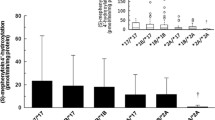
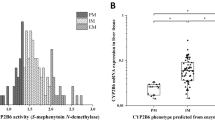
Abstract
Human CYP1A2 is one of the major CYPs in human liver and metabolizes a variety of clinically important drugs (e.g., clozapine, tacrine, tizanidine, and theophylline), a number of procarcinogens (e.g. benzo[a]pyrene and aflatoxin B1), and several important endogenous compounds (e.g. steroids and arachidonic acids). Like many of other CYPs, CYP1A2 is subject to induction and inhibition by a number of compounds, which may provide an explanation for some drug interactions observed in clinical practice. A large interindividual variability in the expression and activity of CYP1A2 and elimination of drugs that are mainly metabolized by CYP1A2 has been observed, which is largely caused by genetic (e.g., SNPs) and epigenetic (e.g., DNA methylation) and environmental factors (e.g., smoking and comedication). CYP1A2 is primarily regulated by the aromatic hydrocarbon receptor (AhR) and CYP1A2 is induced through AhR-mediated transactivation following ligand binding and nuclear translocation. To date, more than 15 variant alleles and a series of subvariants of the CYP1A2 gene have been identified and some of they have been associated with altered drug clearance and response to drug therapy. For example, lack of response to clozapine therapy due to low plasma drug levels has been reported in smokers harboring the −163A/A genotype; there is an association between CYP1A2*1F (−163C>A) allele and the risk for leflunomide-induced host toxicity. The *1F allele is associated with increased enzyme inducibility whereas *1C causes reduced inducibility. Further studies are warranted to explore the clinical and toxicological significance of altered CYP1A2 expression and activity caused by genetic, epigenetic, and environmental factors.




Similar content being viewed by others
References
Corchero J, Pimprale S, Kimura S, Gonzalez FJ. Organization of the CYP1A cluster on human chromosome 15: implications for gene regulation. Pharmacogenetics. 2001;11:1–6.
Ikeya K, Jaiswal AK, Owens RA, Jones JE, Nebert DW, Kimura S. Human CYP1A2: sequence, gene structure, comparison with the mouse and rat orthologous gene, and differences in liver 1A2 mRNA expression. Mol Endocrinol. 1989;3:1399–408.
Jaiswal AK, Nebert DW, McBride OW, Gonzalez FJ. Human P(3)450: cDNA and complete protein sequence, repetitive Alu sequences in the 3′ nontranslated region, and localization of gene to chromosome 15. J Exp Pathol. 1987;3:1–17.
Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP. Interindividual variations in human liver cytochrome P450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals. J Pharmacol Exp Ther. 1994;270:414–23.
Ingelman-Sundberg M, Sim SC, Gomez A, Rodriguez-Antona C. Influence of cytochrome P450 polymorphisms on drug therapies: pharmacogenetic, pharmacoepigenetic and clinical aspects. Pharmacol Ther. 2007;116:496–526.
Zhou SF, Di YM, Chan E, et al. Clinical pharmacogenetics and potential application in personalized medicine. Curr Drug Metab. 2008;9:738–84.
Gunes A, Dahl ML. Variation in CYP1A2 activity and its clinical implications: influence of environmental factors and genetic polymorphisms. Pharmacogenomics. 2008;9:625–37.
Zhou SF, Liu JP, Chowbay B. Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab Rev. 2009;41:89–295.
Lewis DF, Lake BG, Dickins M, Ueng YF, Goldfarb PS. Homology modelling of human CYP1A2 based on the CYP2C5 crystallographic template structure. Xenobiotica. 2003;33:239–54.
Shimizu T, Tateishi T, Hatano M, Fujii-Kuriyama Y. Probing the role of lysines and arginines in the catalytic function of cytochrome P450d by site-directed mutagenesis. Interaction with NADPH-cytochrome P450 reductase. J Biol Chem. 1991;266(6):3372–5.
Liu J, Ericksen SS, Sivaneri M, Besspiata D, Fisher CW, Szklarz GD. The effect of reciprocal active site mutations in human cytochromes P450 1A1 and 1A2 on alkoxyresorufin metabolism. Arch Biochem Biophys. 2004;424:33–43.
Sansen S, Yano JK, Reynald RL, et al. Adaptations for the oxidation of polycyclic aromatic hydrocarbons exhibited by the structure of human P450 1A2. J Biol Chem. 2007;282:14348–55.
