-
PDF
- Split View
-
Views
-
Cite
Cite
Mayoura Keophiphath, Vincent Achard, Corneliu Henegar, Christine Rouault, Karine Clément, Danièle Lacasa, Macrophage-Secreted Factors Promote a Profibrotic Phenotype in Human Preadipocytes, Molecular Endocrinology, Volume 23, Issue 1, 1 January 2009, Pages 11–24, https://doi.org/10.1210/me.2008-0183
Close - Share Icon Share
White adipose tissue (WAT) in obese humans is characterized by macrophage accumulation the effects of which on WAT biology are not fully understood. We previously demonstrated that macrophage-secreted factors impair preadipocyte differentiation and induce inflammation, and we described the excessive fibrotic deposition in WAT from obese individuals. Microarray analysis revealed significant overexpression of extracellular matrix (ECM) genes in inflammatory preadipocytes. We show here an organized deposition of fibronectin, collagen I, and tenascin-C and clustering of the ECM receptor α5 integrin, characterizing inflammatory preadipocytes. Anti-α5 integrin-neutralizing antibody decreased proliferation of these cells, underlining the importance of the fibronectin/integrin partnership. Fibronectin-cultured preadipocytes exhibited increased proliferation and expression of both nuclear factor-κB and cyclin D1. Small interfering RNA deletion of nuclear factor-κB and cyclin D1 showed that these factors link preadipocyte proliferation with inflammation and ECM remodeling. Macrophage-secreted molecules increased preadipocyte migration through an increase in active/phosphorylated focal adhesion kinase. Gene expression and neutralizing antibody experiments suggest that inhibin β A, a TGF-β family member, is a major fibrotic factor. Interactions between preadipocytes and macrophages were favored in a three-dimensional collagen I matrix mimicking the fibrotic context of WAT. Cell-rich regions were immunostained for preadipocytes, proliferation, and macrophages in the vicinity of fibrotic WAT from obese individuals. In conclusion, an inflammatory environment leads to profound modifications of the human preadipocyte phenotype, producing fibrotic components with increased migration and proliferation. This phenomenon might play a role in facilitating the constitution of quiescent preadipocyte pools and eventually in the maintenance and aggravation of increased fat mass in obesity.
Extracellular matrix (ECM) remodeling plays a pivotal role in adipocyte differentiation, which is characterized by the cellular transition of a fibroblast cell called a preadipocyte to an adipocyte, a spherical cell that accumulates triglycerides. Although the molecular mechanisms are not yet fully understood, ECM remodeling occurs concomitantly with the activation or repression of a transcriptional network involved in adipogenesis. This transcriptional network is activated or repressed in response to extracellular stimuli. Adipocyte differentiation, which has mostly been studied in murine cell lines (3T3-L1), is associated with a decrease in fibronectin-rich matrix and formation of basal lamina (1–3). Gene invalidation of the pericellular collagenase MT1-matrix metalloproteinase during mouse development leads to the formation of a rigid network of collagen fibrils, altering in vivo adipogenesis (4) and illustrating the relationship between modification of the ECM structure and the adipocyte differentiation process.
The origin and potential consequences of perturbed ECM remodeling are not well understood in pathological conditions such as human obesity, which can be considered a chronic low-grade inflammatory state. Excess adipose tissues produce a variety of inflammation-related molecules, most of which originate from accumulated macrophages (5–7). We showed recently that ECM genes are coexpressed with inflammatory genes in the transcriptional networks that characterize the transcriptomic signature of the white adipose tissue (WAT) in obese persons. Fibrotic deposits have been found in WAT, but their cellular origin and the mechanisms involved in their constitution are unknown for the most part (8).
The ECM forms a complex network of structural and multifunctional molecules comprising collagens, adhesive glycoproteins, and proteoglycans. This network provides a scaffold for cells and the signaling pathways controlling their migration, proliferation, and differentiation. ECM remodeling, which results from a combination of matrix synthesis and degradation, with the deposition of specific proteins (tenascins and fibrin), occurs in physiological responses such as development and tissue repair, and pathological processes such as inflammation (9). Fibronectin, an abundant component of the ECM, influences cell growth and differentiation through its effects on the expression of genes coding for cell cycle components, including cyclin D1 (10). Fibronectin is required for the assembly of ECM proteins such as collagen I. Heterodimeric αβ-integrins, a family of cell surface receptors, control the formation of the fibrillar fibronectin network (11). Through binding to ECM proteins, integrins promote the assembly of cytoskeleton and signaling complexes at specific junctions called focal adhesions. The clustering of integrins with cytosolic proteins at focal adhesions is critical for cell migration. Focal adhesion kinase (FAK) plays an essential role in this process (12). Whether these proteins are involved in regulating ECM remodeling in the inflamed adipose tissue of obese humans is unknown.
A proinflammatory state has been described for 3T3-L1 adipocytes in response to macrophage cell line-secreted factors (13–15). We chose to develop an experimental model using primary human preadipocytes cultured with secreted factors from monocyte-derived and adipose-tissue macrophages. In these conditions, preadipocytes exhibit impaired adipogenesis and an inflammation state with increased nuclear factor-κB (NF-κB) activity and release of inflammatory mediators (16). By functional profiling of the transcriptomic signature of the inflammatory preadipocytes, we found that genes involved in biological themes related to the extracellular matrix were among those most overexpressed in these cells. We show here that proinflammatory microenvironments are capable of inducing a considerable synthesis of several ECM proteins by human preadipocytes and are associated with their increased proliferation and migration. Several key proteins involved in these phenomena are identified.
Results
Significant overexpression of ECM genes in inflammatory preadipocytes
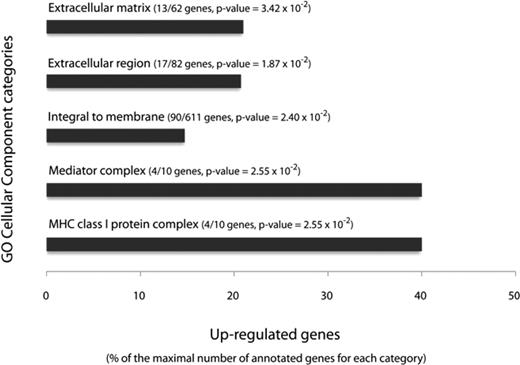
We showed previously that secreted factors from activated macrophages (Ac CM) induced inflammatory changes in human preadipocytes (16). To gain insight into the signaling pathways and processes that are altered in proinflammatory preadipocytes, we performed a pangenomic cDNA microarray analysis to compare gene expression profiles between preadipocytes treated with Ac CM and controls. The Significance Analysis of Microarrays procedure identified 3149 overexpressed cDNAs in proinflammatory preadipocytes with a 5% false discovery ratio (FDR) threshold (fold-change: 1.5–24.9). We performed a functional analysis to identify the biological themes that best characterized the proinflammatory preadipocytes. The most significantly overrepresented gene ontology (GO) Cellular Component categories were Extracellular Matrix, Extracellular Region, Integral to Membrane, Mediator Complex, and Major Histocompatability Complex class I protein complex (Fig. 1), emphasizing the importance of ECM-related genes and processes in changes to the preadipocyte phenotype induced by the inflammatory environment. Detailed results of the functional analysis of the preadipocyte differential expression profiles, inferred separately for each of the three GO ontologies, are published as supplemental data on The Endocrinology Society’s Journals Online web site at http://mend.endojournals.org (available at: http://corneliu.henegar.info/projects/FunCluster/mol_ endocrinol_2008/).
