.4.24.15 in the neurome.J Neurosci Res. 2003;74:456–467.
Smith AI, Clarke IJ, Lew RA. Post-secretory processing of peptide signals: a novel mechanism for the regulation of peptide hormone receptors.Biochem Soc Trans. 1997;25:1011–1014.
Goumon Y, Lugardon K, Gadroy P, et al. Processing of proenkephalin-A in bovine chromaffin cells. Identification of natural derived fragments by N-terminal sequencing and matrix-assisted laser desorption ionization-time of flight mass spectrometry.J Biol Chem. 2000;275:38355–38362.
Wei S, Segura S, Vendrell J, et al. Identification and characterization of three membrers of the human metallocarboxypeptidase gene family.J Biol Chem. 2002;277:14954–14964.
Fontenele-Neto JD, Kalinina E, Feng Y, Fricker LD. Identification and distribution of mouse carboxypeptidase A-6.Brain Res Mol Brain Res.
Emoto N, Yanagisawa M. Endothelin-converting enzyme-2 is a membrane-bound, phosphoramidon-sensitive metalloprotease with acidic pH optimum.J Biol Chem. 1995;270:15262–15268.
Mzhavia N, Pan H, Che F-Y, Fricker LD, Devi, LA. Characterization of endothelin-converting enzyme-2. Implication for a role in the nonclassical processing of regulatory peptides.J Biol Chem. 2003;278:14704–14711.
Srinivasan S, Bunch DO, Feng Y, et al. Deficits in reproduction and pro-gonadotropin-releasing hormone processing in male Cpefat mice.Endocrinology. 2004;145:2023–2034.
Qian Y, Devi LA, Mzhavia N, Munzer S, Seidah NG, Fricker LD. The C-terminal region of proSAAS is a potent inhibitor of prohormone convertase 1.J Biol Chem. 2000;275:23596–23601.
Wei S, Feng Y, Che F-Y, et al. Obesity and diabetes in transgenic mice expressing proSAAS.J Endocrinol. 2004;180:357–368.
Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G-protein-coupled receptors that regulate feeding behavior.Cell. 1998;92:573–585.
Preti A. Orexins (hypocretins): their role in appetite and arousal.Curr Opin Investig Drugs. 2002;3:1199–1206.
Rodgers RJ, Ishii Y, Halford JC, Blundell JE. Orexins and appetite regulation.Neuropeptides. 2002;36:303–325.
Tao WA, Aebersold R. Advances in quantitative proteomics via stable isotope tagging and mass spectrometry.Curr Opin Biotechnol. 2003;14:110–118.
Goshe MB, Smith RD. Stable isotope-coded proteomic mass spectrometry.Curr Opin Biotechnol. 2003;14:101–109.
Che F-Y, Eipper BA, Mains RE, Fricker LD. Quantitative peptidomics of pituitary glands from mice deficient in copper transport.Cell Mol Biol. 2003;49:713–722.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published: October 5, 2005
Rights and permissions
About this article
Cite this article
Fricker, L.D. Neuropeptide-processing enzymes: Applications for drug discovery. AAPS J 7, 44 (2005). https://doi.org/10.1208/aapsj070244
Received:
Accepted:
DOI: https://doi.org/10.1208/aapsj070244