Yano JK, Hsu MH, Griffin KJ, Stout CD, Johnson EF. Structures of human microsomal cytochrome P450 2A6 complexed with coumarin and methoxsalen. Nat Struct Mol Biol. 2005;12:822–3.
Faber MS, Jetter A, Fuhr U. Assessment of CYP1A2 activity in clinical practice: why, how, and when? Basic Clin Pharmacol Toxicol. 2005;97:125–34.
Yuan R, Madani S, Wei XX, Reynolds K, Huang SM. Evaluation of cytochrome P450 probe substrates commonly used by the pharmaceutical industry to study in vitro drug interactions. Drug Metab Dispos. 2002;30:1311–9.
Waxman DJ, Chang TK. Use of 7-ethoxycoumarin to monitor multiple enzymes in the human CYP1, CYP2, and CYP3 families. Methods Mol Biol. 2006;320:153–6.
Rendic S. Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab Rev. 2002;34:83–448.
Zhou SF, Koh HL, Gao YH, Gong ZY, Lee EJD. Herbal bioactivation: the good, the bad and the ugly. Life Sci. 2004;74:935–68.
Ueng YF, Hsieh CH, Don MJ, Chi CW, Ho LK. Identification of the main human cytochrome P450 enzymes involved in safrole 1'-hydroxylation. Chem Res Toxicol. 2004;17:1151–6.
Stiborova M, Frei E, Wiessler M, Schmeiser HH. Human enzymes involved in the metabolic activation of carcinogenic aristolochic acids: evidence for reductive activation by cytochromes P450 1A1 and 1A2. Chem Res Toxicol. 2001;14:1128–37.
Guengerich FP, Shimada T. Activation of procarcinogens by human cytochrome P450 enzymes. Mutat Res. 1998;400:201–13.
Crespi CL, Penman BW, Steimel DT, Smith T, Yang CS, Sutter TR. Development of a human lymphoblastoid cell line constitutively expressing human CYP1B1 cDNA: substrate specificity with model substrates and promutagens. Mutagenesis. 1997;12:83–9.
Skene DJ, Papagiannidou E, Hashemi E, et al. Contribution of CYP1A2 in the hepatic metabolism of melatonin: studies with isolated microsomal preparations and liver slices. J Pineal Res. 2001;31:333–42.
Lambrecht RW, Sinclair PR, Gorman N, Sinclair JF. Uroporphyrinogen oxidation catalyzed by reconstituted cytochrome P450 1A2. Arch Biochem Biophys. 1992;294:504–10.
Yamazaki H, Shaw PM, Guengerich FP, Shimada T. Roles of cytochromes P450 1A2 and 3A4 in the oxidation of estradiol and estrone in human liver microsomes. Chem Res Toxicol. 1998;11:659–65.
Ma Q. Induction of CYP1A1. The AhR/DRE paradigm: transcription, receptor regulation, and expanding biological roles. Curr Drug Metab. 2001;2:149–64.
Ma Q, Lu AY. CYP1A induction and human risk assessment: an evolving tale of in vitro and in vivo studies. Drug Metab Dispos. 2007;35:1009–16.
Backman JT, Granfors MT, Neuvonen PJ. Rifampicin is only a weak inducer of CYP1A2-mediated presystemic and systemic metabolism: studies with tizanidine and caffeine. Eur J Clin Pharmacol. 2006;62:451–61.
Nebert DW, Dalton TP, Okey AB, Gonzalez FJ. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem. 2004;279:23847–50.
Burbach KM, Poland A, Bradfield CA. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc Natl Acad Sci U S A. 1992;89:8185–9.
Carver LA, Bradfield CA. Ligand-dependent interaction of the aryl hydrocarbon receptor with a novel immunophilin homolog in vivo. J Biol Chem. 1997;272:11452–6.
Yueh MF, Huang YH, Hiller A, Chen S, Nguyen N, Tukey RH. Involvement of the xenobiotic response element (XRE) in Ah receptor-mediated induction of human UDP-glucuronosyltransferase 1A1. J Biol Chem. 2003;278:15001–6.
Ohyama K, Nakajima M, Suzuki M, Shimada N, Yamazaki H, Yokoi T. Inhibitory effects of amiodarone and its N-deethylated metabolite on human cytochrome P450 activities: prediction of in vivo drug interactions. Br J Clin Pharmacol. 2000;49:244–53.