The most significantly enriched cluster of GO cellular component categories annotating genes up-regulated in Ac CM-treated preadipocytes. Significantly enriched GO cellular categories are represented in relation to the fraction of up-regulated genes among the genes annotated by each category. MHC, Major histocompatibility complex.
We also found that ECM adhesion proteins such as fibronectin and tenascin-C, and proteoglycans such as lumican [which may be involved stabilizing the collagen I network (17)], as well as proteases (matrix metalloproteinases) and their inhibitors (tissue inhibitor of metalloproteinases 1 and tissue factor pathway inhibitor 2) were among the most overexpressed genes annotated by ECM-related themes. In addition, inhibin βA, a member of TGF-β family that displays profibrotic activity, was also significantly overexpressed. We pursued our phenotypic characterization of the proinflammatory preadipocytes at the molecular and functional levels, particularly by exploring the role of fibronectin, its main receptor α5 integrin, and tenascin-C. Inhibin βA was also considered a potential candidate favoring ECM remodeling.
Modification of ECM components in inflammatory preadipocytes
Because fibronectin is a negative regulator of adipogenesis in murine cell lines (3) and tenascin-C associates with wound repair and inflammation (18), we examined whether these two proteins, as well as α5-integrin, participate in the ECM remodeling that potentially accompanies the inflammatory state of preadipocytes.
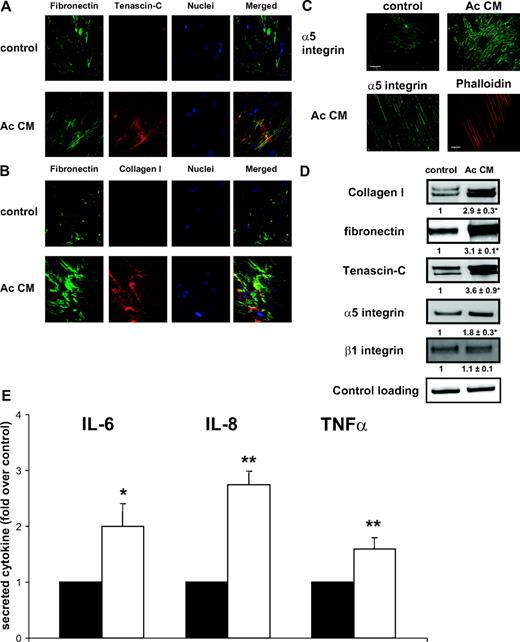
The polymerized fibronectin network in Ac CM-treated preadipocytes partly colocalized with intense tenascin-C staining (Fig. 2A). We observed a dense network of collagen I (Fig. 2B), the deposition of which into ECM is enhanced by fibronectin (19). Ac CM-treated preadipocytes had increased staining for α5-integrin, paralleled with the actin stress fibers in these cells (Fig. 2C); however, we were unable to detect any α-smooth actin staining in Ac CM-treated preadipocytes (data not shown).
ECM changes in inflammatory preadipocytes. Preadipocytes were differentiated with control or Ac CM and immunostained. Cells were examined by confocal (A and B) and immunofluorescence (C) microscopy. A, Fibronectin (green, cy2-conjugated antimouse IgG) and tenascin-C (red, cy3-conjugated antirabbit IgG). B, Fibronectin (green, cy2-conjugated antimouse IgG) and Collagen I (red, cy3-conjugated antirabbit IgG). C, α5-Integrin (green, cy2-conjugated antimouse IgG) (bar, 20 μm) and actin (red, phalloidin-AlexaFluor 546) (bar, 10 μm). D, Immunoblot of cell extracts to detect collagen I, fibronectin, tenascin-C, α5-integrin, and β1-integrin. Mean ± sem of three independent experiments (in bold). *, P < 0.05. E, Preadipocytes were seeded on plastic (black bars) or collagen I/fibronectin matrix (open bars). IL-6, IL-8, and TNFα in media were measured by ELISA. Data were normalized to cell number. Mean ± sem of four separate experiments. *, P < 0.05; **, P < 0.01.
We confirmed the immunofluorescence findings by Western blot analysis and found increased protein expression of tenascin-C, collagen I, and α5-integrin. β1-Integrin expression was unchanged (Fig. 2D). In contrast with observations made in 3T3-L1 adipocytes (20), we observed no change in the expression of α6-integrin during adipogenesis and after exposure to macrophage-secreted factors (data not shown). Proinflammatory preadipocytes exhibited profound qualitative and quantitative changes in ECM components, as witnessed by the significant modifications of fibronectin, tenascin-C, collagen I, and α5-integrin receptor proteins. Strikingly, we observed increased expression of fibrogenic proteins (fibronectin, collagen I) in preadipocytes exposed to secreted factors from macrophage WAT (data not shown).
To gain information regarding the relationship between ECM and preadipocyte inflammation, we tested the effects on inflammatory cytokine production of culturing preadipocytes over a collagen I and fibronectin matrix. As illustrated in Fig. 2E, preadipocytes secreted increased amounts of IL-6, IL-8, and TNFα in this condition; the first two cytokines were previously shown to be produced in human inflammatory preadipocytes (16).
ECM remodeling and preadipocyte proliferation
We previously observed increased proliferation of Ac CM-preadipocytes (16). These cells produced a fibronectin-rich network and high α5-integrin staining (Fig. 2).
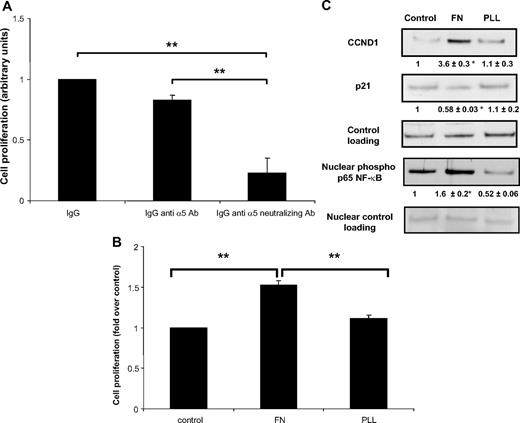
To evaluate the involvement of integrin signaling in inflammatory preadipocyte proliferation, we performed experiments using an anti-α5 integrin neutralizing antibody. As shown in Fig. 3A, inflammatory preadipocytes cultured with α5-integrinneutralizing antibody substantially reduced their proliferative capacity. We further examined the contribution of the fibronectin-integrin partnership in preadipocyte proliferation by culturing preadipocytes on either fibronectin- (integrin dependent) or poly-l-lysine (PLL)-coated (integrin nondependent) wells. As shown in Fig. 3B, fibronectin significantly stimulated preadipocyte proliferation whereas PLL did not.
Preadipocyte proliferation and ECM. A, Preadipocytes were cultured in Ac CM in the absence or the presence of nonimmune IgG (IgG, 40 μg/ml), anti-α5-integrin Ab (IgG anti-α5 Ab, 40 μg/ml), or anti-α5-integrin neutralizing Ab (IgG anti α5-neutralizing Ab, 40 μg/ml). An MTS proliferation assay was then performed. **, P < 0.001. B, Preadipocytes were seeded on fibronectin- or PLL-coated plates and cultured for 5 d, after which an MTS proliferation assay was performed. **, P < 0.001. C, Cell extracts were immunoblotted for CCND1, p21, and nuclear extracts for phospho-p65 NF-κB (ser 536). Mean ± sem of five separate experiments (in bold). *, P < 0.05. Ab, Antibody; FN, fibronectin; PLL, poly-l-lysine.
