- 1Sport Science Program (SSP), College of Arts and Sciences (QU-CAS), University of Qatar, Doha, Qatar
- 2Higher Institute of Sport and Physical Education of Ksar Said, Mannouba, Tunisia
- 3School of Health and Biomedical Sciences, RMIT University, Melbourne, VIC, Australia
- 4Active Ageing Research Group, Department of Medical and Sport Sciences, University of Cumbria, Lancaster, United Kingdom
- 5Cellular and Molecular Immunology Research Center, London Metropolitan University, London, United Kingdom
- 6University eCampus, Novedrate, Italy
- 7Department of Health Sciences (DISSAL), Postgraduate School of Public Health, University of Genoa, Genoa, Italy
Immunosenescence is characterized by deterioration of the immune system caused by aging which induces changes to innate and adaptive immunity. Immunosenescence affects function and phenotype of immune cells, such as expression and function of receptors for immune cells which contributes to loss of immune function (chemotaxis, intracellular killing). Moreover, these alterations decrease the response to pathogens, which leads to several age-related diseases including cardiovascular disease, Alzheimer's disease, and diabetes in older individuals. Furthermore, increased risk of autoimmune disease and chronic infection is increased with an aging immune system, which is characterized by a pro-inflammatory environment, ultimately leading to accelerated biological aging. During the last century, sedentarism rose dramatically, with a concomitant increase in certain type of cancers (such as breast cancer, colon, or prostate cancer), and autoimmune disease. Numerous studies on physical activity and immunity, with focus on special populations (i.e., people with diabetes, HIV patients) demonstrate that chronic exercise enhances immunity. However, the majority of previous work has focused on either a pathological population or healthy young adults whilst research in elderly populations is scarce. Research conducted to date has primarily focused on aerobic and resistance exercise training and its effect on immunity. This review focuses on the potential for exercise training to affect the aging immune system. The concept is that some lifestyle strategies such as high-intensity exercise training may prevent disease through the attenuation of immunosenescence. In this context, we take a top-down approach and review the effect of exercise and training on immunological parameters in elderly at rest and during exercise in humans, and how they respond to different modes of training. We highlight the impact of these different exercise modes on immunological parameters, such as cytokine and lymphocyte concentration in elderly individuals.
Aging and Its Impact on the Immune System
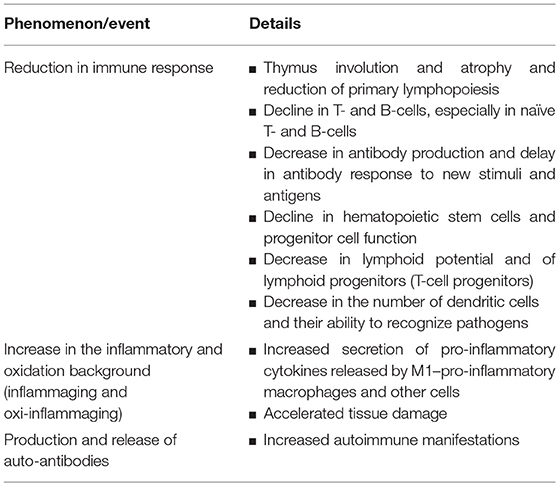
Immunosenescence or immunopause is a complex, multi-factorial aging-related phenomenon characterized by a series of biological events, including (a) alteration of immune function, quantified by a reduction in humoral and cellular immunity, (b) increase in the inflammatory and oxidation background (inflammaging and oxi-inflammaging), and (c) production and release of auto-antibodies leading to the insurgence of autoimmune disorders, as briefly overviewed in Table 1 (1).
Deterioration of the immune system with age is mostly due to biological factors such as genetics and interactions with environmental factors (like exposure to infectious agents, including CMV or cytomegalovirus) imposing metabolic alterations caused by unhealthy lifestyles (poor exercise, inadequate diet) and prolonged physiological stress (2, 3).
Aging primarily influences immunity through changes in thymus structure and activity (i.e., thymus atrophy) and reduction of primary lymphopoiesis (4, 5). Additionally, aging is associated with a decline in naïve T-cells, accumulation of memory T-cells, and a decrease in antibody production (6–8). Aging is also associated with a decline in hematopoietic stem cells (HSCs) and progenitor cell function, which results in increased production of myeloid lineage cells and a decrease in lymphoid potential (9). Thus, the quality and the number of lymphoid progenitor cells reduce with age and the cellular immune compartment becomes skewed toward a myeloid lineage (10, 11).
Moreover, aging perturbs the inflammatory state by increasing secretion of pro-inflammatory cytokines (i.e., interleukin-1 [IL-1], tumor necrosis factor alpha (TNF-α), interleukin-6 [IL-6], and C reactive protein [CRP]) (12). In fact, with advanced age, macrophages become more pro-inflammatory releasing higher amounts of TNF-α and interleukin-12 (IL-12) (13), which can accelerate tissue damage (14). The perturbed secretory state of senescent cells is known as the senescence-associated secretory phenotype and contributes to the aging process (15–17).
A further major aging-related event also occurs in the form of increased production and release of auto-antibodies, leading to a higher number of autoimmune events and manifestations among the elderly (1, 18).
Physical Activity as a Tool to Counteract Immunosenescence
Importantly, some lifestyle interventions can preserve the normal course of aging and, ultimately, prevent premature immunosenescence. Among these interventions, diet and exercise training (multiple single bout of exercise) are the most studied non-pharmacological strategies to fight the age-associated decline in immunity (19–21).
In fact, exercise training has been shown to induce transient changes in immunity responses at rest and in response to efforts (i.e., recovery following efforts). Exercise training or the “chronic exercise” intervention can be defined as a repeated amount of bouts of exercise during a short or long-term period of time) while, the “acute exercise” can be defined as a single bout of exercise.
The available scholarly literature seems to suggest that chronic exercise is a safe mode of intervention to prevent immunosenescence, chronic low-grade inflammation and improve the effectiveness of flu vaccination in elderly populations without harmful side effects (22–24).
Other recent studies have indeed suggested that chronic exercise exerts a positive effect on cardiovascular health (25–27) as well as on the immune system (28–31).
On the other hand, it has been well demonstrated that physiological responses to acute and long-term adaptations of immunity to exercise are dependent on exercise type or dose (low intensity (<40% VO2max), moderate (40-69%VO2max) vigorous (70-90%VO2max), or very high intensity (>90% VO2max)). Following an overly intense workout, some authors have interpreted measurements to show that there is a general decrease in immunity for several hours after exercise, termed the “open-window theory of susceptibility to infections,” showing, on the contrary, that, chronic exercise can enhance immunity rather than suppress immune competency among athletes (32, 33). An array of parameters including fatigue, nutritional deficiency, psychological stress, or environmental exposures and not just exercise per se can, indeed, explain the apparently higher rate of upper respiratory tract infections (URTIs) in athletes compared to general population (32, 34, 35). Exercise training can be considered as a kind of “immunotherapy,” potentially representing a highly cost-effective measure that can dramatically improve human quality of life (36).
As for acute exercise, the changes in immunity response seems to be altered by exercise type and form (i.e., endurance, resistance or sprint training). For example, the endurance training which refers to regular exercises at low to moderate intensity, is generally seen to enhance the aerobic system and cardio-respiratory function together with the exercised muscles. This type of training is essential for sports like running a marathon, swimming a long distance, or climbing mountains, which have recently become more practiced among elderly subjects. The resistance training includes all forms of exercise that forces skeletal muscles (not the involuntary muscles) to contract in response to some type of force that “resists” to the movement with or without equipment (i.e., weight training, isometric exercise, weight machines…etc.). It is commonly used to increase muscular strength and may reduce metabolic, cardiovascular disease, and risk of fall in 70–75 year old subjects (37, 38).
Finally, sprint training which is considered the most intense training mode because it includes short bouts of running exercise at high speed (i.e., race over short distances such as 10, 100, 800 m). Benefits of sprinting for the middle aged (40–50 years) including building muscles, burning fat, relieving stress and also improvement of the endocrine system (39–41) have been well demonstrated, however, few and unclear data were reported in elderly subjects with regard to all the body's system and especially to the immunity response, which was more apparent in other type of training.
One of the major concerns in training adaptation in the immune system concerns changes in catecholamines, with a blunted neuro-endocrine response and adrenergeric receptors being down-regulated. In fact, previous studies have demonstrated that chronic exercise such as sprint and resistance training may counteract the negative effect of age on catecholamines and growth hormones in 40 year-old men (40–42). As catecholamines modulate immune cell function (43, 44), it is therefore important to highlight the impact of these type of exercises training on immunity in the elderly population.
In the subsequent sections, building on an extensive search of the literature based on important discoveries of Nieman and collaborators from the 90's (45–49) to recent data of 2018, we will discuss the current understanding of different modes of exercise training on immunological parameters and mechanisms that are implicated in immunosenescence in older subjects. As there is little research in this area, we have also mentioned in some parts that only studies in young subjects (20–30 years) were conducted. In fact, in the literature, there is a lack of consensus concerning the definition of elderly/older subject: according to the World Health Organization (WHO), this category includes individuals aged >60 or 65 years old, whereas the oldest subject is a person aged >80 years old.
Impact of Acute Exercise on Immune Cells
A single bout of exercise is known to stimulate immune cells during efforts and during recovery. Evidence indicates that mechanisms underlying exercise associated with immune function alteration are related to several factors such as neuro-endocrine system stimulations (catecholamines, cortisol), and metabolic (i.e., carbohydrate, antioxidants, or prostaglandin) (50, 51) as well as to cardiac output, blood flow, blood pressure, and shear forces, among others.