Thelingwani RS, Zvada SP, Hugues D, Ungell AL, Masimirembwa CM. In vitro and in silico identification and characterisation of thiabendazole as a mechanism-based inhibitor of CYP1A2 and simulation of possible pharmacokinetic drug–drug interactions. Drug Metab Dispos. 2009;37:1286–94.
Zhu Q, Liao J, Xie L, Wang GJ, Liu XD. Mechanism-based inhibition of CYP1A2 by antofloxacin, an 8-NH2 derivative of levofloxacin in rats. Xenobiotica. 2009;39:293–301.
Langouet S, Furge LL, Kerriguy N, Nakamura K, Guillouzo A, Guengerich FP. Inhibition of human cytochrome P450 enzymes by 1, 2-dithiole-3-thione, oltipraz and its derivatives, and sulforaphane. Chem Res Toxicol. 2000;13:245–52.
Chang TK, Chen J, Lee WB. Differential inhibition and inactivation of human CYP1 enzymes by trans-resveratrol: evidence for mechanism-based inactivation of CYP1A2. J Pharmacol Exp Ther. 2001;299:874–82.
Shimada T, Yamazaki H, Foroozesh M, Hopkins NE, Alworth WL, Guengerich FP. Selectivity of polycyclic inhibitors for human cytochrome P450s 1A1, 1A2, and 1B1. Chem Res Toxicol. 1998;11:1048–56.
Cho US, Park EY, Dong MS, Park BS, Kim K, Kim KH. Tight-binding inhibition by α-naphthoflavone of human cytochrome P450 1A2. Biochim Biophys Acta. 2003;1648:195–202.
Jensen KG, Poulsen HE, Doehmer J, Loft S. Kinetics and inhibition by fluvoxamine of phenacetin O-deethylation in V79 cells expressing human CYP1A2. Pharmacol Toxicol. 1995;76:286–8.
Rasmussen BB, Maenpaa J, Pelkonen O, et al. Selective serotonin reuptake inhibitors and theophylline metabolism in human liver microsomes: potent inhibition by fluvoxamine. Br J Clin Pharmacol. 1995;39:151–9.
Parker AC, Preston T, Heaf D, Kitteringham NR, Choonara I. Inhibition of caffeine metabolism by ciprofloxacin in children with cystic fibrosis as measured by the caffeine breath test. Br J Clin Pharmacol. 1994;38:573–6.
Granfors MT, Backman JT, Neuvonen M, Neuvonen PJ. Ciprofloxacin greatly increases concentrations and hypotensive effect of tizanidine by inhibiting its cytochrome P450 1A2-mediated presystemic metabolism. Clin Pharmacol Ther. 2004;76:598–606.
Zhang L, Wei MJ, Zhao CY, Qi HM. Determination of the inhibitory potential of 6 fluoroquinolones on CYP1A2 and CYP2C9 in human liver microsomes. Acta Pharmacol Sin. 2008;29:1507–14.
Ueng YF, Jan WC, Lin LC, Chen TL, Guengerich FP, Chen CF. The alkaloid rutaecarpine is a selective inhibitor of cytochrome P450 1A in mouse and human liver microsomes. Drug Metab Dispos. 2002;30:349–53.
Qiu F, Zhang R, Sun J, et al. Inhibitory effects of seven components of danshen extract on catalytic activity of cytochrome P450 enzyme in human liver microsomes. Drug Metab Dispos. 2008;36:1308–14.
Liang HC, Li H, McKinnon RA, et al. CYP1A2 (−/−) null mutant mice develop normally but show deficient drug metabolism. Proc Natl Acad Sci U S A. 1996;93:1671–6.
Liang HC, McKinnon RA, Nebert DW. Sensitivity of CYP1A1 mRNA inducibility by dioxin is the same in Cyp1a2 +/+ wild-type and Cyp1a2 −/− null mutant mice. Biochem Pharmacol. 1997;54:1127–31.
Lasker JM, Huang MT, Conney AH. In vivo activation of zoxazolamine metabolism by flavone. Science. 1982;216:1419–21.
Buters JT, Tang BK, Pineau T, Gelboin HV, Kimura S, Gonzalez FJ. Role of CYP1A2 in caffeine pharmacokinetics and metabolism: studies using mice deficient in CYP1A2. Pharmacogenetics. 1996;6:291–6.
