Uveal melanoma as a target for immune-therapy
Introduction
Uveal melanoma (UM) is a rare disease accounting for 0.1% of all cancer deaths. On the other hand, this is the most common primary intraocular malignant tumor in adults, with an annual incidence of six cases per million in white populations and this rate seems to have remained stable over time (1). Global overall survival at five years is as low as 60%. This elevated mortality rate is caused by a high incidence of metastases (2-5). The clinical and metastatic behavior differs from cutaneous melanoma because of its initially purely hematogenous dissemination and its tendency to metastasize to the liver (6). The reason for this liver-homing is largely unknown but raises the possibility that hepatic environmental factors may be implicated in the growth, dissemination, and progression of this malignancy. When liver metastases develop, the prognosis is poor and life expectancy reduces to less than 6 months in the absence of treatment (7,8).
Systemic chemotherapy is usually unsuccessful in metastatic UM and results in an objective response rate that ranges from 5% to 15%. There is no proof that conventional chemotherapy prolongs survival, which remains between 6 and 10 months with only 15% of patients alive at one year (7,9) [(9) is an excellent review of published chemotherapy trials]. Although many therapies have been developed, the 5-year survival rate of patients with UM has not improved in more than 25 years (9). Available clinical trials are still the best treatment option for these patients.
A better understanding of melanoma molecular biology has been critical in developing successful treatments for cutaneous melanoma, but that knowledge has not yet led to similar developments in regard to its uveal counterpart. Recently, oncogenic mutations in GNAQ or GNA11 (GNAQ/11) have been identified in 80% of primary UMs. GNAQ/11 activate signaling pathways, including the mitogen-activated protein kinase (MAPK) pathway, similar to the way that BRAF mutation does in cutaneous melanoma (10,11). A first trial has shown that blocking MAPK activation with selumetinib modestly improves progression-free survival and response rate without a clear effect in overall survival (12), but that has not been reproduced in a confirmatory phase III trial combining selumetinib with chemotherapy (13). A reasonable explanation for this might be that GNAQ/11 is positioned upstream in the signaling pathway, so when it is mutated, it activates several pathways including MAPK, YAP1, and others. Recent papers have shown that YAP1 is activated by GNAQ/11 mutations and YAP1 is responsible for tumor growth in the tumor models used (14,15). Other pathways such as PKC, and AKT are also activated by GNAQ/11, but the specific implication of the various pathways in UM pathogenesis is still unknown.
An antibody against the Cytotoxic T-lymphocyte-associated protein-4 (CTLA-4), Ipilimumab, has been approved for the treatment of metastatic melanoma due to a slightly but significant improvement in overall survival leading to an increased proportion of patients alive at a later follow up point (16-18). Unfortunately these trials systematically excluded patients with metastatic UM. Experiences with Ipilimumab in metastatic UM are restricted to a couple of phase II trials and retrospective analysis of several expanded-use programs in a heterogeneous population (9,19-27). Recently two blocking antibodies against the T-cell programmed death-1 (PD-1) checkpoint protein have shown impressive activity in metastatic melanoma and both have been approved as first line treatment for this disease (28,29). Again, these trials systematically excluded patients with metastatic UM and, to date, there is no extant data regarding activity of anti-PD-1 or anti-programmed death ligand-1 (PD-L1) in treating metastatic UM patients.
In the present paper we review UM immune-biology with a special focus on those points that can be exploited as targets for immune-therapy.
The immune-privileged nature of the eye and how uveal melanoma (UM) exploits it
The eye is a slightly asymmetrical globe mostly filled with a gel called the vitreous. The front part includes the cornea, the uvea, the pupil, the sclera, and the conjunctiva. The main function of the cornea and the pupil is to absorb the light and focus it through the lens to the back of the eye. The inside lining of the back of the eye is covered by light-sensing cells, called the retina, which convert light into electrical impulses that are carried to the brain by the optic nerve. The uvea, constituted by the iris, the ciliary body and the choroid, has two main functions; nutrition and gas exchange. The uvea divides the front area into two separate spaces: the anterior chamber, which is the space between the cornea and the iris that is filled by aqueous humor; and the posterior chamber, which consists of a small space directly posterior to the iris but anterior to the lens. In the uvea we can find endothelial cells, immune cells and melanocytes. Melanocytes give color to the eye, and are the cells from which UM develops.