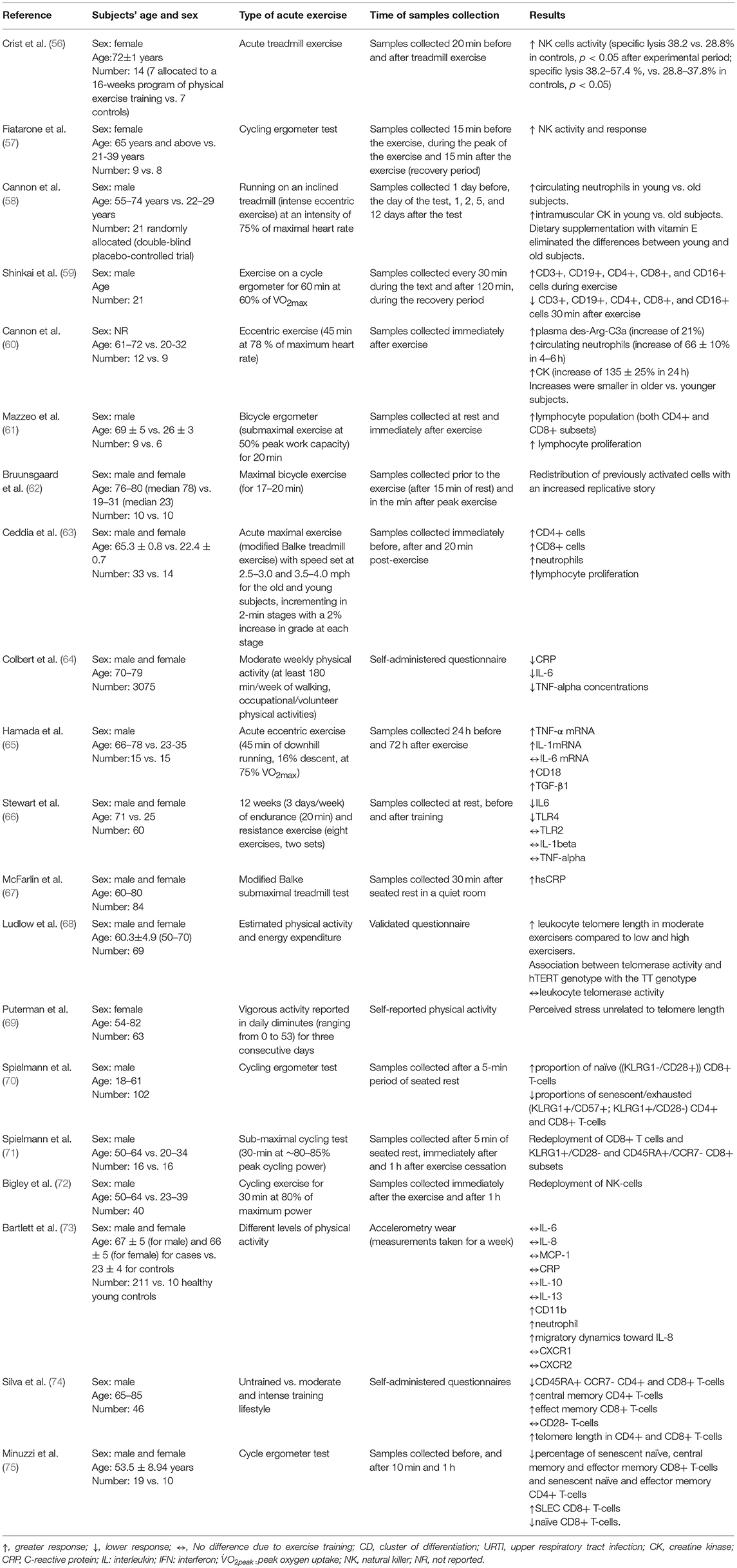
Acute exercise impacts on circulation and leaves blood to travel to tissues where they are more likely to encounter infected cells or body cells that have become cancerous. Some studies seem to suggest that the acute effects of exercise (e.g., apoptosis of some cells) can stimulate a mobilization of hematopoietic stem cells from bone marrow and of senescent immune cells from the peripheral tissues to the circulation (22, 52–55). This could justify and explain why in older subjects, in which these mechanisms are impaired by aging, response to acute exercise is different from younger individuals, as shown by some studies reported in Table 2.
Furthermore, noradrenaline is responsible for the effects of acute exercise on lymphocyte changes, including natural killer (NK)-cell and T-cell activity (76). Increases in catecholamine with growth hormones mediate the changes in neutrophils levels and control lymphopenia and neutrocytosis during long duration exercise. In addition, glutamine, the abundant amino acid found in muscles, is known to stimulate in vitro lymphocyte proliferation, lymphokine activated killer cell action, and cytokine release (77). During intense exercise, blood, and muscle levels of glutamine and glucose tend to fall, which may explain how a possible “immunosuppression” is likely to occur, despite existing data that contradict this relationship between glutamine and immunosuppression (77). According to some authors, exercise-induced apoptosis is a necessary process that accelerates the removal of damaged cells without inducing a pronounced inflammatory status, which may, instead, enhance body function (78).
The magnitude of changes in immune cell levels during acute exercise and if it is dependent on exercise intensity, is still debated (22, 79–81) and most previous work investigated the effect of exercise on immunity generally in young subjects (20–30 years) while no study or very few investigations focused on the elderly population.
The proliferation of lymphocytes is considered to correlate with the response of the adaptive immune system to training. Studies use several types of mitogens to stimulate lymphocytes such as concanavalinA (con-A), poke weed mitogen (PWM), phytohaemagglutinin (PHA). Other studies, have used interleukin-2 (IL-2), purified derivative of tuberculin (PPD), and lipopolysaccharide to induce the proliferation of lymphocytes. The data are, however, equivocal. For instance, Espersen et al. (82) have shown that proliferation of lymphocytes to mitogens (such as, PHA, con A, PWM) increased 2 h after exercise. In contrast, other studies indicate that lymphocyte response to PHA, con-A and especially to PPD was depressed after 2.5–3 h of heavy exercise (a complete 42, 195-km marathon) in a sample of 4 male subjects aged 25–50 years old compared with eight highly conditioned long-distance runners and 59 controls immunized with tetanus toxoid vaccine (83). Samples were collected at 30 min before, 30 min, 3 h and 1 day after the completion of the run. However, despite the depression of lymphocyte transformation response, no changes in lymphocyte count were observed (from 3,196 to 2,451 cells per mm3) and no effects on antibody-forming capacity could be detected. This lymphocyte transformation response was transient (24-h recovery period). Granulocytosis, increased plasma cortisol (from 0.48 to 1.08 μmol/l) and leucocytosis (from 7,600 to 19,609 cells per mm3) were also found.
In order to demonstrate the effects of age and mode of exercise (in terms of intensity and duration), the impact of single exercise bouts on changes in leukocyte subsets and other immunological parameters is summarized in Table 2.
It should be emphasized that assessing cell functions in response to acute exercise may be subject to different confounding factors (such as CMV serostatus), unless is assessed on a per cell and per phenotype basis (32, 84).
In the next paragraphs, we will introduce different cell types and we will briefly explain how they respond to exercise.
Monocytes, which represent 2–12% of circulating leukocytes, can differentiate into macrophages and myeloid lineage dendritic cells (DCs). Aging is responsible for the pro-inflammatory phenotype of these cells such as expression of the Cluster of differentiation 16 (CD16) and increased levels of TNF-α, IL-6, and the Interleukin 1 beta, also known as leukocytic pyrogen, leukocytic endogenous mediator, mononuclear cell factor, or lymphocyte activating factor (IL1-β). Acute aerobic exercises (running, cycling) have been shown to increase monocyte number in young subjects (85, 86) as well as in the elderly (87). In conclusion, there are numerous studies that have examined the acute effects of exercise on monocytes. Interestingly, it seems that brief exercise alters significantly a number of microRNAs (miRNAs) that putatively influence monocytes involvement in vascular health, leading to a novel genomic profile of circulating monocytes, which promotes cardiovascular health. As such, this suggests that, despite the overall stress response and the pro-inflammatory profile, acute exercise exerts a positive effect.
Macrophages play a key role in inflammatory responses and are specialized cells involved in the detection, phagocytosis, and destruction of bacteria and other pathogens, as well as in tissue homeostasis and development (88). They can be subdivided into two populations: namely, the pro-inflammatory, anti-tumorigenic M1 macrophages and the anti-inflammatory, pro-tumorigenic M2 macrophages (89). Many studies demonstrate that aging does not change number of macrophages, but it does alter their properties and function (88, 90). For instance, it seems that macrophages of the adipose tissue and the liver of the elderly exhibit a more M1 phenotype when compared to younger subjects (89). Macrophages also play an important anti-inflammatory role and can decrease immune reactions through release of cytokines: briefly, aging is characterized by “dysregulated macrophage-mediated immunosuppression” (89). A recent study demonstrated that during rest, there was an increase in IL-1 and IL-10 produced by macrophages, yet an unaltered number and function of these cells immediately post-exercise (91).
Like macrophages, neutrophils play an important role in the innate immune system and are the most abundant granulocyte (40% to 75% of WBCs). Neutrophils have many ways of neutralizing microorganisms: namely, chemotaxis, phagocytosis (engulfment of bacteria, other pathogens or tissue fragments), degranulation of cytoplasmic granules, activation of the respiratory burst, and neutrophil extracellular traps (92). It has been revealed that acute exercise has a profound impact on neutrophil count, potentially mediated by the activation of cathecolamines as well as of growth hormones and cortisol (42, 52). For example, Cannon et al. (58) showed that running at 75% of maximum heart rate (HRmax) for 15 min increased neutrophil number (from 3.66 to 3.84 cells/mm3) in older adults (aged from 61 to 72 years). Cannon et al. (60) in a group of 12 old subjects aged 61–72 years vs. a sample of 9 participants aged 20–32 years, eccentric exercise (45 min at 78% of maximum heart rate) led to the increase of circulating neutrophils.
According to numerous studies, neutrophils recruited into circulation during exercise may be responsible for controlling the elevated levels of oxidative stress in plasma after exercise (93). The lower adhesion of neutrophils and platelets elicited by regular exercise could be an important factor in the prevention of vascular and inflammatory diseases, among others (94).
Furthermore, habitual physical activity is associated with the maintenance of neutrophil migratory dynamics in a sample of 211 healthy older adults aged 67 ± 5 years when compared to 10 young participants (aged 23 ± 4 years). There was no difference in expression of the chemokine receptors CXCR1 or CXCR2 (73).
Few studies have investigated the effect of acute exercise on leukocytes in the elderly. For instance, Ludlow et al. (68) found in a sample of 69 subjects aged 50–70 years that the leukocyte telomere length was increased in subjects undergoing moderate physical activity (50-70% VO2max), when compared to groups practicing low and high levels of exercise and training. Specifically, the second exercise energy expenditure (EEE) quartile had longer telomere lengths with respect to the first and fourth quartiles but not to the third one, whereas the telomerase activity did not differ among the groups, remaining preserved and stable.