Tsuneoka Y, Dalton TP, Miller ML, et al. 4-Aminobiphenyl-induced liver and urinary bladder DNA adduct formation in CYP1A2 −/− and CYP1A2 +/+ mice. J Natl Cancer Inst. 2003;95:1227–37.
Smith AG, Davies R, Dalton TP, et al. Intrinsic hepatic phenotype associated with the Cyp1a2 gene as shown by cDNA expression microarray analysis of the knockout mouse. EHP Toxicogenomics. 2003;111:45–51.
Kall MA, Clausen J. Dietary effect on mixed function P450 1A2 activity assayed by estimation of caffeine metabolism in man. Hum Exp Toxicol. 1995;14:801–7.
Relling MV, Lin JS, Ayers GD, Evans WE. Racial and gender differences in N-acetyltransferase, xanthine oxidase, and CYP1A2 activities. Clin Pharmacol Ther. 1992;52:643–58.
Rasmussen BB, Brix TH, Kyvik KO, Brosen K. The interindividual differences in the 3-demethylation of caffeine alias CYP1A2 is determined by both genetic and environmental factors. Pharmacogenetics. 2002;12:473–8.
Aitchison KJ, Gonzalez FJ, Quattrochi LC, et al. Identification of novel polymorphisms in the 5′ flanking region of CYP1A2, characterization of interethnic variability, and investigation of their functional significance. Pharmacogenetics. 2000;10:695–704.
Soyama A, Saito Y, Hanioka N, et al. Single nucleotide polymorphisms and haplotypes of CYP1A2 in a Japanese population. Drug Metab Pharmacokinet. 2005;20:24–33.
Chida M, Yokoi T, Fukui T, Kinoshita M, Yokota J, Kamataki T. Detection of three genetic polymorphisms in the 5′-flanking region and intron 1 of human CYP1A2 in the Japanese population. Jpn J Cancer Res. 1999;90:899–902.
Nakajima M, Yokoi T, Mizutani M, Kinoshita M, Funayama M, Kamataki T. Genetic polymorphism in the 5'-flanking region of human CYP1A2 gene: effect on the CYP1A2 inducibility in humans. J Biochem. 1999;125:803–8.
Sachse C, Brockmoller J, Bauer S, Roots I. Functional significance of a C>A polymorphism in intron 1 of the cytochrome P450 CYP1A2 gene tested with caffeine. Br J Clin Pharmacol. 1999;47:445–9.
Ghotbi R, Christensen M, Roh HK, Ingelman-Sundberg M, Aklillu E, Bertilsson L. Comparisons of CYP1A2 genetic polymorphisms, enzyme activity and the genotype–phenotype relationship in Swedes and Koreans. Eur J Clin Pharmacol. 2007;63:537–46.
Han XM, Ouyang DS, Chen XP, et al. Inducibility of CYP1A2 by omeprazole in vivo related to the genetic polymorphism of CYP1A2. Br J Clin Pharmacol. 2002;54:540–3.
Aklillu E, Carrillo JA, Makonnen E, et al. Genetic polymorphism of CYP1A2 in Ethiopians affecting induction and expression: characterization of novel haplotypes with single-nucleotide polymorphisms in intron 1. Mol Pharmacol. 2003;64:659–69.
Huang JD, Guo WC, Lai MD, Guo YL, Lambert GH. Detection of a novel cytochrome P450 1A2 polymorphism (F21L) in Chinese. Drug Metab Dispos. 1999;27:98–101.
Sachse C, Bhambra U, Smith G, et al. Polymorphisms in the cytochrome P450 CYP1A2 gene (CYP1A2) in colorectal cancer patients and controls: allele frequencies, linkage disequilibrium and influence on caffeine metabolism. Br J Clin Pharmacol. 2003;55:68–76.
Pucci L, Geppetti A, Maggini V, Lucchesi D, Maria Rossi A, Longo V. CYP1A2 F21L and F186L polymorphisms in an Italian population sample. Drug Metab Pharmacokinet. 2007;22:220–2.
Chevalier D, Cauffiez C, Allorge D, et al. Five novel natural allelic variants—951A>C, 1042G>A (D348N), 1156A>T (I386F), 1217G>A (C406Y) and 1291C>T (C431Y)—of the human CYP1A2 gene in a French Caucasian population. Hum Mutat. 2001;17:355–6.
Bilgen T, Tosun O, Luleci G, Keser I. Frequencies of four genetic polymorphisms in the CYP1A2 gene in Turkish population. Genetika. 2008;44:1133–6.