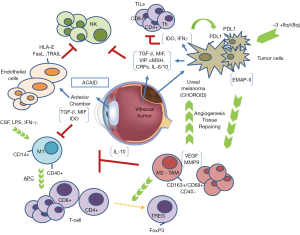
The eye is considered an immune-privileged organ; it possesses some singularities in regard to immune response. It has a unique ability to defend itself against uncontrolled inflammation that could damage eyesight. This immune privilege influences the immune response against UM cells and provides escape mechanisms for UM (30). Different factors play a role in the immune-privilege of the eye. For example, the aqueous humor is rich in immunosuppressive proteins such as transforming growth factor β (TGF-β), vasoactive intestinal peptide (VIP), α-melanocyte-stimulating hormone (α-MSH), and complement regulatory proteins (CRP’s); the blood-eye barrier restricts inflammatory cell access to the eye; eye cells reduce major histocompatibility complex (MHC) class Ia expression to escape cytotoxic mediated lyses; and, ocular cells express PD-L1 which inhibits T cell response (31). Furthermore this immune response inhibition is not limited to the eye, and by means of a process called anterior chamber-associated immune deviation (ACAID) active immune cells interact with the immune system to induce unusual suppression of the systemic immune response when an antigen is detected in the anterior chamber. The eye, the thymus, the spleen, and the sympathetic nervous system are also involved in ACAID (32). The objective is to moderate inflammation against antigens presented into the eye as an evolutionary adaptation to prevent the loss of eyesight.
Immunotherapy has proven extraordinarily effective in the treatment of several kinds of tumors (18,28,29,33). In order to achieve the maximal benefit of this treatment, it is crucial to have a deeper understanding of the interaction between the immune system and cancer cells. The immunoediting theory explains how tumors modify themselves and their microenvironment with the purpose of evading destruction by the immune cells (34). In other words, immunoediting is characterized by changes in the immunogenicity of tumors due to the anti-tumor response of the immune system, resulting in modification of the tumor microenvironment and the emergence of immune-resistant variants of the tumor. Recently, the existence of four different types of tumor microenvironments has been proposed based on the presence or absence of tumor-infiltrating lymphocytes (TILs) and PD-L1 expression (Table 1), and this classification might have therapeutic implications (35).
Immune-escape mechanisms exploited by cancer can be far different between various diseases (35,36), and there is strong evidence that UM cells mimic the mechanisms that enable normal ocular cells to be immune-privileged, both in the eye and other metastatic locations (30). Deeper knowledge of the immune-escape mechanism of UM will improve our therapeutic approach in regard to this deadly disease.
Innate immunity might be more important than we expect
The innate immune system is the nonspecific part of the immune system that responds to pathogens in a generic way without conferring long-lasting or protective immunity on the host. Two cell types of the innate immune system might play an important role in UM; the natural killer (NK) cells, and the macrophages.
NK cells protect us against viral infections and neoplasms. Their main function is to recognize and kill any cell failing to express MHC class I molecules (37). Characteristically, endothelial cells that line the anterior chamber of the eye as well as UM cells seem to express little or no MHC class I molecules (30,38). In fact, it has been observed in vitro that tumor cells’ susceptibility to cytolysis by NK cells is inversely correlated with the expression of MHC class I molecules. Although this may suggest that endothelial and tumor cells would be the perfect targets for NK, that is not the case. In 1990, a large immunohistochemical study of immune-cell populations demonstrated that there was no NK infiltrate in primary UM nor in the anterior chamber of the eye (39). NK cells are believed to have an important influence on the growth and metastatic potential of UM, however their specific role is still unclear. Several mechanisms have been described in order to explain how UM cells avoid NK destruction. One of them is the presence of macrophage migration inhibitory factor (MIF) and TGF-β in the aqueous humor of the anterior chamber, two cytokines capable of inhibiting NK cell-mediated cytolytic activity, as it has been observed in vitro and in vivo studies. Apte et al. found that NK-sensitive human UM cells transplanted subcutaneously to nude mice were rejected by NK cell-dependent processes whereas when transplanted into the anterior chamber tumors grew progressively in the eye (40,41). Interestingly, TILs have been observed in primary UM, and as much as 40% of them express NK membrane cell markers (42). Not only in the primary site, but also once in the peripheral blood and especially in the liver, UM metastatic cells find themselves in an environment that has the highest concentration of NK cells of any organ in the body (43). It seems that these cells are able to produce higher amounts of MIF than primary tumor cells, allowing them to escape this anti-tumor immune step (44).