Fibronectin modulates the expression of cell cycle proteins such as cyclin D1 and their inhibitors, e.g. p21, in several cell types (10). Data in Fig. 3C clearly show that in human preadipocytes, fibronectin, but not PLL, strongly enhanced cyclin D1 protein expression (∼360%) and, conversely, decreased p21 protein expression (−50%). Fibronectin provoked increased nuclear accumulation of the active phosphorylated form of p65 NF-κB (∼160%). These results demonstrate a strong relationship between the fibronectin-α5 integrin partnership and preadipocyte proliferation through the promotion of cell cycle progression.
ECM remodeling, proliferation, and p65 NF-κB deletion in preadipocyte
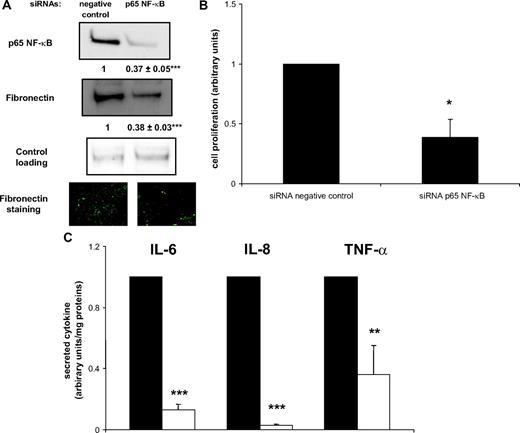
Because the activity of NF-κB was previously shown to be increased in Ac CM-treated preadipocytes (16) and fibronectin is a molecular target of NF-κB, we evaluated the role of NF-κB in ECM remodeling and preadipocyte proliferation. Using small interfering RNA (siRNA), we knocked down the NF-κB p65 subunit in Ac CM-treated preadipocytes (Fig. 4A). Decreased expression of NF-κB p65 was associated with decreased expression of fibronectin and diminished formation of the extracellular network (Fig. 4A). Importantly, the proliferation rate of Ac CM-treated preadipocytes was significantly decreased by exposure to siRNA p65 NF-κB (Fig. 4B). As expected, the secretion of the inflammatory molecules IL-6 and IL-8 was almost abolished in siRNA p65 NF-κB-exposed preadipocytes (Fig. 4C). These results establish the relevant contribution of p65 NF-κB to preadipocyte proliferation associated with fibronectin network remodeling.
ECM remodeling and p65 NF-κB deletion. Ac CM-treated preadipocytes were exposed to either negative control or p65 NF-κB siRNA. A, Cell extracts were immunoblotted for p65 NF-κB and fibronectin. Mean ± sem of five separate experiments (in bold). Cells were also examined for fibronectin staining (green, cy2-conjugated antimouse IgG). ***, P < 0.0001. B, MTS proliferation assay was performed. Mean ± sem of three separate experiments. *, P < 0.05. C, IL-6, IL-8, and TNFα secreted from negative control (black bars) or p65 NF-κB siRNA (open bars) preadipocyte media were measured by ELISA. Mean ± sem of four separate experiments. **, P < 0.02; ***, P < 0.0001.
Cyclin D1 deletion and preadipocyte ECM remodeling and inflammation
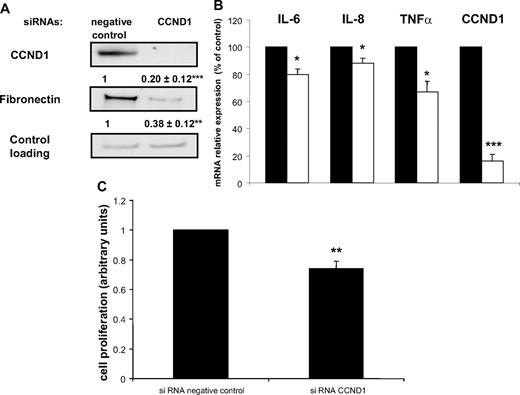
We hypothesized that Cyclin D1 could constitute a molecular link between proliferation, inflammation, and ECM remodeling. We therefore explored its contribution to the altered phenotype of inflammatory preadipocytes. Knockdown of Cyclin D1 by siRNA in Ac CM-treated preadipocytes decreased fibronectin levels by 60% (Fig. 5A) and substantially suppressed the expression of proinflammatory cytokines IL-6 (−20%), IL-8 (−15%), and TNFα (−25%) (Fig. 5B). Preadipocyte proliferation was reduced by cyclin D1 (CCND1) siRNA treatment (−25%) (Fig. 5C).
CCND1 deletion affects preadipocyte ECM remodeling and inflammation. Ac CM-treated preadipocytes were exposed to either negative control or CCND1 siRNA. A, Cell extracts were immunoblotted for CCND1 and fibronectin. Mean ± sem of four separate experiments (in bold). **, P < 0.005; ***, P < 0.001. B, IL-6, IL-8, TNFα, and CCND1 were quantified by real-time PCR. Data are mean ± sem of five separate experiments. *, P < 0.02; ***, P < 0.001. C, MTS proliferation assay was performed. Mean ± sem of four separate experiments. **, P < 0.005.
Adhesion and migration of inflammatory preadipocytes
Cell migration is regulated, in part, by connections among ECM proteins and the actin cytoskeleton. Considering that Ac CM-treated preadipocytes displayed actin cytoskeleton modifications (i.e. increased actin stress fibers) (Fig. 2C), one might expect that other phenotypic properties would be modified, including the capacity to migrate.
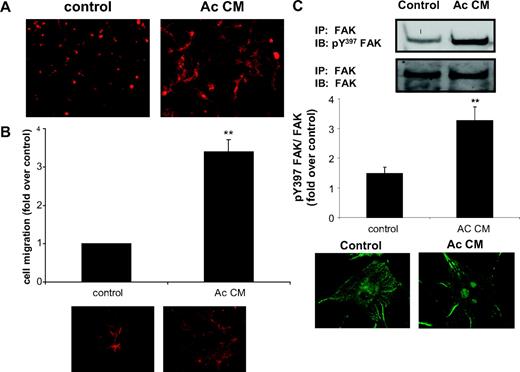
In a first set of experiments, preadipocyte adhesion properties were evaluated by seeding control and Ac CM-treated preadipocytes over a collagen I matrix. Cell shape, as determined by their actin cytoskeleton, was visualized after a 4-h spreading period. On the collagen matrix, control preadipocytes adopted a round shape whereas Ac CM-treated preadipocytes spread out with numerous dendritic extensions, thereby demonstrating their increased migration capacity (Fig. 6A). Matrices with different molecular compositions may affect the morphology of control and Ac CM-treated preadipocytes differently; the latter cells appeared more prone to migrate on a fibrosis-like matrix. Furthermore, experiments using fibronectin-coated inserts showed a 3-fold increase in the migration rate of Ac CM-treated preadipocytes compared with control cells (Fig. 6B). Actin staining of migrated cells on the bottom face of the inserts confirmed the higher number of Ac CM-treated preadipocytes.