Several studies have demonstrated that acute exercise induces recruitment of lymphocyte subpopulations from marginated leukocyte pools from organs into general circulation. Two of these types of lymphocytes are critical for specific immune responses; B lymphocytes (B cells) and T lymphocytes (T cells), for humoral and cellular responses, respectively. Both B and T cells are notably impacted, with elevated serum levels post exercise. Mobilization of these cells is due to redistribution of activated cells with elevated replicative history rather than cells isolated from blood at rest such as CD4+ T cells, CD8+ T cells, CD16+ NK, and CD56+ NK cells (95).
Lymphocytosis is known to occur during exercise or immediately thereafter during the early stages of the recovery phase and is proportional to the intensity and the duration of acute exercise, with an increase in the number of T lymphocytes (and to a lesser extent B lymphocytes) compared to the values measured before exercise (96, 97). Subsequently, during the 24 h following the effort, data reported the same values at rest. Mobilization of these subgroups of cells (T and B) is largely influenced by the action of catecholamines which is the response of the nervous system to energy demand (98). After intense long duration exercise, lymphopenia is reported, with the lymphocyte number decreasing rapidly after exercise. However this phenomenon is transient and, rather than being a real reduction, ig is secondary to lymphocyte redeployment to peripheral side (51).
Mooren et al. (99), reported a continuous exhaustive at 80% VO2max (progressive exercise test on a treadmill ergometer) induces apoptosis in peripheral blood lymphocytes in young (20–30 years) while the moderate intensity exercise performed at 60% of VO2max did not. As indicated in the last study, the subjects were young but there are no studies that have investigated apoptosis in elderly/older subjects depending on exercise intensity which make it difficult to really understand the responses of each type of exercise on this population.
In a sample of 14 young (aged ~22 years) and 33 older adults (aged ~ 65 years) acute maximal exercise induces leukocytosis, mainly due to lymphocytosis and neutrophilia (100–102). In particular, an increase both in CD8+ lymphocytes (153 and 112% respectively) and in CD4+ lymphocytes (57 and 22% respectively) could be detected (63). Specifically, older subjects exhibited higher percentages of memory CD45RO+ CD4+ cells and CD8+ cells pre-exercise and lower percentages of naive CD45RA+ CD4+ and CD8+ cells pre-exercise when compared to younger individuals, even though both groups recruited equal number of naïve and memory cells in response to exercise (103). Despite the higher number of CD3+ cells, the lymphoproliferative response was lower in the elderly subjects. It can be concluded that exercise-induced leukocytosis can occur both in young and old individuals, even though this is less frequent among the elderly, whereas the lympho-proliferative response was different (101, 102).
In addition, Mazzeo et al. (61) examined the changes induced by an acute 20-min bout of aerobic exercise (cycling at 50% of peak power) on lymphocytes in older adults (aged ~69 years) and observed an increase in CD4+ and CD8+ T-cells post-exercise. Thus, during exercise, CD4+ T-cells, CD8+ T-cells, CD19 B-cells, CD16 NK cells, and CD56 NK cells increase in number after intense exercise, whilst they decrease 1-h post-exercise.
Silva et al. (74) recruited a sample of 46 subjects aged 65–85 years, subdivided into three groups (untrained subjects vs. moderately and intensely trained individuals). A significant redeployment of T-cells could be noticed: namely, a decrease in CD45RA+ C-C chemokine receptor type 7 (CCR7)- CD4+ and CD8+ T-cells, an increase in central memory CD4+ T-cells and in effector memory CD8+ T-cells, whilst the number of CD28- T-cells remained unvaried. Furthermore, telomere length in CD4+ and CD8+ T-cells correlated with training intensity.
Minuzzi et al. (75) investigated a sample of 19 male old subjects (aged ~54 years) undergoing the cycle ergometer test and found that acute exercise can lead to a decrease in the percentage of both CD4+ and CD8+ T-cells. Specifically, this effect was due to an increase in short-lived effector cells (SLECs) and to a decrease of senescent naïve, central memory, and effector memory cells. A similar redeployment of CD8+ T cell subsets, induced by acute exercise, was described by Bruunsgaard et al. (62) in a sample of 10 individuals (aged 76–80 years) undergoing a maximal bicycle exercise for 17–20 min, by Spielmann et al. (70) in a group of 102 subjects undergoing the cycling ergometer test, and by Spielmann et al. (71) in a sample of 32 individuals undergoing a 30-min sub-maximal cycling test (at ~80% peak cycling power).
Inconsistent findings were obtained related to cytokines and interleukins (64, 65, 104). After a bout of isokinetic exercise in a sample of 16 subjects (8 participants aged ~67 years vs. 8 individuals aged ~20 years), an increase of monocyte chemoattractant protein-1 (MCP-1, known also as CCL2) (105), IL-6, and IL-8 could be detected whereas the serum levels of IL-4, IL-10, and interleukin-13 (IL-13) remained stable (104). In another study, in a sample of 15 subjects aged 66–78 years, acute eccentric exercise (45 min of downhill running, 16% descent, at 75% VO2max) led to increase of IL-1, TNF-α, whereas, IL-6 did not change before and after the exercise (65). Finally, Colbert and colleagues (64) interviewed 3075 subjects aged 70-79 years concerning their previous-week household, walking, exercise, and occupational/volunteer physical activities. Moderate weekly physical activity (defined as, at least 180 min/week of walking, occupational/volunteer physical activities) was found to correlate with lower levels of CRP, IL-6, and TNF-α.
Natural killer cells (also known as NK cells, K cells, or killer cells) are lymphocytes that share a common progenitor with all subsets of T and B lymphocytes. NK cells are a class of cytotoxic lymphocytes that control several microbial infections and tumor cells by limiting their spread and removing damaged tissue (106, 107). They express surface markers, CD16 and CD56, but do not express CD3. On the basis of the level of CD56 expression, they can be roughly subdivided into two major subsets: namely, CD56bright and CD56dim cells, which exhibit different phenotypical and functional characteristics. CD56dim represents a mature subset of NK cells, with exclusive migratory potential for non-lymphoid tissue and potent effector capabilities, such as the capacity to produce and release high amounts of perforin and granzyme. CD56bright usually reside in secondary lymphoid organs, and express cell-surface molecules such as the lymphoid tissue homing makers L-selectin (CD62L) and CCR7 (32, 108, 109). CD56bright and CD56dim cells also differ in terms of response to exercise, with CD56bright being less responsive to physical activity compared to CD56dim (110). Whilst interferon alpha (IFN-α) (111) and interleukin-2 (IL-2) (112) increase cytolytic activity of NK cells, immune perturbations, some prostaglandins and infections such as CMV can decrease activity of NK cells (112, 113).
Aging induces changes to the phenotype and function of NK cells. Studies found that the number of NK cells increases in elderly individuals caused by expansion of the CD56dim subset and the accumulation of CD57+ long-lived NK cells (114). CD56bright cells, instead, tend to decrease (114), as well as NK capacity for killing decreases with age (115). Others have shown that low to moderate cycling exercise leads to an increased NK cells cytotoxicity (NKCC) (116). In particular, Targan and collaborators (116) found that in a sample of 10 healthy volunteers (of unreported age and gender composition) bicycle ergometer (pedaling at speeds of approximately 25 m.p.h. for 5 min) leads to the recruitment of some NK subsets: cells which can bind targets but are non-cytotoxic and to the increased capacity of IFN of inducing overall lytic ability.
Generally, most types of exercise can increase NK cell function and number (57, 72, 117, 118). Brahmi and colleagues (117) recruited both trained and sedentary individuals, who underwent a progressive cycle ergometer test using an incremental work load of 15 W (90 kpm), increasing every minute. NK activity against K562 reached maximum levels immediately after exercise, decreased 120 min later, and then slowly came back to pre-exercise levels within 20 h. Fiatarone et al. (57) observed an increase in NK activity and response in a sample of 9 subjects aged 65 years and above (vs. a group of 8 participants aged 21–39 years) undergoing the cycling ergometer test.
A mechanism explaining NK mobilization and redistribution could be that the binding of epinephrine as well as increased serum serotonin releases NK cells from endothelial tissue via a decrease in adhesion molecules following acute exercise (119, 120). Shear stress could play a major role too (119).
Among young subjects, Bigley and co-workers (121) had found that three 30-min cycling bouts at −5%, +5%, and +15% of lactate threshold can lead to redeployment of NK cells in a sample of 16 healthy cyclists. This redeployment is stepwise and preferential in the sense that it involves NK-subsets exhibiting a high differentiation phenotype (KIR+/NKG2A– vs. medium-differentiated KIR+/NKG2A+ and low-differentiated KIR–/NKG2A+). Bigley and co-authors (72) were able to replicate this finding in a sample of 40 subjects (50–64 vs. 23–39 years) undergoing 30 min of cycling exercise at 80% of maximum power. Interestingly, the post-exercise response to CMV was similar between young and old individuals (72).
Summarizing, acute exercise in older subjects increases NK cell both in terms of number and activity/function, mobilizing and redistributing them. Furthermore, exercise increases circulating numbers of neutrophils; potentially increases secondary antibody response to booster injections; changes circulating T-cell populations (decreasing naïve CD8+ T-cells, increasing SLEC CD8+ T-cells), induces leukocytosis; and modulates T-cell proliferation (95). Inconsistent findings related to cytokines and interleukins are, instead, reported in the extant scholarly literature.
However, as can be seen from Table 2, most studies did not analyze the effect of gender on the changes in NK cell properties during exercise. This is particularly important in younger women due to the known impact of the cycle of immune parameters, and could also be a mediating factor for the acute effects of exercise in older women (122, 123). Another limitation that should be emphasized is that studies focused on older subjects but not on oldest individuals (aged >80 years old). Taking into account the global phenomenon of aging and the lengthening of the human life span, this is an important gap in our knowledge that should be properly addressed by future research. Furthermore, the studies summarized so far did not assess CMV serostatus, so the results are difficult to interpret in an unambiguous way (32).