Following this step, another way to escape NK cells is related to the expression of HLA-E, a MHC class Ib molecule that specifically inhibits NK cells, and other inhibitory molecules such as FasL, PD-L1, or TRAIL on tumor cell membranes in response to different pro-inflammatory cytokines such TNF-β and IFN-α (30,38). In UM cells, loss of heterozygosity at the MHC class I locus is a frequent event found in approximately 50% of primary tumors (45-47). Similar to the endothelial cells of the anterior chamber, HLA-E is overexpressed and significantly enhanced by INF-γ in UM cells (48). Additionally, there is a lack of HLA-A or -B expression on the surface of UM cells, and that has been associated with longer patient survival (36,45-47). This seems to be discordant with what we observe in most malignant tumors where down-regulation or mutations on antigen-presenting machinery are associated to poor prognosis (36,49,50). This might be related to the pure hematogenous dissemination of UM. A high amount of NK cells are found in the blood, so UM cells need to increase expression of MHC class I molecules prior to dissemination in an attempt to avoid being recognized by NK cells. The fact that UM remodels the expression of MHC class I molecules in a way that is different to most solid tumors, and the presence of NK inhibitory molecules locally and in the metastatic site, might be related to the predominant role that NK cells have during the immunoediting process in this disease. To support this, it has been shown that neoadjuvant treatment with INF-α2b in a murine model has resulted in decreased hepatic micro-metastasis and increased survival time through increasing intrinsic hepatic NK cell-mediated tumor apoptosis (51,52). There are no available treatments that target NK cell activation but pharmaceutical and biotechnological companies are working on compounds that potentiate NK cell activation and tumor rejection. UM should be a major target for new immunotherapy treatments that activate NK cells.
Macrophages have different functions depending on the subtype. The M1 subtype has an immunostimulatory role acting as antigen-presenting cells (APCs), while the M2 subtype favors angiogenesis and works as myeloid-derived suppressor cells (MDSC), inhibiting the development of effective immune responses and participating in the induction of immune tolerance. Tumor-associated macrophages (TAMs) are responsible for the immune-suppression within the tumor (37) and some studies claim that in UM, TAMs are mainly M2 and their presence confers poor prognosis (53). The first observation was performed by Mäkitie et al. They observed that a high density of macrophages in the tumor was associated with poor prognosis (54). A second study by Maat et al., determined the density of TAMs in 149 choroidal and ciliar body melanomas. The group with moderate to high density constituted 83% of the tumors and was associated with more patients dying from UM metastasis (55). Interestingly, it was also related to bad prognostic pathologic features such as larger basal tumor dimensions, epithelioid cell type, heavy pigmentation, and high microvascular density. Inflammatory infiltrates in the tumor microenvironment are critical for the development of malignancies, and UM cells might take advantage of this inflammatory environment through the recruitment of macrophages in the tumor that drive the pro-angiogenic and immunosuppressive function. TAMs-driven angiogenesis is vital to tumor growth in UM as shown by Ly et al. In this study, when macrophages were removed from an intraocular tumor model in old mice, tumor growth was almost completely prevented (56).
High infiltration by TAMs has also been associated to the presence of chromosome 3 monosomy, a genetic alteration related to worse tumor prognosis. This seems to be asociated with a loss of function mutations in the BRCA1-associated protein 1 (BAP1) gene (57-59). This genetic alteration is thought to contribute to the generation of this characteristic inflammatory microenvironment in which macrophages play an important role (57). In a recent work Jager et al. proposed the term “inflammatory phenotype” to define uveal malignant tumors carrying a high density of macrophages and T-lymphocytes, a high expression of MHC class I and II, a high microvascular density, and a presence of epithelioid cells (60). This might be mediated by cytokines produced not only by the tumor cells but also by normal cells in the eye such as IL-6, IL-10, TGF-β, MIF, GM-CSF, and VEGF. Most of those are immunosuppressive and are known to polarize macrophages to the M2 subtype. For example, the EMAP-II is a chemotactic cytokine with an important effect on macrophages that has been identified in several primary UM tumor cells (61). Other soluble factors are produced by macrophages themselves, such as melanoma inhibitory activity (MIA), that facilitates the detachment of the tumor cells from the extracellular matrix (62). M2 TAMs can modify T-lymphocyte responses as they drive the conversion of naïve T-cells to T-regulatory lymphocytes (T-reg) and regulate their recruitment. The presence of T-reg in the tumor has been strongly correlated to the amount of M2 macrophages. A loss of certain markers expressed in eye tissues and TAM’s, like CD40, a marker necessary for the lymphocyte T-mediated immune-response has been observed (63). This loss seems to impede a proper T cell-mediated anti-tumor response due to an incorrect APC function.