Altered spreading and migration properties of inflammatory preadipocytes. Preadipocytes were differentiated in the presence of control medium or Ac CM. A, Immunofluorescence detection of actin (red, phalloidin) showing control and Ac CM-treated preadipocytes seeded on top of a collagen I matrix. B, Equal numbers of control and Ac CM-treated preadipocytes were placed in fibronectin-coated inserts and allowed to migrate. Mean ± sem of four individual experiments. Cells were also stained for actin (red, phalloidin) on insert bottoms. **, P < 0.01. C, Cell extracts were immunoblotted for Y397 FAK after immunoprecipitation of total FAK. Graphs show quantification of the immunoblots. Mean ± sem of four independent experiments. Cells were examined for phospho-Y397 FAK staining (green, Cy2-conjugated antimouse IgG). **, P < 0.01. IB, Immunoblot; IP, immunoprecipitation.
FAK was previously shown to be involved in cell migration through the formation of focal contacts when induced by ECM signals via phosphorylation at Y397 (12). To establish a molecular basis for the increased migration of inflammatory preadipocytes, we evaluated the FAK activation level in these cells. As illustrated in Fig. 6C, Ac CM-treated preadipocytes exhibited a 3-fold increase in phosphorylated Y397 FAK level. Immunofluorescence analysis also revealed a characteristic spotted distribution of phosphorylated Y397 FAK in these cells.
Factors involved in the preadipocyte profibrotic phenotype
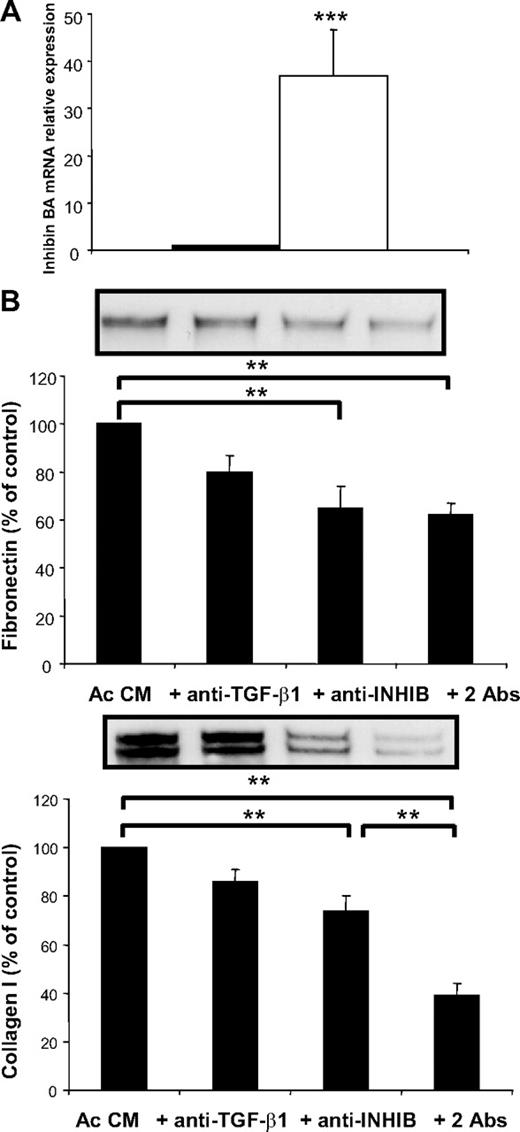
Gene expression profiling has demonstrated that inhibin βA is one of the most overexpressed genes in Ac CM-treated preadipocytes. Inhibins belong to the TGF-β family and are considered to be profibrotic proteins that act synergistically (21, 22). Real-time PCR experiments confirmed a strong inhibin βA overexpression (∼35-fold) in Ac CM-treated preadipocytes (Fig. 7A). We investigated the profibrotic role of these proteins by using anti-TGF-β1 and anti-inhibin βA neutralizing antibody treatments. The promoting effect of Ac CM on fibronectin and collagen I production was not modified by blocking TGF-β1. In contrast, inhibin β A neutralizing antibody treatment markedly decreased fibronectin and collagen I levels. It should be noted that a synergistic effect was observed between TGF-β1 and inhibin βA only for collagen I production (Fig. 7B). This set of experiments suggests that inhibin β A might be involved in the synthesis of fibrotic molecules by the human preadipocytes.
Identification of fibrotic factor(s). A, Preadipocytes were differentiated in the presence of control medium (black bars) or Ac CM (open bars). Inhibin βA was quantified by real-time PCR. Data are mean ± sem of seven separate experiments. ***, P < 0.005. B, Preadipocytes were differentiated in the presence of Ac CM with nonimmune IgG or anti-TGF-β1 (anti TGF-β1, 4 μg/ml), anti-inhibin βA (anti-INHIB, 2 μg/ml), or both antibodies (2Abs: anti TGF-β1, 2 μg/ml; and anti-INHIB, 1 μg/ml). Data are mean ± sem of six separate experiments. **, P < 0.01. Abs, Antibodies; INHIB, inhibin.
Direct preadipocyte and macrophage interactions in a three-dimensional (3D) matrix
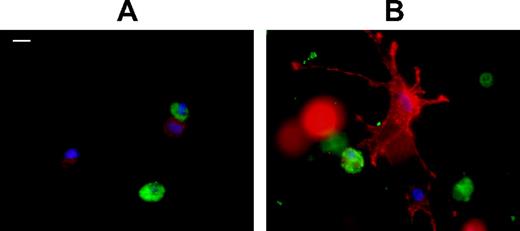
In the first set of experiments, a proinflammatory phenotype was induced in preadipocytes by incubating them with macrophage-secreted factors. At the tissue level, adipose tissue macrophages, in addition to their paracrine action, could also directly interact with preadipocytes depending on the cellular microenvironment. To evaluate the importance of cell-cell contacts, we cocultured preadipocytes and macrophages isolated from the WAT stroma-vascular fraction (SVF) of the same obese patients. Cellular interactions were compared in two different types of 3D matrices. The actin cytoskeleton of the two cell types was visualized by phalloidin staining, whereas macrophages were stained with a macrophage-specific marker (Ham 56). Whereas matrigel, which is largely composed of basement membrane proteins, constitutes a loose microenvironment, the collagen I matrix reproduces fibrosis conditions. When cultured in matrigel, macrophages and preadipocytes remained rounded in shape (Fig. 8A). In marked contrast, in collagen I matrix, preadipocytes produced dendritic extensions and directed numerous filipodia toward the round macrophages (Fig. 8B). The results of this experiment suggest that interactions between preadipocytes and macrophages are highly influenced by the microenvironment surrounding these cells.
Interactions between macrophages and preadipocytes in 3D matrix. Preadipocytes and macrophages were isolated from adipose tissue from the same patient. Cells were seeded in 3D matrigel (A) or collagen I (B) matrices. Cells were then stained for actin (red, phalloidin) and Ham 56 (green, cy2-conjugated antimouse IgG). Bar, 10 μm. The experiment was repeated three times with identical results.