Impact of Chronic Exercise on Immune Cells
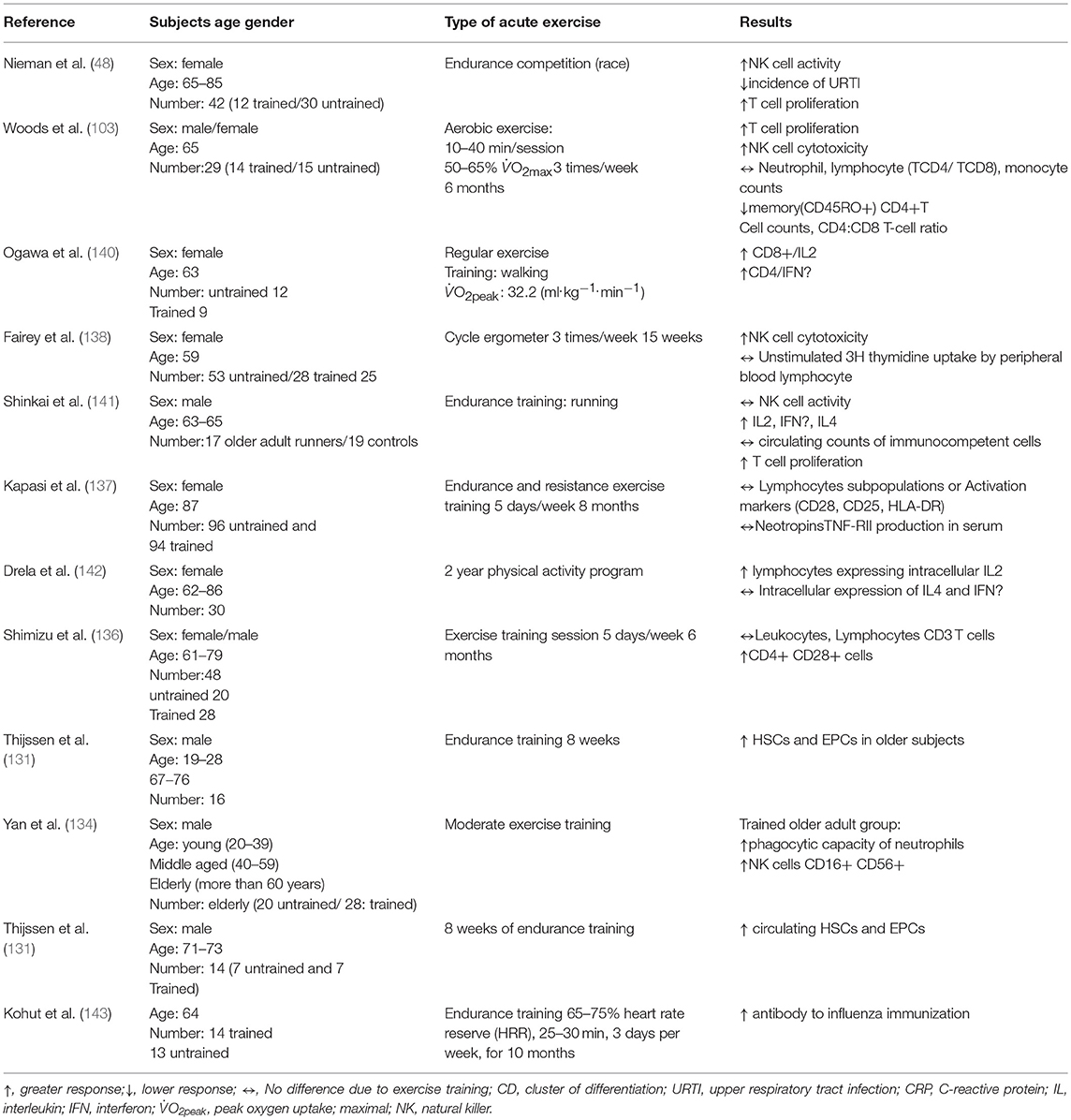
In general, studies on the effect of chronic exercise on immune cells and immunity function have been focused mostly on healthy young people, athletes, elderly, oncologic or HIV patients, with the overall goals of ascertaining the extent of immune decline in athletes due to extreme exercise training (overtraining/ excessive training) or finding the responsible factor that help to improve immunity responses in the elderly or the immunocompromised without clear evidence of the impact of exercise training type itself. Long-term effect on immune function in the elderly is less debated. In their systematic review, Cao Dinh et al. (22) reported that most studies were conducted in young (~20–40 years) and middle-aged (~40–50 years) and documented an increase in NK cells activity and T lymphocytes occurring without apoptosis.
The intriguing observation is that (1) most studies debated the effect of aerobic and resistance training, while no evidence on the effect of anaerobic training such as sprint training on resting or in response to acute exercise were found in the elderly population (>60 years) and (2) the exercise induced apoptosis in senescent cells in the elderly is still not debated.
On the other hand, it is noteworthy that intensity of training required to achieve a certain goal differs according to age groups [American College of Sports Medicine (ACSM)]. Thus, for better interpretation of results it is essential to consider that older subjects (> 60 years) have low fitness levels compared with more young (20–40 years), and they can only reach the significant exercise training effect with a training heart rate as low as 40–50% of heart rate compared with a person with high fitness levels. Therefore, these methodological considerations indicate that the findings of studies need to be interpreted with caution.
Endurance Training on Immune Cells
A healthy amount of regular exercise provides an overall benefit to the immune system. However, more than one component of the immune system may be weakened by excessive training (124). In fact, long-term intensive training may result in a decline in function of innate immune cells' capacity to respond to acute challenges, contributing to an elevated risk of infection (125). Hence, immune response depends on training intensity and duration (45, 48, 126). Despite the immune system's vulnerability to prodigious exercise training, the overall anti-inflammatory effect of exercise may reduce the risk of age-related chronic disease characterized by chronic low-grade inflammation (e.g., cancer, type 2 diabetes, heart, and Alzheimer's disease) (127).
Dendritic cells differentiate from monocytes to become professional antigen presenting cells (APCs). They form a part of the dendritic cell/macrophage continuum that presents antigen material on their MHC I/II to both T CD8+ and CD4+ cells, respectively, and play an important role as a messenger between innate and adaptive immune system. Two subsets of DCs are recognized: namely, DCs of myeloid origin (including the conventional DCs in the blood, interstitial DCs in tissues, Langerhans cells in the skin and monocyte-derived DCs) and the DCs of lymphoid origin (or plasmacytoid DCs) (128). Aging does not seem to affect the overall number of DCs, even though it could lead to decrease in the number of Langerhans cells in the skin and of plasmacytoid DCs (128). Della Bella and co-authors (129) showed that in a sample of 70 healthy subjects aged 20–92 years the number of myeloid DCs progressively declines with age, together with a decrease of CD34+ precursors and an increase of circulating monocytes, suggesting that the entire differentiation process of antigen presenting cells (APCs) is partially dysregulated in the elderly. Furthermore, DCs from aged individuals appeared to have a more mature phenotype and an impaired ability to produce and release IL-12 upon stimulation.
Recent data suggests that Tai Chi Chuan elevates the number of circulating myeloid DCs but not the plasmacytoid DCs in middle-aged individuals ~53 years (130). However, few studies have examined the effects of endurance training on stem cells number and function.
Concerning stem cells, Thijssen et al. (131) found that endurance training induced significant increases in numbers of hematopoietic stem cells (HSCs) and endothelial progenitor cells (EPCs) in older subjects.
Other studies have shown that moderate exercise increases neutrophil function (chemotaxis, phagocytosis, and oxidative burst activity). In contrast, severe or heavy exercise diminished these cell activities without affecting chemotaxis and degranulation (92, 132).
In older individuals, some studies that have explored the effect of endurance training on immunity response, have found no changes in circulating neutrophil count (103, 133). Yan et al. (134) found that neutrophil phagocytic ability in older adults is attenuated by moderate exercise compared to a control group. Given that few studies have investigated the impact of endurance training on neutrophils; it is difficult to make conclusive statements regards the role of endurance training on neutrophils in the elderly.
Eosinophils are a type of white blood cells that defend against parasites and infectious agents. Aging induces several changes in eosinophils such as decreases in blood eosinophils number in the elderly. However, there is no alteration to the function of these cells in young and older group with asthma (135).
Basophils are a type of leukocyte which represent 1% of circulating WBC. These cells play a key role in the inflammatory response. The effect of endurance training on eosinophils and basophils is rather unexplored but endurance training has been found to induce no change in eosinophils and basophils levels (122).
Shimizu et al. (136) reported that 6 months of moderate exercise training performed by elderly individuals (61–79 years) led to increased expression of CD28 on CD4+ T cells. Moreover, Nieman et al. (48) compared the immune response before and after training and they found that 12 weeks of training did not induce changes in T cell proliferation in older women (65–85 years). In contrast, Woods et al. (103) examined the effects after 6 months aerobic exercise on T cell compartment in older adult participants. They found that T cell proliferation increased in the experimental group compared to a control group and was responsive to mitogenic stimulation. Few studies indicate that endurance training has no effect on T cell function and cell frequencies in the elderly population (62). Future studies may wish to elucidate why endurance training fails to improve T cell function and determine whether there is a genetic predisposition, controlling the inter-individual variation in responses to immune function after different training regimes.
Kapasi et al. (137) found that 32 weeks' moderate aerobic training had no effect on T-cell proliferative responses and the soluble production of cytokine activity in frail elderly nursing home residents. Fairey et al. (138) showed that aerobic exercise training increased T cell proliferation (by 218 per dpm × 106 cells) in post-menopausal breast cancer survivors, whereas . van der Geest et al. (139) found that in a sample of people aged 80 years, walking 30 km per day lead to an increase of CD4+ T-cells.
Nieman et al. (48) has shown a higher NK cell activity in older woman (67-85 years) after walking for 12 weeks compared to a callisthenic control group. Moreover, it has also been shown that 6 months of moderate aerobic training practiced by older adult men aged 65 years, increased NK cell cytolysis (103). Recently Della Bella et al. (134), showed that concentration of NK cells (CD16+ and CD56+) increased in the older adults (>60 year) who performed regular moderate exercise for 1 h twice per week.
Distinct types of exercise and prescriptions (frequency, duration, and intensity) and sex discrepancies could be responsible for these discordant results Table 3. Also, the changes of composition of NK cells caused by aging could be responsible for discrepancies amongst results, such as CD57, cluster of differentiation 158 (CD158), killer lectin-like receptor 61 (KLR61) (144) and cluster of differentiation 94 (CD94) receptors (145). NK tumor cytoxicity increased following a 16-week exercise intervention in 14 elderly women, due to increased number of NK cells (56).