Finally, treatment of the primary tumor may activate recruitment of immune-related cells with anti-tumor effect. Irradiated tumors have moderate to high numbers of infiltrating TAMs carrying pigment, also called melanophages. Moreover, a study comparing primarily- and secondarily-enucleated eyes after brachytherapy showed significantly more necrosis and low micro-vascular density in the second group even though the number of macrophages was similar in both groups. This observation suggests that the role of macrophages in that situation is mostly to clear damaged tumor cells and to repair tissue, but little is known if that observation has any clinical impact (64).
There are no available treatments that target the function of macrophages but extensive efforts are being made to achieve compounds that allow us to modulate the activation of macrophages from an immunosuppressive M2 to a more antitumoral M1 class.
Adaptive immune system, friend or foe?
The adaptive immune system is composed of highly specialized cells. As the name itself says, cells are able to adapt to different pathogens and generate immunological memory after an initial response to a specific antigen. This adaptation leads to an enhanced response to subsequent encounters with that pathogen. The adaptive immune system includes both humoral immunity and cell-mediated immunity. Given the goal of this review, we are going to focus on cell-mediated immunity, and the main key player, the T-lymphocyte (65).
Activated T-cells, both T-helper and T-cytotoxic, are present in UM, and they can be inhibited by tumor cells using a variety of processes. One of them is the production of indoleamine 2,3-dioxygenase (IDO), which is an enzyme that leads to tryptophan depletion, impairing lymphocyte proliferation (66). Furthermore, metabolites produced by this enzyme act as immune suppressors at other cellular levels such as NK-cells and macrophages. Expression of an altered Fas Ligand, resistance to perforin action via INF-γ secretion, and alteration of CRPs are three ways in which the lysis of tumor cells can be avoided (30).
The most relevant mechanism to inhibit T-cell action by tumor cells is the overexpression of PD-L1 receptor, and the same can be said for UM. Multiple in vitro studies demonstrate that UM cell lines express this ligand when exposed to IFN-γ, and this expression seems to be functional (67,68). In a work performed on primary UM tumors and metastatic UM cell lines authors demonstrated that expression of PD-L1 by UM cells regulates T-cell function by suppressing IL-2 production. Furthermore, the presence of INF-γ in the tumor local microenvironment promotes up-regulation of the PD-L1 expression by UM, promoting immune escape by impairing T-cell function (31). Another work demonstrated that UM cell lines constitutively express PD-L1 mRNA and negatively regulate T-cell immune response through the inhibition of T-cell activation (68). A more recent work has demonstrated that PD-L1 expression is dynamic and tied to INF-γ expression, and IL-2 production from purified CD3+ T-cells co-stimulated with INF-γ treated UM cells was significantly enhanced by the addition of anti-PD-L1 monoclonal antibody (67). This observation suggests that PD-L1 contributes to suppression of T-cells by decreasing IL-2 production. UM cells do not constitutively express PD-L1 while they are in the immunoprivileged ocular microenvironment, but when they metastasize, these cells come in contact with IFN-γ produced in the new organ and consequently, PD-L1 is up-regulated leading to T-cell apoptosis and a decrease in the production of cytokines. Furthermore INF-γ, an immunostimulatory cytokine, has an inhibitory effect since it boosts the MHC class I presentation machinery but suppresses their MHC class I-restricted destruction by CD8+ lymphocytes (30). According to that data, UM not only benefits from the immune privilege of the eye but also has adopted many of the mechanisms that contribute to ocular immune privilege as a strategy for protecting UM cells once they leave the sanctuary of the eye and are disseminated systemically in the form of metastases.
Contrary to what is found in other tumors, lymphocytic infiltration is related to poor prognosis in UM (69,70). TILs in UM are mainly CD8+ cytotoxic T cells (39) and they were present in all 43 cases analyzed by Bronkhorst et al. In addition, CD4+ T-helper cells could also be found in 91% of the samples, and approximately half of these were FoxP3+ T-reg cells (57). It is also noteworthy that a well-characterized prognostic factor in UM, such as chromosome 3 monosomy, seems to be strongly correlated with larger lymphocytic infiltrate. The key question here is why TILs confer poor prognosis. One of the hypothesis is that metastatic dissemination is required to create a T-cell response due to the peculiar immune ocular characteristics (70). If that hypothesis is true, only the UM that disseminate outside the eye should have TILs in the primary tumor. The immunosuppressive microenvironment of the primary site, along with inhibitory characteristics displayed by UM cells would render this infiltrate non-effective when it comes to immune-surveillance. Another, more intriguing, possibility is that TILs not only fail to eliminate tumor cells but also help tumor growth (9). Many examples demonstrate that inflammation can promote proliferation and survival of cancer cells (71). Activated TILs can produce inflammatory mediators, generating a cancer-related inflammatory microenvironment. In this tumor promoting ambient, secondary activated macrophages play a key role.