Preadipocytes located in fibrotic areas in WAT of obese individuals
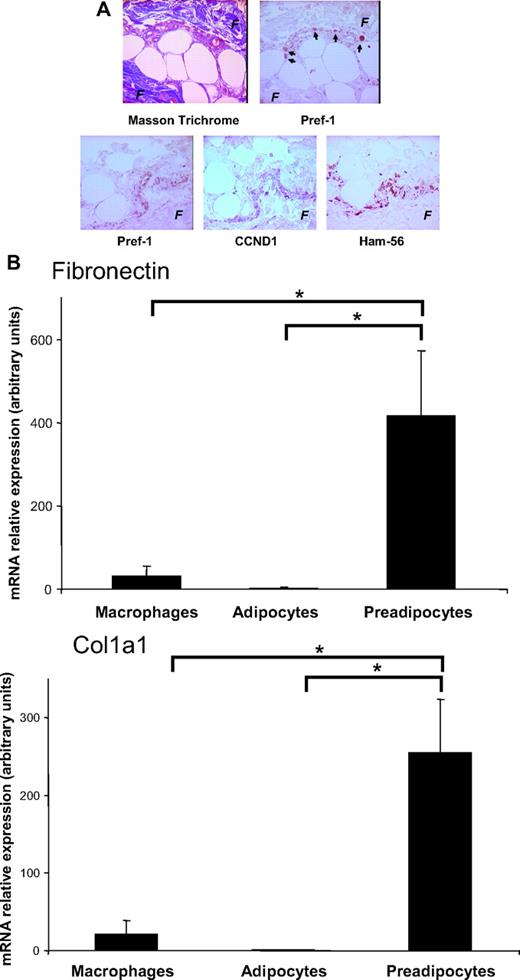
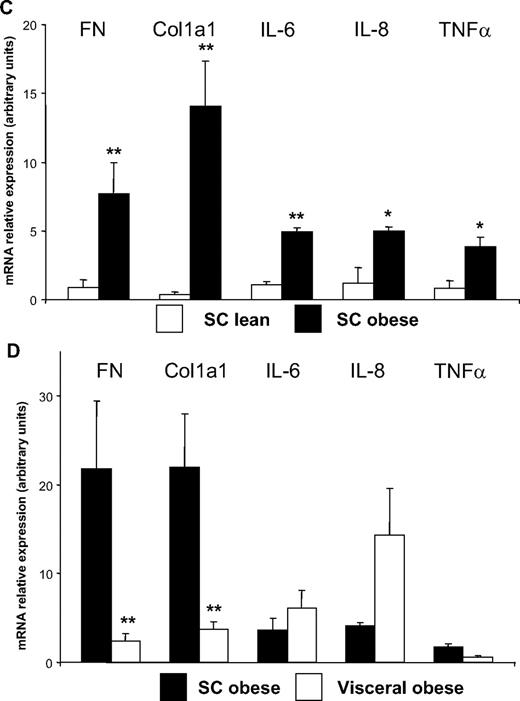
To further explore the involvement of human preadipocytes in synthesizing fibrosis components, we examined the preadipocyte localization by immunochemistry analysis of WAT sections obtained from several obese individuals. The preadipocytes, detected by immunostaining of Pref-1, a specific marker for preadipocytes (23), were found not only between adipocytes (data not shown) but also in the fibrotic areas. This cell-rich region was also positive for Cyclin D1 and the macrophage marker Ham-56 (Fig. 9A). To confirm the contribution of human preadipocytes to fibrosis, we determined the expression of fibronectin and the α-1 chain of collagen I (col1a1) by real-time PCR in macrophages, adipocytes, and preadipocytes isolated from obese WAT. As shown in Fig. 9B, fibronectin and col1a1 were found to be more highly expressed in preadipocytes than in other WAT cell types. To give stronger support to our in vitro data, we next performed a functional analysis of sc preadipocytes from lean and obese individuals. We observed, as also reported by another group in cells obtained from Pima Indians (24), that inflammatory (IL-6, IL-8, and TNFα) and ECM (fibronectin and col1a1) genes were up-regulated in sc preadipocytes from obese WAT (Fig. 9C). Visceral preadipocytes from obese individuals, although expressing similar expression levels of inflammatory genes, appeared less fibrotic than their sc counterparts (Fig. 9D).
Localization of preadipocytes near fibrosis in human adipose tissue. A, Serial sections of human obese WAT were stained, respectively, for fibrosis (F) (Masson trichrome coloration) and for markers of preadipocytes (Pref-1), proliferation (CCND1), and macrophages (Ham-56). These sections were obtained from five severely obese individuals (mean ± sem; BMI, 48.5 ± 3.5 kg/m2; age, 47.2 ± 2.4 yr). B, Adipocytes, macrophages, and preadipocytes were isolated from WAT of severely obese individuals. Fibronectin and col1 a1 were quantified by real-time PCR. Data are mean ± sem of six separate experiments. *, P < 0.05. C, Preadipocytes were isolated from sc WAT of six lean (BMI < 25 kg/m2, open bars) and six obese (BMI > 30 kg/m2, black bars) individuals. Fibronectin, col1 a1, IL-6, IL-8, and TNFα were quantified by real-time PCR. *, P < 0.05; **, P < 0.02. D, Preadipocytes were isolated from sc (black bars) and visceral (open bars) WAT of six obese (BMI > 30 kg/m2) individuals. Fibronectin, col1 a1, IL-6, IL-8, and TNFα were quantified by real-time PCR. **, P < 0.02. FN, Fibronectin.
Discussion
The incubation of human preadipocytes with macrophage-secreted factors has been shown to induce profound alterations of their phenotype (e.g. impaired differentiation, increased proliferation, and synthesis of cytokines and ECM components) (16). Using a series of human cell studies driven by the results of microarray experiments, we identified a set of molecular players involved in the formation of excessive amounts of ECM by inflammatory preadipocytes with increased adhesive and migratory capacities. We showed that one of these players, inhibin βA, has a mediating role in the production of profibrotic proteins by preadipocytes in contact with an inflammatory microenvironment.
In agreement with the gene expression findings, we found that inflammatory preadipocytes synthesize a highly ordered and abundant ECM composed of collagen I, as well as of tenascin-C and fibronectin, both of which are involved in the formation of the collagen network. α5-Integrin, the main receptor of fibronectin, was coordinately expressed with its ligand, reinforcing the molecular link between this ECM component and outside-in signaling. The gene expression profiles, confirmed by real-time PCR, showed that inhibin βA was one of the most overexpressed genes in preadipocytes cultured with macrophage-secreted factors. Although inhibin βA belongs to the TGF-β family, members of which are potent profibrotic molecules that act synergistically, it has never, to our knowledge, been shown to play a role in adipose tissue biology (21, 22). In contrast, TGF-β1 has been reported to be associated with human obesity (25, 26) with increased release by WAT from obese individuals (27). However, its role in the local biology of adipose tissue remains unclear. Using neutralizing antibody experiments, we showed that inhibin βA, but not TGF-β1 alone, mediated the production of fibronectin and collagen I, both of which are important components of the ECM network. We cannot exclude the contribution of other profibrotic factors acting coordinately with inhibin β A to the synthesis of ECM components and to the generation of fibrosis, as illustrated by the synergistic effect of inhibin βA and TGF-β1 in the production of collagen I. In the inflammatory and profibrotic context of obesity, cells such as macrophages exert paracrine actions on adipocyte metabolism and development via the production of a large number of inflammatory molecules (13–16, 28). Macrophages could also establish direct interactions with preadipocytes and adipocytes in vivo. 3D Matrices constitute appropriate models in which to study the influence of the ECM environment on cell interactions, rather than 2D cultures (29). Our coculture assays of macrophages and preadipocytes indicate that cellular interactions are mostly observed with cells cultured in a 3D collagen I matrix, a fibrosis-mimicking environment. These findings are consistent with previous observations showing selective induction of macrophage inflammation by ECM molecules such as collagen I (30). In further support of these data, we observed that preadipocytes cultured in a collagen I/fibronectin matrix secreted an increased amount of inflammatory cytokines. This finding suggests that the proinflammatory state of macrophages and preadipocytes provoked by this fibrotic microenvironment favors cross talk between these two cell types.