Hamdy SI, Hiratsuka M, Narahara K, et al. Genotyping of four genetic polymorphisms in the CYP1A2 gene in the Egyptian population. Br J Clin Pharmacol. 2003;55:321–4.
Skarke C, Kirchhof A, Geisslinger G, Lotsch J. Rapid genotyping for relevant CYP1A2 alleles by pyrosequencing. Eur J Clin Pharmacol. 2005;61:887–92.
Obase Y, Shimoda T, Kawano T, et al. Polymorphisms in the CYP1A2 gene and theophylline metabolism in patients with asthma. Clin Pharmacol Ther. 2003;73:468–74.
Eap CB, Bender S, Jaquenoud Sirot E, et al. Nonresponse to clozapine and ultrarapid CYP1A2 activity: clinical data and analysis of CYP1A2 gene. J Clin Psychopharmacol. 2004;24:214–9.
Ozdemir V, Kalow W, Okey AB, et al. Treatment-resistance to clozapine in association with ultrarapid CYP1A2 activity and the C>A polymorphism in intron 1 of the CYP1A2 gene: effect of grapefruit juice and low-dose fluvoxamine. J Clin Psychopharmacol. 2001;21:603–7.
Melkersson KI, Scordo MG, Gunes A, Dahl ML. Impact of CYP1A2 and CYP2D6 polymorphisms on drug metabolism and on insulin and lipid elevations and insulin resistance in clozapine-treated patients. J Clin Psychiatry. 2007;68:697–704.
Berecz R, de la Rubia A, Dorado P, Fernandez-Salguero P, Dahl ML, Llerena A. Thioridazine steady-state plasma concentrations are influenced by tobacco smoking and CYP2D6, but not by the CYP2C9 genotype. Eur J Clin Pharmacol. 2003;59:45–50.
Mihara K, Kondo T, Suzuki A, et al. Effects of genetic polymorphism of CYP1A2 inducibility on the steady-state plasma concentrations of trazodone and its active metabolite m-chlorophenylpiperazine in depressed Japanese patients. Pharmacol Toxicol. 2001;88:267–70.
Hartter S, Korhonen T, Lundgren S, et al. Effect of caffeine intake 12 or 24 hours prior to melatonin intake and CYP1A2*1F polymorphism on CYP1A2 phenotyping by melatonin. Basic Clin Pharmacol Toxicol. 2006;99:300–4.
Bohanec Grabar P, Rozman B, Tomsic M, Suput D, Logar D, Dolzan V. Genetic polymorphism of CYP1A2 and the toxicity of leflunomide treatment in rheumatoid arthritis patients. Eur J Clin Pharmacol. 2008;64(9):871–6.
Lewis DF, Lake BG. Molecular modelling of CYP1A subfamily members based on an alignment with CYP102: rationalization of CYP1A substrate specificity in terms of active site amino acid residues. Xenobiotica. 1996;26:723–53.
Mayuzumi H, Sambongi C, Hiroya K, Shimizu T, Tateishi T, Hatano M. Effect of mutations of ionic amino acids of cytochrome P450 1A2 on catalytic activities toward 7-ethoxycoumarin and methanol. Biochemistry. 1993;32:5622–8.
Dai R, Zhai S, Wei X, Pincus MR, Vestal RE, Friedman FK. Inhibition of human cytochrome P450 1A2 by flavone: a molecular modeling study. J Protein Chem. 1998;17:643–50.
Hadjokas NE, Dai R, Friedman FK, et al. Arginine to lysine 108 substitution in recombinant CYP1A2 abolishes methoxyresorufin metabolism in lymphoblastoid cells. Br J Pharmacol. 2002;136:347–52.
Lozano JJ, Pastor M, Cruciani G, et al. 3D-QSAR methods on the basis of ligand–receptor complexes. Application of COMBINE and GRID/GOLPE methodologies to a series of CYP1A2 ligands. J Comput Aided Mol Des. 2000;14:341–53.
Parikh A, Josephy PD, Guengerich FP. Selection and characterization of human cytochrome P450 1A2 mutants with altered catalytic properties. Biochemistry. 1999;38:5283–9.
Mayuzumi H, Shimizu T, Sambongi C, Hiroya K, Hatano M. Essential role of His163 of cytochrome P450 1A2 in catalytic functions associated with cytochrome b 5. Arch Biochem Biophys. 1994;310:367–72.