Is there a role for immunotherapy in metastatic uveal melanoma (UM)?
Among patients with advanced ocular melanoma, the development of autoimmune phenomena or hypothyroidism during therapy has been associated with a favorable outcome. In a recent study, among the patients affected with UM, it was reported that 8.8% had a systemic autoimmune disease and 13.2% had autoimmune hypothyroidism (72). The same study concluded that there is a trend toward longer survival from the date of metastasis in UM patients with a systemic autoimmune disorder, suggesting that systemic autoimmunity may play a role in modifying the activity of established metastases.
There are not many clinical trials in metastatic UM. Recently all data from the few phases II and the only one phase III clinical trials available have been reviewed. Overall response rate (ORR) was 4.6% (95% CI: 3.3–6.3%), and no responses were seen in more than 50% of the trials included. Interestingly, the best responses were seen for chemo-immunotherapy (ORR 10.3%; 95% CI: 4.8–18.7%), though mainly in first-line patients (9). In addition, some of the longest overall survivals reported are in the chemo-immunotherapy group as well. This new era of immunotherapy has provided new drugs that have been tested already in UM. Ipilimumab, an antibody that blocks CTLA-4 has been approved to treat patients with metastatic melanoma based on better OS and long-term survival in a proportion of patients (16-18). Once again, metastatic UM patients were systematically excluded from those trials. Experience with Ipilimumab in metastatic UM is limited to data from few expanded-use programs and two phase II clinical trials (Table 2) (19-27). A first phase II trial from the DeCOG group in Germany has been reported (26). No patients experienced responses, and 1- and 2-year OS were 22% and 7% respectively. The authors concluded that ipilimumab has very limited clinical activity in this setting. A second phase II trial has been presented by the Spanish Melanoma Group (GEM) (27). Partial responses were seen in close to 10% of the patients, and responses were seen even in liver metastases. Overall survival was 10 months and 1- and 2-year OS were 48% and 25%, respectively. There are several differences between both studies. The DeCOG trial used ipilimumab at a dose of 3 mg/kg, and 85% of patients were pretreated. In the GEM trial ipilimumab was administered at a higher dose of 10 mg/kg, and all patients were treatment-naïve. Results in the GEM trial are in line with clinical experience with first line ipilimumab in cutaneous melanoma. Toxicity was manageable in both trials and did not differ from other clinical trials with ipilimumab in other tumors. There is still no available results with anti-PD1 or anti-PD-L1 in metastatic UM, and a few experiences are limited to patients treated in phase I trials. As we previously explained, PD-L1 is inducible in UM cell lines, and seems to be functional (67,68), so treatment with anti-PD1, anti-PD-L1, or combinations may be effective. There are currently three clinical trials exploring this strategy; pembrolizumab in monotherapy (NCT02359851) and the combination of ipilimumab and nivolumab (NCT01585194 and NCT01585914). The results from these clinical trials are expected to be available no earlier than 2017. Another interesting immunotherapeutic approach in this disease, different to checkpoint inhibitors, is the infusion of autologous TILs, which is being tested in a clinical trial at the NIH (NCT01814046).

Full table
Conclusions
In conclusion, there is a need to improve systemic treatment in metastatic UM patients. Still today, there is no evidence that any treatment prolongs overall survival in metastatic UM and results obtained with chemotherapy are unsatisfactory. UM cells evade immune surveillance through several mechanisms that involve inhibiting both innate and adaptive responses. The knowledge of this processes leads to new therapeutic approaches, some that are already being tested like PD1 inhibition and other potential treatments like checkpoint activating antibodies, IDO and TGF-β Figure 1. To improve the knowledge about how UM, a tumor that arises in an immune-privileged site, evade the immune system is mandatory and can lead to the development of new therapeutic strategies in this disease. Also new clinical trials are needed to apply this knowledge. Therefore close collaboration between basic and clinical scientists is presumably the best way to improve treatments to further help patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflict of interest to declare.