Furthermore, our immunostaining experiments revealed a strict paralleling of α5-integrin with actin stress fibers in Ac CM-treated preadipocytes. Biomechanical forces between the rigid ECM, sensed through integrins, and the actin cytoskeleton could influence both cell proliferation and migration, as observed in several other cell types (31). This phenomenon might extend to human preadipocytes. Indeed, we observed both proliferative and migratory phenotypes in inflammatory preadipocytes producing ECM components. Increased proliferation depends on fibronectin/integrin signaling, because the disruption of this partnership considerably abolishes the proliferative state. We show that this process depends on at least two molecular players: NF-κB, the nuclear mediator of cell inflammation, and cyclin D1, which is involved in cell cycle progression and arrest.
By siRNA knockdown of p65 NF-κB, we demonstrated that NF-κB activation is essential for the ECM remodeling and proliferation of human preadipocytes in response to macrophage-secreted factors. Because fibronectin itself increased the expression of p65 NF-κB (Fig. 3), it is possible that ECM remodeling could contribute, through NF-κB activation, to the maintenance of preadipocyte inflammation and its associated proliferative state. Fibronectin could also increase preadipocyte proliferation by up-regulating cyclin D1 whereas down-regulating p21, one of its inhibitors. Cyclin D1 and p21 are known to be associated with adipogenesis through interactions with peroxisome prolferator-activated receptor-γ, the main adipogenic transcription factor (32). Cyclin D1 is also up-regulated in inflammation sites, a feature that seems to be cell cycle independent (33). Cyclin D1 knockdown in inflammatory preadipocytes provoked a marked decrease in fibronectin production and, to a lesser extent, in the expression of inflammatory cytokines IL-6, IL-8, and TNFα. Thus, NF-κB and cyclin D1 could represent important molecular players in a regulatory loop controlling proliferation, inflammation, and ECM remodeling of human preadipocytes.
Moreover, cell migration is initiated in response to extracellular cues such as chemokines and signals from the ECM. This coordinated process involves changes in the reorganization of actin filaments together with the formation of cell adhesion sites. Focal contacts are initiated at ECM-integrin junctions, which bring together cytoskeletal and signaling proteins during cell adhesion, spreading, and migration (12). Cell spreading reflects cytoskeletal tension (34) transmitted by integrin signaling. The specific behavior of inflammatory preadipocytes during spreading on a rigid collagen I matrix could be associated with the presence of the integrin clusters and actin stress fibers that characterize these cells. FAK is a crucial component that promotes cell migration through a mechanical linkage between the ECM and the actin cytoskeleton. FAK activation is associated with the formation of focal contacts that contain a subset of proteins, one of which is α5-integrin (12). Here, we demonstrated that inflammatory preadipocytes display considerably increased migratory properties, integrin clustering, and a high level of FAK activation.
It is worth noting that tenascin-C, a ECM protein that shows a restricted pattern of expression in areas of active cell proliferation and tissue reorganization, is known to promote cell migration by acting on focal adhesion structures (35). Interestingly, tenascin-C, large amounts of which is deposited in the ECM of inflammatory preadipocytes, is induced by the same factors as FAK, including adhesion molecules and biochemical forces (36). Tenascin-C could also link ECM remodeling and the migration of inflammatory preadipocytes.
Our cellular study suggests that preadipocytes in contact with inflammatory cells within the adipose tissue could contribute to the synthesis of selective ECM molecules, most likely in concert with other cell types, such as fibroblasts, which remain to be definitively demonstrated. To connect our human cell findings with the conditions found within tissue, we performed experiments in human adipose tissue obtained from obese individuals. Our in vitro observations were strengthened by our immunohistochemistry findings, in which preadipocytes were labeled in fibrosis regions of sc WAT from obese individuals, a feature that we do not believe has been shown before.
Importantly, up-regulation of inflammation-related genes were found in sc preadipocytes from obese compared with nonobese Pima Indians (24). We also observed an inflammatory profile in addition to a fibrotic status in preadipocytes from sc obese WAT. Interestingly, visceral preadipocytes from obese WAT did not exhibit higher fibrogenic properties than sc ones. Some experimental evidence shows that, at least in vivo, the amount of fibrosis is not necessarily linked with the intensity of inflammation, suggesting that distinct mechanisms regulate fibrosis and inflammation (37).
Together, our results suggest that preadipocytes could act in the formation of interstitial fibrosis, composed mostly of collagen I and fibronectin, as seen in WAT from obese individuals. As such, they may contribute to the pathological state of human adipose tissue in obese humans (8, 38).
Pathophysiological links between ECM remodeling favoring adipose tissue fibrosis and obesity-related complications have not yet been established, but recent data suggest relationships between the macrophage-mediated pathological state of WAT and the metabolic complications of obesity in both mice and humans. Within intraabdominal WAT, murine obesity induced by a high-fat diet leads to a progressive pattern of adipocyte death, collagen deposition, macrophage inflammation associated with whole-body insulin resistance, and liver steatosis (39). We recently demonstrated correlative associations between the amount of sc WAT fibrosis and hepatic fibroinflammation in morbidly obese humans (8). Local tissue adaptations to fibrosis might also occur. For example, we showed that the cysteine protease Cathepsin S, the production of which is increased in WAT in obese humans, promotes in vitro preadipocyte differentiation caused, at least in part, by fibronectin degradation (38). Thus, this phenomenon could occur locally in an attempt to eliminate WAT fibrosis and restore impaired adipogenesis and the metabolic functions of hypertrophied WAT.
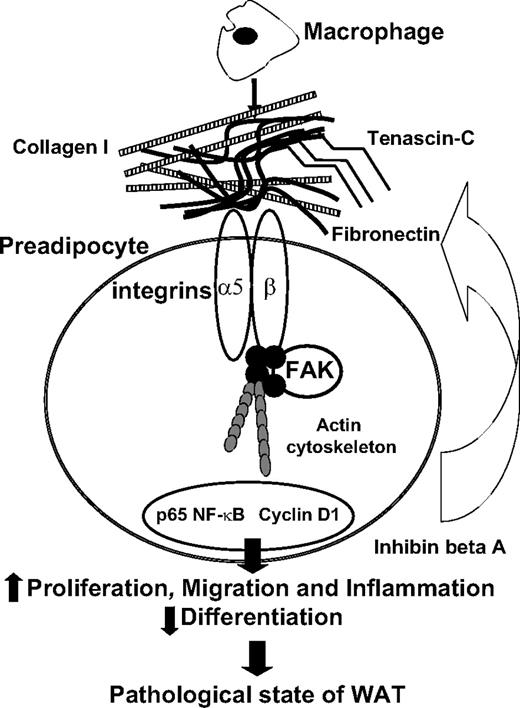
In conclusion, we showed that human preadipocytes exposed to molecules secreted by activated macrophages exhibited profound ECM remodeling. This particular microenvironment was associated with major modifications of preadipocyte phenotype, including inflammation, increased proliferation, and migration, as well as decreased differentiation (Fig. 10). Therefore, in the kinetic context of WAT changes such as those observed during weight loss and regain, where inflammation varies considerably (40), we suggest that if preadipocytes exhibit these altered properties, they could contribute to the formation of new fat depots.
Schematic representation of the biological effects of macrophage-secreted factors on human preadipocytes.