Cvrk T, Strobel HW. Photoaffinity labeling of cytochrome P4501A1 with azidocumene: identification of cumene hydroperoxide binding region. Arch Biochem Biophys. 1998;349:95–104.
Krainev AG, Shimizu T, Hiroya K, Hatano M. Effect of mutations at Lys250, Arg251, and Lys253 of cytochrome P450 1A2 on the catalytic activities and the bindings of bifunctional axial ligands. Arch Biochem Biophys. 1992;298:198–203.
Shimizu T, Murakami Y, Hatano M. Glu318 and Thr319 mutations of cytochrome P450 1A2 remarkably enhance homolytic O-O cleavage of alkyl hydroperoxides. An optical absorption spectral study. J Biol Chem. 1994;269:13296–304.
Shimizu T, Ito O, Hatano M, Fujii-Kuriyama Y. CO binding studies of engineered cytochrome P450ds: effects of mutations at putative distal sites in the presence of polycyclic hydrocarbons. Biochemistry. 1991;30:4659–62.
Krainev AG, Shimizu T, Ishigooka M, Hiroya K, Hatano M, Fujii-Kuriyama Y. Absorption spectral study of cytochrome P450d–phenyl isocyanide complexes: effects of mutations at the putative distal site on the conformational stability. Biochemistry. 1991;30:11206–11.
Hiroya K, Murakami Y, Shimizu T, Hatano M, de Montellano PR Ortiz. Differential roles of Glu318 and Thr319 in cytochrome P450 1A2 catalysis supported by NADPH-cytochrome P450 reductase and tert-butyl hydroperoxide. Arch Biochem Biophys. 1994;310:397–401.
Nakano R, Sato H, Watanabe A, Ito O, Shimizu T. Conserved Glu318 at the cytochrome P450 1A2 distal site is crucial in the nitric oxide complex stability. J Biol Chem. 1996;271:8570–4.
Pavanello S, Pulliero A, Lupi S, Gregorio P, Clonfero E. Influence of the genetic polymorphism in the 5'-noncoding region of the CYP1A2 gene on CYP1A2 phenotype and urinary mutagenicity in smokers. Mutat Res. 2005;587:59–66.
Nordmark A, Lundgren S, Ask B, Granath F, Rane A. The effect of the CYP1A2 *1F mutation on CYP1A2 inducibility in pregnant women. Br J Clin Pharmacol. 2002;54:504–10.
Chen X, Wang L, Zhi L, et al. The G-3113A polymorphism in CYP1A2 affects the caffeine metabolic ratio in a Chinese population. Clin Pharmacol Ther. 2005;78:249–59.
Han XM, Ou-Yang DS, Lu PX, et al. Plasma caffeine metabolite ratio (17X/137X) in vivo associated with G-2964A and C734A polymorphisms of human CYP1A2. Pharmacogenetics. 2001;11:429–35.
Dandara C, Basvi PT, Bapiro TE, Sayi J, Hasler JA. Frequency of −163 C>A and 63 C>G single nucleotide polymorphism of cytochrome P450 1A2 in two African populations. Clin Chem Lab Med. 2004;42:939–41.
B'Chir F, Pavanello S, Knani J, Boughattas S, Arnaud MJ, Saguem S. CYP1A2 genetic polymorphisms and adenocarcinoma lung cancer risk in the Tunisian population. Life Sci. 2009;84(21–22):779–84.
Acknowledgements
The authors appreciate the grant support of RMIT Health Innovations Research Institute, RMIT University, Bundoora, Victoria 3083, Australia and National Institute of Complementary Medicine, New South Wales, Australia. Mr. Zhi-Wei Zhou is a holder of RMIT University International Postgraduate Scholarship, Victoria, Australia. Dr. Li-Ping Yang was and Mr. Ya-He Liu is a holder of the Australian Postgraduate (PhD) Award funded by the Commonwealth Government of Australia. We also appreciate the support of Professor Hualiang Jiang, Center for Drug Discovery and Design, State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zuchongzhi Road, Shanghai 201203, China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, SF., Yang, LP., Zhou, ZW. et al. Insights into the Substrate Specificity, Inhibitors, Regulation, and Polymorphisms and the Clinical Impact of Human Cytochrome P450 1A2. AAPS J 11, 481–494 (2009). https://doi.org/10.1208/s12248-009-9127-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-009-9127-y