References
- Osterlind A. Trends in incidence of ocular malignant melanoma in Denmark 1943-1982. Int J Cancer 1987;40:161-4. [Crossref] [PubMed]
- Caminal JM, Ribes J, Cleries R, et al. Relative survival of patients with uveal melanoma managed in a single center. Melanoma Res 2012;22:271-7. [Crossref] [PubMed]
- Bergman L, Seregard S, Nilsson B, et al. Uveal melanoma survival in Sweden from 1960 to 1998. Invest Ophthalmol Vis Sci 2003;44:3282-7. [Crossref] [PubMed]
- Singh AD, Topham A. Survival rates with uveal melanoma in the United States: 1973-1997. Ophthalmology 2003;110:962-5. [Crossref] [PubMed]
- Burr JM, Mitry E, Rachet B, et al. Survival from uveal melanoma in England and Wales 1986 to 2001. Ophthalmic Epidemiol 2007;14:3-8. [Crossref] [PubMed]
- Bakalian S, Marshall JC, Logan P, et al. Molecular pathways mediating liver metastasis in patients with uveal melanoma. Clin Cancer Res 2008;14:951-6. [Crossref] [PubMed]
- Pons F, Plana M, Caminal JM, et al. Metastatic uveal melanoma: is there a role for conventional chemotherapy? - A single center study based on 58 patients. Melanoma Res. 2011;21:217-22. [Crossref] [PubMed]
- Mariani P, Piperno-Neumann S, Servois V, Berry MG, Dorval T, Plancher C, et al. Surgical management of liver metastases from uveal melanoma: 16 years' experience at the Institut Curie. Eur J Surg Oncol 2009;35:1192-7. [Crossref] [PubMed]
- Buder K, Gesierich A, Gelbrich G, et al. Systemic treatment of metastatic uveal melanoma: review of literature and future perspectives. Cancer Med 2013;2:674-86. [Crossref] [PubMed]
- Van Raamsdonk CD, Bezrookove V, Green G, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature 2009;457:599-602. [Crossref] [PubMed]
- Van Raamsdonk CD, Griewank KG, Crosby MB, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med 2010;363:2191-9. [Crossref] [PubMed]
- Carvajal RD, Sosman JA, Quevedo JF, et al. Effect of selumetinib vs chemotherapy on progression-free survival in uveal melanoma: a randomized clinical trial. JAMA 2014;311:2397-405. [Crossref] [PubMed]
- Available online: http://www.astrazeneca.com/Media/Press-releases/Article/20150722--astrazeneca-provides-update-on-selumetinib
- Feng X, Degese MS, Iglesias-Bartolome R, et al. Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry. Cancer Cell 2014;25:831-45. [Crossref] [PubMed]
- Vaqué JP, Dorsam RT, Feng X, et al. A genome-wide RNAi screen reveals a Trio-regulated Rho GTPase circuitry transducing mitogenic signals initiated by G protein-coupled receptors. Mol Cell 2013;49:94-108. [Crossref] [PubMed]
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [Crossref] [PubMed]
- Maio M, Grob JJ, Aamdal S, et al. Five-year survival rates for treatment-naive patients with advanced melanoma who received ipilimumab plus dacarbazine in a phase III trial. J Clin Oncol 2015;33:1191-6. [Crossref] [PubMed]
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517-26. [Crossref] [PubMed]
- Alexander M, Mellor JD, McArthur G, et al. Ipilimumab in pretreated patients with unresectable or metastatic cutaneous, uveal and mucosal melanoma. Med J Aust 2014;201:49-53. [Crossref] [PubMed]
- Danielli R, Ridolfi R, Chiarion-Sileni V, et al. Ipilimumab in pretreated patients with metastatic uveal melanoma: safety and clinical efficacy. Cancer Immunol Immunother 2012;61:41-8. [Crossref] [PubMed]
- Kelderman S, van der Kooij MK, van den Eertwegh AJ, et al. Ipilimumab in pretreated metastastic uveal melanoma patients. Results of the Dutch Working group on Immunotherapy of Oncology (WIN-O). Acta Oncol 2013;52:1786-8. [Crossref] [PubMed]
- Khattak MA, Fisher R, Hughes P, et al. Ipilimumab activity in advanced uveal melanoma. Melanoma Res 2013;23:79-81. [Crossref] [PubMed]
- Luke JJ, Callahan MK, Postow MA, et al. Clinical activity of ipilimumab for metastatic uveal melanoma: a retrospective review of the Dana-Farber Cancer Institute, Massachusetts General Hospital, Memorial Sloan-Kettering Cancer Center, and University Hospital of Lausanne experience. Cancer 2013;119:3687-95. [Crossref] [PubMed]