Materials and Methods
The following is a list of the antibodies used in the study: monoclonal antihuman fibronectin and cyclin D1 antibodies (BD Transduction Laboratories, San Jose, CA), rabbit antihuman collagen I (Novus Biologicals, Littleton, CO; mouse antihuman integrin β1 [CD29] adhesion blocking monoclonal antibody (6S6) (Chemicon, Temecula, CA) neutralizing antihuman α5-integrin subunit (CD49e) antibody (AF 1864) by (R&D Systems, Minneapolis, MN); antihuman integrin α5-subunit antibody (P1D6, H104, and P19) and rabbit antihuman tenascin-C (Santa Cruz Biotechnology, Santa Cruz, CA); rabbit antihuman Pref-1 from (Protein Tech Group, Chicago, IL), rabbit antihuman FAK and mouse phospho-NF-κB p65 (Ser536) (Cell Signaling Technology, Danvers, MA), mouse antihuman macrophage HAM56 (DAKO, Glostrup, Denmark) and rabbit antihuman FAK [pY397] (Biosource Technologies, Inc., Camarillo, CA).
Isolation of human preadipocytes
We obtained sc WAT biopsies from nonobese [body mass index (BMI) <30 kg/m2] young female patients undergoing elective surgery. None of the patients had diabetes or metabolic disorders, and none were taking medication. This study was approved by the Ethical Committee of Hôtel-Dieu Hospital (Paris, France). Human preadipocytes were isolated and cultured as described elsewhere (16, 41, 42). Cells were used at passage 2 to eliminate contamination by nonpreadipocyte cells; this was confirmed by negative staining for macrophage markers (Ham 56 and Mac-1).
Preparation of human blood monocyte-derived macrophages and conditioned medium
Blood from female patients was processed for plasma blood mononuclear cell isolation (16). Briefly, we differentiated macrophages from monocytes as described previously with slight modifications (43). CD14-positive cells were selected from the plasma blood mononuclear cells (Stemcell Technologies, Grenoble, France). After differentiation in RPMI and 10% fetal bovine serum (FBS) for 8 d, macrophages were incubated for 24 h with 100 ng/ml lipopolysaccharides (from Escherichia coli 0127:B8, Sigma, St. Louis, MO) before collecting the medium (Ac CM).
Preparation of different cell type from WAT
Isolations of macrophages (adipose tissue macrophages) and preadipocytes from the SVF of WAT from obese individuals were performed as previously described (7, 44) by using immunoselection cocktails (Stemcell Technologies).
Differentiation of human preadipocytes
Preadipocytes were cultured for 24 h in DMEM and 10% FBS and then incubated with control RPMI or with Ac CM (1V corresponding to 1 × 105 activated macrophages) and DMEM/F12 (3V) induction medium for 4 d. The medium was then replaced with either control RPMI or Ac CM and DMEM/F12 culture medium as described in Ref. 16 .
Direct cocultures of preadipocytes and macrophages in 3D matrices
Preadipocytes and macrophages were prepared from the SVF of WAT from the same obese patients as described above and in Refs. 16 and 44 . Cells were then cocultured in either 3D collagen cell culture system (Chemicon) or matrigel (BD Biosciences, Palo Alto, CA) at a concentration of 1 × 105 cells of each cell type per 0.2 ml of gel. The cell suspensions were then transferred into 48-well plates and fed by RPMI and 1% FBS. After 48 h, gels were fixed, stained as described (45), and examined for immunofluorescence.
Immunochemistry in human WAT
Immunochemistry analyses were performed on serial sections of WAT from five obese patients (mean ± sem; BMI, 48.5 ± 3.5 kg/m2, age, 47.2 ± 2.4 yr) as described in Ref. 38 . DLK1/Pref-1 and Cyclin D1 proteins were detected with antihuman Pref-1 and cyclin D1 monoclonal antibodies. Mouse antimacrophage Ham 56 was used to detect macrophages. WAT fibrosis was revealed using the Masson trichrome coloration kit (Sigma-Aldrich, St. Louis, MO).
Preadipocyte transfection siRNAs
We transfected human preadipocytes as described in Ref. 46 , with slight modifications. Briefly, after a 24-h culture, preadipocytes were treated with 100 nm on target plus siRNA or negative control (Dharmacon, Lafayette, CO) complexed with a low cell-toxicity reagent, lipofectamine RNAi MAX (Invitrogen, Carlsbad, CA). After 5 h, Ac CM media were added, and different experiments were performed after 5 d. We verified that the siRNA negative control (Dharmacon nontargeting sRNA no. 2) did not affect fibronectin, p65NF-κB, and CCND1 protein levels (data not shown).
mRNA preparation and real-time PCR
RNA extraction was performed with the RNeasy RNA Mini Kit (QIAGEN, Courtaboeuf, France). Reverse-transcription and real-time PCR were conducted as described in Ref. 16 . Primers for the tested genes are listed in Table 1. All values were normalized with regard to 18S expression.
List of primer sequences used for real-time PCR
| Gene . | Forward . | Reverse . |
|---|---|---|
| hCol1 a1 | GGGATTCCCTGGACCTAAAG | GGAACACCTCGCTCTCCAG |
| hCyclin D1 | GAAGATCGTCGCACCTG | GACCTCCTCCTCGGACTTCT |
| hFibronectin | CTGGCCGAAAATACATTGTAAA | CCACAGTCGGGTCAGGAG |
| hIL-6 | GCCCAGCTATGAACTCCTTCT | GAAGGCAGCAGGCAACAC |
| hIL-8 | AGACAGCAGAGCACACAAGC | ATGGTTCCTTCCGGTGGT |
| hInhibin βA | CTCGGAGATCATCACGTTTG | CCTTGGAAATCTCGAAGTGC |
| hTNFα | CAGCCTCTTCTCCTTCCTGA | GCCAGAGGGCTGATTAGAGA |
| Gene . | Forward . | Reverse . |
|---|---|---|
| hCol1 a1 | GGGATTCCCTGGACCTAAAG | GGAACACCTCGCTCTCCAG |
| hCyclin D1 | GAAGATCGTCGCACCTG | GACCTCCTCCTCGGACTTCT |
| hFibronectin | CTGGCCGAAAATACATTGTAAA | CCACAGTCGGGTCAGGAG |
| hIL-6 | GCCCAGCTATGAACTCCTTCT | GAAGGCAGCAGGCAACAC |
| hIL-8 | AGACAGCAGAGCACACAAGC | ATGGTTCCTTCCGGTGGT |
| hInhibin βA | CTCGGAGATCATCACGTTTG | CCTTGGAAATCTCGAAGTGC |
| hTNFα | CAGCCTCTTCTCCTTCCTGA | GCCAGAGGGCTGATTAGAGA |
h, Human.