Effect of Resistance Training on Immune Cells
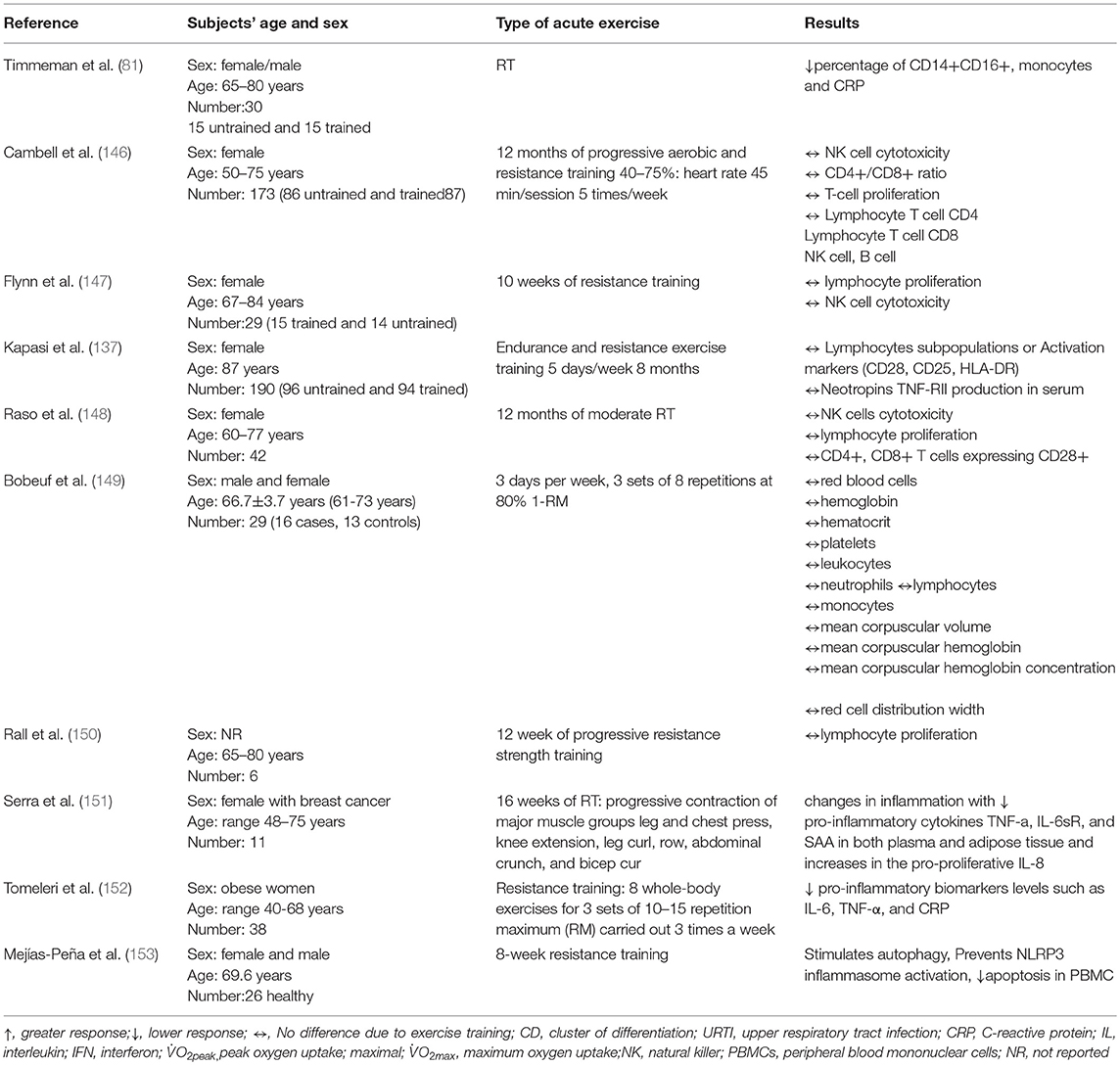
Currently, ambiguity remains over the influence of resistance training on immunity in older adults (Table 4) (154). Some studies (147–150, 155) found that short-term resistance training intervention (8–12 weeks) in the healthy elderly (between 65–84 years of age) had no beneficial or detrimental effect on (lymphocytic counts, apoptosis, proliferation…etc), while other studies have found beneficial effects on inflammatory status in older subjects with breast cancer or obesity syndrome (151, 152, 156) or healthy older ones (69 years) (153), and the combination of resistance and aerobic or endurance training reported a concrete change in immune cells levels in the elderly. For example, Timmerman et al. (81) reported that 12 weeks of aerobic and resistance exercise training lowered (CD14+, CD16+) monocyte frequencies in blood of older adults, while no changes were observed in toll-like receptor 4 (TLR4) expression, a type of protein playing an important role in innate immunity. TLR4 is expressed on sentinel cells and recognizes molecules derived from microbes. McFarlin et al. (157) found that TLR4 expression is lower in resistance trained women (65–80 years) compared to untrained peers. Up-regulated TLR activation is implicated in the manifestation of hypertension and chronic low-grade inflammation (158, 159). Stewart et al. (66) showed also that 12 weeks of resistance exercise (50% 1RM) decreased the TLR4 expression on monocytes in elderly subjects.
For Neutrophils, only a few studies investigated the effect of resistance exercises on their circulating levels. Recently, Bartholomeu-Neto et al. (156) explored the effect of resistance training (moderate-intensity physical training (70% of 1RM) with session including nine exercises: horizontal leg press, knee extension, knee flexion, bench press, triceps extension in the pulley, biceps curling, seated rowing, plantar flexion, and abdominals. They found higher phagocytic activity in healthy older women. This phagocytic activity is determined by the phagocytosis index of neutrophils (but not of monocytes). In contrast, the training did not significantly influence the oxidative activity of either neutrophils or monocytes in elderly women.
Kapasi et al. (137) found no change in activation markers (CD28, CD45RA, CD45RO, or HLA.DR) and subpopulation lymphocytes (CD4+ or CD8+ T cells) in older adult nursing home residents who performed 32-weeks of combined endurance and resistance exercise. Similarly, Flynn et al. (147) reported 10 weeks' resistance training did not alter T cell proliferation in older women (67–84 years). Moreover, recent studies (146, 148) showed that 12 months' moderate resistance training failed to induce changes in T cell proliferation and NK cells in older women. Raso et al. (148) found that a 12-month, moderate resistance training program produces significant improvement in muscle strength of healthy, elderly women without significant differences between the two groups at any time point for total lymphocyte number or its subsets (CD3+, CD4+, CD8+, CD19+, and CD56+ cells). In addition, Rall et al. (150) found that 12 weeks' progressive resistance training practiced by elderly volunteers (aged 65–80 years) did not affect lymphocyte proliferation. The inconsistent results could be due to different doses of mitogen, types of exercise (running, cycling, walking), exercise prescription and the duration of proliferation (95).
Campbell et al. (146) found that 12 months' progressive aerobic and resistance training practiced by postmenopausal women (50-75 years) has no effect on NK cell cytotoxicity. Bermon et al. (155) investigated the effect of 8-week strength training in elderly individuals (aged ~70 years) on NK cells and found that resistance training did not induce changes in NK cell function. Bermon et al. (160) found that n sedentary older adults, unlike young subjects, strength exercises can induce a transient decrease in NK cell count which can be canceled by a short-term strength conditioning. However, Fairey et al. (138) found that 10 weeks' resistance training improved NK cell function in post-menopausal breast cancer survivors aged (65–85 years).
Effect of Sprint Training on Immune Cells
Sprint training (ST) is considered as repeated bouts of exercise at supramaximal intensity. Sprint training is time efficient method of improving performance (161), and cardio-metabolic health (162). Moreover, it induces alterations in immunity such depression of neutrophil function and an increase of cytokines. However, immune responses depend on exercise training duration, intensity, and subject population.
Regarding inflammatory markers, inconsistent findings have been reported in the literature. Allen and colleagues (163) performed a randomized controlled trial, randomly allocating 55 sedentary adults (aged 49.2 ± 6.1 years). Twenty were randomized into high intensity interval training, whereas 21 and 14 to prolonged intermittent ST (PIST) and to a sedentary control group, respectively. HIIT and PIST groups performed three training sessions per week for 9 weeks. On a cycle ergometer, at the end of the trial, markers of systemic inflammation (CRP and TNF-α) remained unchanged across all groups. However, Harnish and Sabo (164) reported increased levels of IL-6, IL-10, and TNF-α after SIT in a sample of 13 men and two women aged 23.8 ± 3.5 years.
The influence of ST on neutrophils has been described in few studies, yet a single session of ST typically causes a decrease in neutrophil function (oxidative burst and degranulation). However, the depression of neutrophils is caused by oxidative stress and stress hormones (165).
The effect of ST on lymphocytes and lymphocyte proliferation has been investigated in rats whereby ST during for 5 weeks increased FeG+ lymphocyte number (166). Although, the effect of ST on lymphocytes in the elderly is rarely investigated, a recent study has shown in 53 sprinters (22.2 ± 1.1 years) that any supplementary increase in lactic acid level does not cause an additional increase in lymphocyte subsets after four sprint intervals (1,000 meters at 85% of a subject's maximal velocity) (167). In addition, there are studies concerning the effect of anaerobic exercise in general, such as the study of Hack et al. (168) that investigated the influence of 8-week anaerobic exercise training program in healthy untrained subjects. They indicate impairment of the number and activity of CD4+ T cells, which might be linked to metabolic factors such as glutamine.
On other hand, there is limited information concerning the influence of ST on NK cells. Marshall-Gradisnik et al (169) performed a 10-s all-out cycle sprint test in healthy young males at week 0 and at week 6 and found a significant increase in NK cytotoxic activity (NKCA), whilst the NK number did not significantly increase. Another recent study found that the recruitment of NK cells depends on the levels of lactic acid, higher levels of lactate increase NK cell activity and number after four sprint intervals accumulating to 1,000 meters at 85% of a subject's maximal velocity in 53 sprinters (aged 22.2 ± 1.1 years) (167).
Conclusion
The present review has synthesized and discussed the current evidence for the role of exercise interventions in influencing lymphocytes activity in older subjects. The most marked changes occur in NK cells and the general proliferation of immune cells (i.e., the cell proliferative capability and involution of tissues and organs). This is important because advanced age induces decreased proliferative response.