- Maio M, Danielli R, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab in patients with pre-treated, uveal melanoma. Ann Oncol 2013;24:2911-5. [Crossref] [PubMed]
- Moser JC, Pulido JS, Dronca RS, et al. The Mayo Clinic experience with the use of kinase inhibitors, ipilimumab, bevacizumab, and local therapies in the treatment of metastatic uveal melanoma. Melanoma Res 2015;25:59-63. [Crossref] [PubMed]
- Zimmer L, Vaubel J, Mohr P, et al. Phase II DeCOG-study of ipilimumab in pretreated and treatment-naive patients with metastatic uveal melanoma. PLoS One 2015;10:e0118564. [Crossref] [PubMed]
- Piulats Rodriguez J, Ochoa de Olza M, Codes M, et al. Phase II study evaluating ipilimumab as a single agent in the first-line treatment of adult patients (Pts) with metastatic uveal melanoma (MUM): The GEM-1 trial. J Clin Oncol 2014;32:abstr 9033.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320-30. [Crossref] [PubMed]
- Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 2015;372:2521-32. [Crossref] [PubMed]
- Niederkorn JY. Immune escape mechanisms of intraocular tumors. Prog Retin Eye Res 2009;28:329-47. [Crossref] [PubMed]
- Yang W, Chen PW, Li H, et al. PD-L1: PD-1 interaction contributes to the functional suppression of T-cell responses to human uveal melanoma cells in vitro. Invest Ophthalmol Vis Sci 2008;49:2518-25. [Crossref] [PubMed]
- Stein-Streilein J, Streilein JW. Anterior chamber associated immune deviation (ACAID): regulation, biological relevance, and implications for therapy. Int Rev Immunol 2002;21:123-52. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002;3:991-8. [Crossref] [PubMed]
- Teng MW, Ngiow SF, Ribas A, et al. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer Res 2015;75:2139-45. [Crossref] [PubMed]
- Beatty GL, Gladney WL. Immune escape mechanisms as a guide for cancer immunotherapy. Clin Cancer Res 2015;21:687-92. [Crossref] [PubMed]
- Murphy K. Janeway's Immunobiology. Part I: An Introduction to Immunobiology and Innate Immunity. Chapters 1-3. USA, New York: Garland Science, 2012:1-127.
- Luke JJ, Triozzi PL, McKenna KC, et al. Biology of advanced uveal melanoma and next steps for clinical therapeutics. Pigment Cell Melanoma Res 2015;28:135-47. [Crossref] [PubMed]
- Durie FH, Campbell AM, Lee WR, et al. Analysis of lymphocytic infiltration in uveal melanoma. Invest Ophthalmol Vis Sci 1990;31:2106-10. [PubMed]
- Apte RS, Niederkorn JY. Isolation and characterization of a unique natural killer cell inhibitory factor present in the anterior chamber of the eye. J Immunol 1996;156:2667-73. [PubMed]
- Apte RS, Mayhew E, Niederkorn JY. Local inhibition of natural killer cell activity promotes the progressive growth of intraocular tumors. Invest Ophthalmol Vis Sci 1997;38:1277-82. [PubMed]
- de Waard-Siebinga I, Hilders CG, Hansen BE, et al. HLA expression and tumor-infiltrating immune cells in uveal melanoma. Graefes Arch Clin Exp Ophthalmol 1996;234:34-42. [Crossref] [PubMed]
- Nemeth E, Baird AW, O'Farrelly C. Microanatomy of the liver immune system. Semin Immunopathol 2009;31:333-43. [Crossref] [PubMed]
- Repp AC, Mayhew ES, Apte S, et al. Human uveal melanoma cells produce macrophage migration-inhibitory factor to prevent lysis by NK cells. J Immunol 2000;165:710-5. [Crossref] [PubMed]
- Jager MJ, Hurks HM, Levitskaya J, et al. HLA expression in uveal melanoma: there is no rule without some exception. Hum Immunol 2002;63:444-51. [Crossref] [PubMed]
- Ericsson C, Seregard S, Bartolazzi A, et al. Association of HLA class I and class II antigen expression and mortality in uveal melanoma. Invest Ophthalmol Vis Sci 2001;42:2153-6. [PubMed]
- Blom DJ, Luyten GP, Mooy C, et al. Human leukocyte antigen class I expression. Marker of poor prognosis in uveal melanoma. Invest Ophthalmol Vis Sci 1997;38:1865-72. [PubMed]
- Campoli M, Ferrone S. HLA antigen changes in malignant cells: epigenetic mechanisms and biologic significance. Oncogene 2008;27:5869-85. [Crossref] [PubMed]