List of primer sequences used for real-time PCR
| Gene . | Forward . | Reverse . |
|---|---|---|
| hCol1 a1 | GGGATTCCCTGGACCTAAAG | GGAACACCTCGCTCTCCAG |
| hCyclin D1 | GAAGATCGTCGCACCTG | GACCTCCTCCTCGGACTTCT |
| hFibronectin | CTGGCCGAAAATACATTGTAAA | CCACAGTCGGGTCAGGAG |
| hIL-6 | GCCCAGCTATGAACTCCTTCT | GAAGGCAGCAGGCAACAC |
| hIL-8 | AGACAGCAGAGCACACAAGC | ATGGTTCCTTCCGGTGGT |
| hInhibin βA | CTCGGAGATCATCACGTTTG | CCTTGGAAATCTCGAAGTGC |
| hTNFα | CAGCCTCTTCTCCTTCCTGA | GCCAGAGGGCTGATTAGAGA |
| Gene . | Forward . | Reverse . |
|---|---|---|
| hCol1 a1 | GGGATTCCCTGGACCTAAAG | GGAACACCTCGCTCTCCAG |
| hCyclin D1 | GAAGATCGTCGCACCTG | GACCTCCTCCTCGGACTTCT |
| hFibronectin | CTGGCCGAAAATACATTGTAAA | CCACAGTCGGGTCAGGAG |
| hIL-6 | GCCCAGCTATGAACTCCTTCT | GAAGGCAGCAGGCAACAC |
| hIL-8 | AGACAGCAGAGCACACAAGC | ATGGTTCCTTCCGGTGGT |
| hInhibin βA | CTCGGAGATCATCACGTTTG | CCTTGGAAATCTCGAAGTGC |
| hTNFα | CAGCCTCTTCTCCTTCCTGA | GCCAGAGGGCTGATTAGAGA |
h, Human.
mRNA amplification and microarrays
Total RNA (1μg) from control and Ac CM-treated preadipocytes was amplified using the MessageAmp RNA kit (Ambion, Austin, TX), and 3 μg of amplified RNA (aRNA) was dye labeled with Cy using the Cyscribe first-strand cDNA labeling kit (GE Healthcare Biosciences, Little Chalfont, UK) (47). aRNA from control preadipocytes was labeled with Cy3 dye, whereas the aRNA from Ac CM-treated preadipocytes was labeled with Cy5 dye. The hybridization, washing, and scanning procedures were performed as described elsewhere (47). Several quality checks (total RNA quality, aRNA quality, and dye incorporation efficiency) were performed as previously described (47).
Microarray data analysis
Eight cDNA microarrays were performed to characterize the transcriptomic signature of human preadipocytes (gene expression ontology accession no. GSE9017). A print-tip loess normalization was performed after the log transformation of the background-corrected microarray expression measurements (48). Significant gene expression changes were identified by imposing a 5% FDR threshold in the Significance Analysis of Microarrays procedure (49). A functional analysis was performed using the FunCluster algorithm (50) to identify annotating Gene Ontology categories (GO, available at http://www.geneontology.org) enriched in genes differentially expressed in Ac CM-treated preadipocytes vs. control preadipocytes. Individual gene enrichment P values were calculated for each GO category by using a unilateral Fisher’s exact test and then adjusted for multiple testing errors with the Storey FDR correction approach (51). Afterward, a specific clustering procedure was used to group annotating GO categories sharing a significant number of coexpressed genes (50) so that we could explore the regulatory patterns of enriched biological themes.
Western blot analysis
Cell extracts were prepared in buffer containing a cocktail of protease and phosphatase inhibitors. The membranes were probed with the appropriate primary antibodies. Specific signals were detected with ECL detection solution (GE Healthcare) and immediately exposed to x-ray film. Signals were quantified by densitometry. Loading controls are presented as a protein band stained with Ponceau red.
Immunofluorescence analysis and confocal microscopy
Preadipocytes were differentiated on glass coverslips with or without Ac CM for 10 d. Coverslips were incubated with the appropriate primary antibody, and then the corresponding Cy2-conjugated antimouse IgG or Cy3-conjugated antirabbit IgG (GE Healthcare). Neutral lipids were stained with Bodipy 493/503, and actin was stained with AlexaFluor 546 phalloidin (Molecular Probes, Eugene, OR) during the secondary antibody incubation. Nuclei were counterstained with DAPI or TO-PRO-3 iodide (Invitrogen). Negative controls were performed by omitting primary antibodies. Coverslips were examined with an Olympus BX 41fluorescence microscope (Olympus Corp., Lake Success, NY) or a Zeiss 510 confocal laser scanning microscope(Carl Zeiss, Inc., Thornwood, NY) using a plan-neofluar ×40 objective.
Other cellular determinations
Proliferation studies were performed by culturing preadipocytes for 5 d at a cell density of 2 × 103 cells per well in 96-well plates either coated with fibronectin (5 μg/cm2) or (PLL) (10 μg/cm2) or left bare. At the end of the culture, the number of viable cells was measured using the [3–(4,5-dimethylthiazol-2-yl)-5–(3-carboxymethoxyphenyl)-2–(4-sulfophenyl)]-2H-tetrazolium inner salt (MTS) proliferation assay (Promega Corp., Madison, WI).
Nuclear extracts from preadipocytes were prepared as described in Ref. 52 . Cytokine measurements in preadipocyte media were performed using human IL-6 and TNFα immunoassays (R&D Systems, Minneapolis, MN) according to the manufacturer’s protocol. The data were normalized according to cell number estimated by nuclei counting.
Preadipocyte migration assays were performed using an insert system of 8 μm pore size coated with fibronectin (5 μg/cm2). An equal number of control and Ac CM-treated preadipocytes were placed in the upper well, and the lower well was filled with DMEM and 10% FBS. After 16 h at 37 C, nonmigrated cells were removed by scraping, and migrated cells were quantified by the crystal violet technique (53). Immunofluorescence detection of actin was also performed in the same experimental conditions using fluoblock inserts (Costar, Cambridge, MA).
Statistical analysis
The experiments were performed at least three times, each using preadipocytes from different human subjects and distinct macrophage-conditioned media. Statistical analysis was performed using Student’s t test. Comparisons among more than two groups were carried out using a one-way ANOVA, where a P < 0.05 was considered to be statistically significant.
Acknowledgments
We thank Martine Pinçon-Raymond (INSERM Unité 872) for helpful discussions concerning ECM studies, Adeline Divoux for help in immunochemistry experiments, David Mutch for critical reading of the manuscript, and Christophe Klein for providing immunofluorescence and confocal microscopy facilities (Plateau Imagerie Cellulaire de l’Institut des Cordeliers). We thank the patients and physicians of the nutrition department of Pitié-Salpêtrière hospital, Paris, for patients’ recruitment. For cellular studies, ethical authorization was obtained from Comité de Protection des Personnes Hôtel-Dieu. Human adipose tissues pieces were obtained thanks to Assistance Publique/direction de la Recherche Clinique No. 02076.
This work was supported by the French National Agency of Research (Agence Nationole de la Recherche, RIOMA No. ANR-05-PCOD-030-02) and a grant from the European Community seventh framework program, Adipokines as Drug to Combat Adverse Effects of Excess Adipose Tissue project (Grant Agreement No. 201100).
Disclosure Statement: The authors have nothing to declare.
M.K. and V.A. contributed equally to the work.
Abbreviations
- Ac CM
Activated macrophages;
- aRNA
Amplified RNA;
- BMI
body mass index;
- CCND1
cyclin D1;
- Col1a1
collagen type I α-1 chain;
- 3D
three dimensional;
- ECM
extracellular matrix;
- FAK
focal adhesion kinase;
- FBS
fetal bovine serum;
- FDR
false discovery ratio;
- GO
gene ontology;
- MTS
[3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)]-2H-tetrazolium inner salt;
- NF-κB
nuclear factor-κB;
- PLL
poly-l-lysine;
- siRNA
small interfering RNA;
- SVF
stroma-vascular fraction;
- WAT
white adipose tissue.