Many studies show the positive effect of exercise on the immune system such as elevation in T-cell proliferative capacity, increased neutrophil function, and NK cell cytotoxic activity.
The magnitude of exercise-induced immune changes in older adults were different between studies, possibly due to the different protocols, methodologies, ages, sexes, and testing procedures.
Future detailed studies should examine the impact of exercise on immune cell function, such as neutrophils and elucidate the underlying mechanisms responsible for the alterations and the subsequent effect on immunity and health. It will also be critical to elucidate the inter-individual responses in changes to immune parameters (e.g., T-cell activity) after different forms of exercise training, which could account for some of the findings amongst the current literature.
Ultimately, these studies will contribute to our understanding of the role exercise training may have in preventing immunosenescence and disease, or improving health span.
Author Contributions
MS designed and conceived the study. MS and NB drafted the study. MS, MG, JD, LH, DS, JP, and NB critically revised the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ACSM, American College of Sports Medicine; APC, antigen presenting cell; CCR7, C-C chemokine receptor type 7; CD4, cluster of differentiation 4; CD8, cluster of differentiation 8; CD11b, cluster of differentiation 11b; CD18, cluster of differentiation 18; CD19, cluster of differentiation 19; CD28, cluster of differentiation 28; CD45RA, cluster of differentiation 45RA; CD45RO, cluster of differentiation 45RO; CD56, cluster of differentiation 56; CD62L, cluster of differentiation 62 ligand; CD94, cluster of differentiation 94; CD158, cluster of differentiation 158; CK, creatine kinase; CMV, cytomegalovirus; con-A, concanavalin A; CRP, C-reactive protein; CXCR1, C-X-C chemokine receptor type 1; CXCR2, C-X-C chemokine receptor type 2; DC, dendritic cell; EEE, exercise energy expenditure; EPC, endothelial progenitor cells; HLA-DR, Human Leukocyte Antigen – antigen D Related; HR, heart rate; HRmax, maximum heart rate; HRR, heart rate reserve; HSC, hematopoietic stem cells; hsCRP, highly sensitive C-reactive protein; hTERT, human telomerase reverse transcriptase; IFN, interferon; IFN-γ, interferon-gamma; IL-1, interleukin 1; IL1-β, interleukin 1-beta; IL-2, interleukin 2; IL-4, interleukin 4; IL-6, interleukin 6; IL-12, interleukin 12; IL-13, interleukin 13; KLR, killer lectin-like receptor; KLR61, killer lectin-like receptor 61; miRNA, microRNA; mRNA, RNA messenger; NK, natural killer; NKCA, natural killer cytotoxic activity; NKCC, natural killer cellular citotoxicity; NR, not reported; PBMC, peripheral blood mononuclear cell; PHA, phitoemagglutinin; PIST, prolonged intermittent sprinting training; PPD, purified protein derivative; PWM, poke weed mitogen; RM, repetition maximum; RT, resistance training; SLEC, short-lived effector cells; ST, sprint training; TGF, transforming growth factor; TGF-β1, transforming growth factor beta 1; TLR2, Toll like receptor 2; TLR4, Toll like receptor 4; TNF-α, tumor necrosis factor alpha; URTI, upper respiratory tract infection; WBC, white blood cell; WHO, World Health Organization.
References
1. Watad A, Bragazzi NL, Adawi M, Amital H, Toubi E, Porat BS, et al. Autoimmunity in the elderly: insights from basic science and clinics - a mini-review. Gerontology (2017) 63:515–23. doi: 10.1159/000478012
2. Janeway C. Immunobiology: The Immune System in Health and Disease. London, UK: Current Biology Publications (1999).
3. Reed RG, Raison CL. Stress and the immune system. In: E Charlotte editor. Environmental Influences on the Immune System. Vienna: Springer (2016). 97–126. doi: 10.1007/978-3-7091-1890-0_5
4. Aw D, Silva AB, Palmer DB. The effect of age on the phenotype and function of developing thymocytes. J Comp Pathol. (2010) 142 Suppl 1:S45–59. doi: 10.1016/j.jcpa.2009.10.004
5. Palmer DB. The effect of age on thymic function. Front Immunol. (2013) 4:316. doi: 10.3389/fimmu.2013.00316
6. Agrawal A, Agrawal S, Gupta S. Dendritic cells in human aging. Exp Gerontol. (2007) 42:421–6. doi: 10.1016/j.exger.2006.11.007
7. Agrawal A, Agrawal S, Tay J, Gupta S. Biology of dendritic cells in aging. J Clin Immunol. (2008) 28:14–20. doi: 10.1007/s10875-007-9127-6
8. Montecino-Rodriguez E, Berent-Maoz B, Dorshkind K. Causes, consequences, and reversal of immune system aging. J Clin Invest. (2013) 123:958–65. doi: 10.1172/JCI64096
9. Akunuru S, Geiger H. Aging, clonality, and rejuvenation of hematopoietic stem cells. Trends Mol Med. (2016) 22:701–12. doi: 10.1016/j.molmed.2016.06.003
10. Min H, Montecino-Rodriguez E, Dorshkind K. Reduction in the developmental potential of intrathymic T cell progenitors with age. J Immunol. (2004) 173:245–50. doi: 10.4049/jimmunol.173.1.245
11. Min H, Montecino-Rodriguez E, Dorshkind K. Effects of aging on the common lymphoid progenitor to pro-B cell transition. J Immunol. (2006) 176:1007–12. doi: 10.4049/jimmunol.176.2.1007
12. Michaud M, Balardy L, Moulis G, Gaudin C, Peyrot C, Vellas B, et al. Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc. (2013) 14:877–82. doi: 10.1016/j.jamda.2013.05.009
13. Franceschi C. Inflammaging as a major characteristic of old people: can it be prevented or cured? Nutr Rev. (2007) 65(12 Pt 2):S173–6. doi: 10.1301/nr.2007.dec.S173-S176
14. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. (2008) 8:958–69. doi: 10.1038/nri2448
15. De la Fuente M, Miquel J. An update of the oxidation-inflammation theory of aging: the involvement of the immune system in oxi-inflamm-aging. Curr Pharm Des. (2009) 15:3003–26. doi: 10.2174/138161209789058110
16. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell (2013) 153:1194–217. doi: 10.1016/j.cell.2013.05.039
17. van Deursen JM. The role of senescent cells in ageing. Nature (2014) 509:439–46. doi: 10.1038/nature13193
18. Ramos-Casals M, García-Carrasco M, Brito MP, López-Soto A, Font J. Autoimmunity and geriatrics: clinical significance of autoimmune manifestations in the elderly. Lupus (2003) 12:341–55. doi: 10.1191/0961203303lu383ed
19. Bigley AB, Spielmann G, LaVoy EC, Simpson RJ. Can exercise-related improvements in immunity influence cancer prevention and prognosis in the elderly? Maturitas (2013) 76:51–6. doi: 10.1016/j.maturitas.2013.06.010
20. Müller L, Pawelec G. Aging and immunity - impact of behavioral intervention. Brain Behav Immun. (2014) 39:8–22. doi: 10.1016/j.bbi.2013.11.015
21. Romano-Spica V, Macini P, Fara GM, Giammanco G, GSMS—Working group on movement sciences for Health Italian Society of Hygiene Preventive Medicine and Public Health. Adapted Physical Activity for the Promotion of Health and the Prevention of Multifactorial Chronic Diseases: the Erice Charter. Ann Ig. (2015) 27:406–14. doi: 10.7416/ai.2015.2028
22. Cao Dinh H, Beyer I, Mets T, Onyema OO, Njemini R, Renmans W, et al. Effects of physical exercise on markers of cellular immunosenescence: a systematic review. Calcif Tissue Int. (2017) 100:193–215. doi: 10.1007/s00223-016-0212-9
23. Sardeli AV, Tomeleri CM, Cyrino ES, Fernhall B, Cavaglieri CR, Chacon-Mikahil MPT. Effect of resistance training on inflammatory markers of older adults: a meta-analysis. Exp Gerontol. (2018) 111:188–96. doi: 10.1016/j.exger.2018.07.021
24. Valdiglesias V, Sánchez-Flores M, Maseda A, Lorenzo-López L, Marcos-Pérez D, López-Cortón A, et al. Immune biomarkers in older adults: Role of physical activity. J Toxicol Environ Health (2017) 80:605–20. doi: 10.1080/15287394.2017.1286898
25. Gill JM. Physical activity, cardiorespiratory fitness and insulin resistance: a short update. Curr Opin Lipidol. (2007) 18:47–52. doi: 10.1097/MOL.0b013e328012b8bd
26. Hayes LD, Sculthorpe N, Herbert P, Baker JS, Spagna R, Grace FM. Six weeks of conditioning exercise increases total, but not free testosterone in lifelong sedentary aging men. Aging Male (2015). 18:195–200. doi: 10.3109/13685538.2015.1046123
27. Williams MA, Haskell WL, Ades PA, Amsterdam EA, Bittner V, Franklin BA, et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation (2007) 116:572–84. doi: 10.1161/CIRCULATIONAHA.107.185214
28. Bouassida A, Chamari K, Zaouali M, Feki Y, Zbidi A, Tabka Z. Review on leptin and adiponectin responses and adaptations to acute and chronic exercise. Br J Sports Med. (2010) 44:620–30. doi: 10.1136/bjsm.2008.046151
29. Hwang Y, Park J, Lim K. Effects of pilates exercise on salivary secretory immunoglobulin a levels in older women. J Aging Phys Act. (2016) 24:399–406. doi: 10.1123/japa.2015-0005
30. Pedersen BK, Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiol Rev. (2000) 80:1055–81. doi: 10.1152/physrev.2000.80.3.1055
32. Campbell JP, Turner JE. Debunking the myth of exercise-induced immune suppression: redefining the impact of exercise on immunological health across the lifespan. Front Immunol. (2018) 9:648. doi: 10.3389/fimmu.2018.00648
33. Kakanis MW, Peake J, Brenu EW, Simmonds M, Gray B, Hooper SL, et al. The open window of susceptibility to infection after acute exercise in healthy young male elite athletes. Exerc Immunol Rev. (2010) 16:119–37. doi: 10.1016/j.jsams.2010.10.642
34. Gleeson M, Pyne DB. Special feature for the Olympics: effects of exercise on the immune system: exercise effects on mucosal immunity. Immunol Cell Biol. (2000) 78:536–44. doi: 10.1111/j.1440-1711.2000.t01-8-.x
35. Gleeson M, McDonald WA, Pyne DB, Cripps AW, Francis JL, Fricker PA, et al. Salivary IgA levels and infection risk in elite swimmers. Med Sci Sports Exerc. (1999) 31:67–73. doi: 10.1097/00005768-199901000-00012
36. de Araújo AL, Silva LCR, Fernandes JR, Benard G. Preventing or reversing immunosenescence: can exercise be an immunotherapy? Immunotherapy (2013) 5:879–93. doi: 10.2217/imt.13.77
37. Liu-Ambrose T, Khan KM, Eng JJ, Janssen PA, Lord SR, McKay HA. Resistance and agility training reduce fall risk in women aged 75 to 85 with low bone mass: a 6-month randomized, controlled trial. J Am Geriatr Soc. (2004) 52:657–65. doi: 10.1111/j.1532-5415.2004.52200.x
38. Csapo R, Alegre LM. Effects of resistance training with moderate vs heavy loads on muscle mass and strength in the elderly: A meta-analysis. Scand J Med Sci Sports (2016) 26:995–1006. doi: 10.1111/sms.12536
39. Sellami M, Abderrahman AB, Casazza GA, Kebsi W, Lemoine-Morel S, Bouguerra L, et al. Effect of age and combined sprint and strength training on plasma catecholamine responses to a Wingate-test. Eur J Appl Physiol. (2014) 114:969–82. doi: 10.1007/s00421-014-2828-7
40. Sellami M, Ben Abderrahman A, Kebsi W, De Sousa MV, Zouhal H. Original research: effect of sprint and strength training on glucoregulatory hormones: effect of advanced age. Exp Biol Med. (2017) 242:113–23.