- Chang CC, Campoli M, Ferrone S. Classical and nonclassical HLA class I antigen and NK Cell-activating ligand changes in malignant cells: current challenges and future directions. Adv Cancer Res 2005;93:189-234. [Crossref] [PubMed]
- Hurks HM, Valter MM, Wilson L, et al. Uveal melanoma: no expression of HLA-G. Invest Ophthalmol Vis Sci 2001;42:3081-4. [PubMed]
- Dithmar S, Rusciano D, Lynn MJ, et al. Neoadjuvant interferon alfa-2b treatment in a murine model for metastatic ocular melanoma: a preliminary study. Arch Ophthalmol 2000;118:1085-9. [Crossref] [PubMed]
- Yang H, Dithmar S, Grossniklaus HE. Interferon alpha 2b decreases hepatic micrometastasis in a murine model of ocular melanoma by activation of intrinsic hepatic natural killer cells. Invest Ophthalmol Vis Sci 2004;45:2056-64. [Crossref] [PubMed]
- Talmadge JE, Donkor M, Scholar E. Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev 2007;26:373-400. [Crossref] [PubMed]
- Mäkitie T, Summanen P, Tarkkanen A, et al. Tumor-infiltrating macrophages (CD68(+) cells) and prognosis in malignant uveal melanoma. Invest Ophthalmol Vis Sci 2001;42:1414-21. [PubMed]
- Maat W, Ly LV, Jordanova ES, et al. Monosomy of chromosome 3 and an inflammatory phenotype occur together in uveal melanoma. Invest Ophthalmol Vis Sci 2008;49:505-10. [Crossref] [PubMed]
- Ly LV, Baghat A, Versluis M, et al. In aged mice, outgrowth of intraocular melanoma depends on proangiogenic M2-type macrophages. J Immunol 2010;185:3481-8. [Crossref] [PubMed]
- Bronkhorst IH, Vu TH, Jordanova ES, et al. Different subsets of tumor-infiltrating lymphocytes correlate with macrophage influx and monosomy 3 in uveal melanoma. Invest Ophthalmol Vis Sci 2012;53:5370-8. [Crossref] [PubMed]
- Bronkhorst IH, Jager MJ. Uveal melanoma: the inflammatory microenvironment. J Innate Immun 2012;4:454-62. [Crossref] [PubMed]
- Harbour JW, Onken MD, Roberson ED, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 2010;330:1410-3. [Crossref] [PubMed]
- Jager MJ, Ly LV, El Filali M, et al. Macrophages in uveal melanoma and in experimental ocular tumor models: Friends or foes? Prog Retin Eye Res 2011;30:129-46. [Crossref] [PubMed]
- Clarijs R, Schalkwijk L, Ruiter DJ, et al. EMAP-II expression is associated with macrophage accumulation in primary uveal melanoma. Invest Ophthalmol Vis Sci 2003;44:1801-6. [Crossref] [PubMed]
- Callejo SA, Marshall JC, Cools-Lartigue J, et al. Macrophage-derived soluble factor enhances melanoma inhibitory activity expression by uveal melanoma cells in vitro. Melanoma Res 2004;14:91-5. [Crossref] [PubMed]
- Polak ME, Borthwick NJ, Johnson P, et al. Presence and phenotype of dendritic cells in uveal melanoma. Br J Ophthalmol 2007;91:971-6. [Crossref] [PubMed]
- Vu TH, Bronkhorst IH, Versluis M, et al. Analysis of inflammatory cells in uveal melanoma after prior irradiation. Invest Ophthalmol Vis Sci 2013;54:360-9. [Crossref] [PubMed]
- Murphy K. Janeway's Immunobiology. Part IV: The Adaptive Immune Response. Chapters 9-12. USA, New York: Garland Science, 2012:335-508.
- Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest 2007;117:1147-54. [Crossref] [PubMed]
- Ma J, Usui Y, Kezuka T, et al. Costimulatory molecule expression on human uveal melanoma cells: functional analysis of CD40 and B7-H1. Exp Eye Res 2012;96:98-106. [Crossref] [PubMed]
- Jia R, Jiao Z, Xu X, et al. Functional significance of B7-H1 expressed by human uveal melanoma cells. Mol Med Rep 2011;4:163-7. [PubMed]
- de la Cruz PO Jr, Specht CS, McLean IW. Lymphocytic infiltration in uveal malignant melanoma. Cancer 1990;65:112-5. [Crossref] [PubMed]
- Whelchel JC, Farah SE, McLean IW, et al. Immunohistochemistry of infiltrating lymphocytes in uveal malignant melanoma. Invest Ophthalmol Vis Sci 1993;34:2603-6. [PubMed]
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436-44. [Crossref] [PubMed]
- Ellerhorst JA, Cooksley CD, Grimm EA. Autoimmunity and hypothyroidism in patients with uveal melanoma. Melanoma Res 2001;11:633-7. [Crossref] [PubMed]