41. Sellami M, Dhahbi W, Hayes LD, Padulo J, Rhibi F, Djemail H, et al. Combined sprint and resistance training abrogates age differences in somatotropic hormones. PLoS ONE (2017) 12:e0183184. doi: 10.1371/journal.pone.0183184
42. Pedersen BK, Bruunsgaard H, Klokker M, Kappel M, MacLean DA, Nielsen HB, et al. Exercise-induced immunomodulation–possible roles of neuroendocrine and metabolic factors. Int J Sports Med. (1997) 18 (Suppl 1):S2–7. doi: 10.1055/s-2007-972695
43. Cosentino M, Marino F, Kustrimovic N. Endogenous Catecholamines in Immune Cells: Discovery, Functions and Clinical Potential as Therapeutic Targets (2013). Available from: http://brainimmune.com/endogenous-catecholamines in immune cells: discovery-functions-and-clinical-potential-as-pharmacotherapeutic-targets-3/ [Accessed July 30, 2018].
44. de Abreu Mello A, Fernandes de Souza J, Nunes da Fonseca R, Allodi S, Monteiro de Barros C. Catecholamines are produced by ascidian immune cells: The involvement of PKA and PKC in the adrenergic signaling pathway. Brain Behav Immun. (2017) 61:289–96. doi: 10.1016/j.bbi.2017.01.002
45. Nieman DC. Exercise, infection, and immunity. Int J Sports Med. (1994) 15 (Suppl 3):S131–41. doi: 10.1055/s-2007-1021128
46. Nieman DC. Prolonged aerobic exercise, immune response, and risk of infection. In: L. Hoffman-Goetz editor. Exercise and Immune Function. Boca Raton, FL: CRC Press (1996). 143–61.
47. Nieman DC, Nehlsen-Cannarella SL. The effects of acute and chronic exercise of immunoglobulins. Sports Med. (1991) 11:183–201. doi: 10.2165/00007256-199111030-00003
48. Nieman DC, Henson DA, Gusewitch G, Warren BJ, Dotson RC, Butterworth DE, et al. Physical activity and immune function in elderly women. Med Sci Sports Exerc. (1993) 25:823–31. doi: 10.1249/00005768-199307000-00011
49. Nieman DC, Miller AR, Henson DA, Warren BJ, Gusewitch G, Johnson RL, et al. Effect of high- versus moderate-intensity exercise on lymphocyte subpopulations and proliferative response. Int J Sports Med. (1994) 15:199–206. doi: 10.1055/s-2007-1021047
50. Mazdarani FH, Khaledi N, Hedayati M. Effects of official basketball competition on the levels of cortisol and salivary immunoglobulin (A) among female children. J Childhood Obesity (2016) 1:12. doi: 10.21767/2572-5394.100013
51. Pedersen BK, Toft AD. Effects of exercise on lymphocytes and cytokines. Br J Sports Med. (2000) 34:246–51. doi: 10.1136/bjsm.34.4.246
52. Bruunsgaard H, Pedersen BK. Special feature for the Olympics: effects of exercise on the immune system: effects of exercise on the immune system in the elderly population. Immunol Cell Biol. (2000) 78:523–31. doi: 10.1111/j.1440-1711.2000.t01-14-.x
53. Navalta JW, Mohamed R, El-Baz A, McFarlin BK, Lyons TS. Exercise-induced immune cell apoptosis: image-based model for morphological assessment. Eur J Appl Physiol. (2010) 110:325–31. doi: 10.1007/s00421-010-1504-9
54. Senchina DS, Kohut ML. Immunological outcomes of exercise in older adults. Clin Interv Aging. (2007) 2:3–16. doi: 10.2147/ciia.2007.2.1.3
55. Simpson RJ, Cosgrove C, Chee MM, McFarlin BK, Bartlett DB, Spielmann G, et al. Senescent phenotypes and telomere lengths of peripheral blood T-cells mobilized by acute exercise in humans. Exerc Immunol Rev. (2010) 16:40–55.
56. Crist DM, Mackinnon LT, Thompson RF, Atterbom HA, Egan PA. Physical exercise increases natural cellular-mediated tumor cytotoxicity in elderly women. Gerontology (1989) 35:66–71. doi: 10.1159/000213001
57. Fiatarone MA, Morley JE, Bloom ET, Benton D, Solomon GF, Makinodan T. The effect of exercise on natural killer cell activity in young and old subjects. J Gerontol. (1989) 44:M37–45.
58. Cannon JG, Orencole SF, Fielding RA, Meydani M, Meydani SN, Fiatarone MA, et al. Acute phase response in exercise: interaction of age and vitamin E on neutrophils and muscle enzyme release. Am J Physiol. (1990) 259(6 Pt 2):R1214–9. doi: 10.1152/ajpregu.1990.259.6.R1214
59. Shinkai S, Shore S, Shek PN, Shephard RJ. Acute exercise and immune function. Relationship between lymphocyte activity and changes in subset counts. Int J Sports Med. (1992) 13:452–61. doi: 10.1055/s-2007-1021297
60. Cannon JG, Fiatarone MA, Fielding RA, Evans WJ. Aging and stress-induced changes in complement activation and neutrophil mobilization. J Appl Physiol. (1994) 76:2616–20. doi: 10.1152/jappl.1994.76.6.2616
61. Mazzeo RS, Rajkumar C, Rolland J, Blaher B, Jennings G, Esler M. Immune response to a single bout of exercise in young and elderly subjects. Mech Ageing Dev. (1998) 100:121–32. doi: 10.1016/S0047-6374(97)00130-9
62. Bruunsgaard H, Jensen MS, Schjerling P, Halkjaer-Kristensen J, Ogawa K, Skinhøj P, et al. Exercise induces recruitment of lymphocytes with an activated phenotype and short telomeres in young and elderly humans. Life Sci. (1999) 65:2623–33. doi: 10.1016/S0024-3205(99)00531-7
63. Ceddia MA, Price EA, Kohlmeier CK, Evans JK, Lu Q, McAuley E, et al. Differential leukocytosis and lymphocyte mitogenic response to acute maximal exercise in the young and old. Med Sci Sports Exerc. (1999) 31:829–36. doi: 10.1097/00005768-199906000-00011
64. Colbert LH, Visser M, Simonsick EM, Tracy RP, Newman AB, Kritchevsky SB, et al. Physical activity, exercise, and inflammatory markers in older adults: findings from the Health, Aging and Body Composition Study. J Am Geriatr Soc. (2004) 52:1098–104. doi: 10.1111/j.1532-5415.2004.52307.x
65. Hamada K, Vannier E, Sacheck JM, Witsell AL, Roubenoff R. Senescence of human skeletal muscle impairs the local inflammatory cytokine response to acute eccentric exercise. FASEB J. (2005) 19:264–6. doi: 10.1096/fj.03-1286fje
66. Stewart LK, Flynn MG, Campbell WW, Craig BA, Robinson JP, McFarlin BK, et al. Influence of exercise training and age on CD14+ cell-surface expression of toll-like receptor 2 and 4. Brain Behav Immun. (2005) 19:389–97. doi: 10.1016/j.bbi.2005.04.003
67. McFarlin BK, Flynn MG, Campbell WW, Craig BA, Robinson JP, Stewart LK, et al. Physical activity status, but not age, influences inflammatory biomarkers and toll-like receptor 4. J Gerontol A Biol Sci Med Sci. (2006) 61:388–93. doi: 10.1093/gerona/61.4.388
68. Ludlow AT, Zimmerman JB, Witkowski S, Hearn JW, Hatfield BD, Roth SM. Relationship between physical activity level, telomere length, and telomerase activity. Med Sci Sports Exerc. (2008) 40:1764–71. doi: 10.1249/MSS.0b013e31817c92aa
69. Puterman E, Lin J, Blackburn E, O'Donovan A, Adler N, Epel E. The power of exercise: buffering the effect of chronic stress on telomere length. PLoS ONE (2010) 5:e10837. doi: 10.1371/journal.pone.0010837
70. Spielmann G, McFarlin BK, O'Connor DP, Smith PJ, Pircher H, Simpson RJ. Aerobic fitness is associated with lower proportions of senescent blood T-cells in man. Brain Behav Immun. (2011) 25:1521–9. doi: 10.1016/j.bbi.2011.07.226
71. Spielmann G, Bollard CM, Bigley AB, Hanley PJ, Blaney JW, LaVoy EC, et al. The effects of age and latent cytomegalovirus infection on the redeployment of CD8+ T cell subsets in response to acute exercise in humans. Brain Behav Immun. (2014) 39:142–51. doi: 10.1016/j.bbi.2013.05.003
72. Bigley AB, Spielmann G, Agha N, Simpson RJ. The Effects of Age and Latent Cytomegalovirus Infection on NK-Cell Phenotype and Exercise Responsiveness in Man. Oxid Med Cell Longev. 2015:979645. doi: 10.1155/2015/979645
73. Bartlett DB, Fox O, McNulty CL, Greenwood HL, Murphy L, Sapey E, et al. Habitual physical activity is associated with the maintenance of neutrophil migratory dynamics in healthy older adults. Brain Behav Immun. (2016) 56:12–20. doi: 10.1016/j.bbi.2016.02.024
74. Silva LC, de Araújo AL, Fernandes JR, Matias Mde S, Silva PR, Duarte AJ, et al. Moderate and intense exercise lifestyles attenuate the effects of aging on telomere length and the survival and composition of T cell subpopulations. Age (2016) 38:24. doi: 10.1007/s11357-016-9879-0
75. Minuzzi LG, Rama L, Chupel MU, Rosado F, Dos Santos JV, Simpson R, et al. Effects of lifelong training on senescence and mobilization of T lymphocytes in response to acute exercise. Exerc Immunol Rev. (2018) 24:72–84.
76. Pedersen BK, Nieman DC. Exercise immunology: integration and regulation. Immunol Today. (1998) 19:204–6. doi: 10.1016/S0167-5699(98)01255-9
77. Wasinski F, Gregnani MF, Ornellas FH, Bacurau AV, Câmara NO, Araujo RC, et al. Lymphocyte glucose and glutamine metabolism as targets of the anti-inflammatory and immunomodulatory effects of exercise. Mediators Inflamm. (2014) 2014:326803. doi: 10.1155/2014/326803
78. Phaneuf S, Leeuwenburgh C. Apoptosis and exercise. Med Sci Sports Exerc. (2001) 33:393–6. doi: 10.1097/00005768-200103000-00010
79. Edwards KM, Burns VE, Allen LM, McPhee JS, Bosch JA, Carroll D, et al. Eccentric exercise as an adjuvant to influenza vaccination in humans. Brain Behav Immun. (2007) 21:209–17. doi: 10.1016/j.bbi.2006.04.158
80. Edwards KM, Ziegler MG, Mills PJ. The potential anti-inflammatory benefits of improving physical fitness in hypertension. J Hypertens (2007) 25:1533–42. doi: 10.1097/HJH.0b013e328165ca67
81. Timmerman KL, Flynn MG, Coen PM, Markofski MM, Pence BD. Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: a role in the anti-inflammatory influence of exercise? J Leukoc Biol. (2008) 84:1271–8. doi: 10.1189/jlb.0408244
82. Espersen GT, Toft E, Ernst E, Kaalund S, Grunnet N. Changes of polymorphonuclear granulocyte migration and lymphocyte proliferative responses in elite runners undergoing intense exercise. Scand J Med Sci Sports. (1991) 1:158–62. doi: 10.1111/j.1600-0838.1991.tb00289.x
83. Eskola J, Ruuskanen O, Soppi E, Viljanen MK, Järvinen M, Toivonen H, et al. Effect of sport stress on lymphocyte transformation and antibody formation. Clin Exp Immunol. (1978) 32:339–45.
84. Pistillo M, Bigley AB, Spielmann G, LaVoy EC, Morrison MR, Kunz H, et al. (2013). The effects of age and viral serology on γδ T-cell numbers and exercise responsiveness in humans. Cell Immunol. 284:91–7. doi: 10.1016/j.cellimm.2013.07.009
85. Radom-Aizik S, Zaldivar FP Jr, Haddad F, Cooper DM. Impact of brief exercise on circulating monocyte gene and microRNA expression: implications for atherosclerotic vascular disease. Brain Behav Immun. (2014) 39:121–9. doi: 10.1016/j.bbi.2014.01.003
86. Ulven SM, Foss SS, Skjølsvik AM, Stadheim HK, Myhrstad MC, Raael E, et al. An acute bout of exercise modulate the inflammatory response in peripheral blood mononuclear cells in healthy young men. Arch Physiol Biochem. (2015) 121:41–9. doi: 10.3109/13813455.2014.1003566
87. Bueno V, Lord J, Jackson T. The Ageing Immune System and Health. Cham: Springer International Publishing (2016).
88. Linehan E, Fitzgerald DC. Ageing and the immune system: focus on macrophages. Eur J Microbiol Immunol. (2015) 5:14–24. doi: 10.1556/EuJMI-D-14-00035
89. Jackaman C, Tomay F, Duong L, Abdol Razak NB, Pixley FJ, Metharom P, et al. Aging and cancer: the role of macrophages and neutrophils. Ageing Res Rev. (2017) 36:105–16. doi: 10.1016/j.arr.2017.03.008
90. Linehan E, Dombrowski Y, Snoddy R, Fallon PG, Kissenpfennig A, Fitzgerald DC. Aging impairs peritoneal but not bone marrow-derived macrophage phagocytosis. Aging Cell (2014) 13:699–708. doi: 10.1111/acel.12223
91. Malaguarnera L, Cristaldi E, Vinci M, Malaguarnera M. The role of exercise on the innate immunity of the elderly. Eur Rev Aging Physical Activ. (2008) 5:43–9. doi: 10.1007/s11556-007-0028-8
92. Pyne DB. Regulation of neutrophil function during exercise. Sports Med. (1994) 17:245–58. doi: 10.2165/00007256-199417040-00005
93. Quindry JC, Stone WL, King J, Broeder CE. The effects of acute exercise on neutrophils and plasma oxidative stress. Med Sci Sports Exerc. (2003) 35:1139–45. doi: 10.1249/01.MSS.0000074568.82597.0B
94. Cuzzolin L, Lussignoli S, Crivellente F, Adami A, Schena F, Bellavite P, et al. Influence of an acute exercise on neutrophil and platelet adhesion, nitric oxide plasma metabolites in inactive and active subjects. Int J Sports Med. (2000) 21:289–93. doi: 10.1055/s-2000-13308
95. Kohut ML, Senchina DS. Reversing age-associated immunosenescence via exercise. Exerc Immunol Rev. (2004) 10:6–41.
96. Peake JM, Neubauer O, Walsh NP, Simpson RJ. Recovery of the immune system after exercise. J Appl Physiol. (2017) 122:1077–87. doi: 10.1152/japplphysiol.00622.2016
97. Walsh NP, Gleeson M, Shephard RJ, Gleeson M, Woods JA, Bishop NC, et al. Position statement. Part one: Immune function and exercise. Exerc Immunol Rev. (2011) 17:6–63.
98. Gabriel H, Schwarz L, Steffens G, Kindermann W. Immunoregulatory hormones, circulating leucocyte and lymphocyte subpopulations before and after endurance exercise of different intensities. Int J Sports Med. (1992) 13:359–66. doi: 10.1055/s-2007-1021281
99. Mooren FC, Blöming D, Lechtermann A, Lerch MM, Völker K. Lymphocyte apoptosis after exhaustive and moderate exercise. J Appl Physiol. (2002) 93:147–53. doi: 10.1152/japplphysiol.01262.2001
100. MacNeil B, Hoffman-Goetz L, Kendall A, Houston M, Arumugam Y. Lymphocyte proliferation responses after exercise in men: fitness, intensity, and duration effects. J Appl Physiol. (1991) 70:179–85. doi: 10.1152/jappl.1991.70.1.179
101. McCarthy DA, Dale MM. The leucocytosis of exercise. A review and model. Sports Med. (1988) 6:333–63. doi: 10.2165/00007256-198806060-00002
102. McCarthy DA, Perry JD, Melsom RD, Dale MM. Leucocytosis induced by exercise. Br Med J. (1987) 295:636. doi: 10.1136/bmj.295.6599.636
103. Woods JA, Ceddia MA, Wolters BW, Evans JK, Lu Q, McAuley E. Effects of 6 months of moderate aerobic exercise training on immune function in the elderly. Mech Ageing Dev. (1999) 109:1–19. doi: 10.1016/S0047-6374(99)00014-7
104. Della Gatta PA, Garnham AP, Peake JM, Cameron-Smith D. Effect of exercise training on skeletal muscle cytokine expression in the elderly. Brain Behav Immun. (2014) 39:80–6. doi: 10.1016/j.bbi.2014.01.006
105. Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. (2009) 29:313–26. doi: 10.1089/jir.2008.0027
106. Gleeson M, Bishop NC. The T cell and NK cell immune response to exercise. Ann Transplant (2005) 10:43–8.
107. Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. (2008) 9:503–10. doi: 10.1038/ni1582
108. Melsen JE, Lugthart G, Lankester AC, Schilham MW. Human circulating and tissue-resident CD56(bright) natural killer cell populations. Front Immunol. (2016) 7:262. doi: 10.3389/fimmu.2016.00262
109. Poli A, Michel T, Thérésine M, Andrès E, Hentges F, Zimmer J. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology (2009) 126:458–65. doi: 10.1111/j.1365-2567.2008.03027.x
110. Timmons BW, Cieslak T. Human natural killer cell subsets and acute exercise: a brief review. Exerc Immunol Rev. (2008) 14:8–23.
111. Ortaldo JR, Mantovani A, Hobbs D, Rubinstein M, Pestka S, Herberman RB. Effects of several species of human leukocyte interferon on cytotoxic activity of NK cells and monocytes. Int J Cancer (1983) 31:285–9. doi: 10.1002/ijc.2910310306
112. O'Shea J, Ortaldo JR. The biology of natural killer cells: insights into the molecular basis of function. In: CE Lewis and JO McGee, editors. The Natural Killer Cell. (1992) 4:316.
113. Bigley AB, Spielmann G, Agha N, O'Connor DP, Simpson RJ. Dichotomous effects of latent CMV infection on the phenotype and functional properties of CD8+ T-cells and NK-cells. Cell Immunol. (2016) 300:26–32. doi: 10.1016/j.cellimm.2015.11.005
114. Solana R, Campos C, Pera A, Tarazona R. Shaping of NK cell subsets by aging. Curr Opin Immunol. (2014) 29:56–61. doi: 10.1016/j.coi.2014.04.002
115. Borrego F, Alonso MC, Galiani MD, Carracedo J, Ramirez R, Ostos B, et al. NK phenotypic markers and IL2 response in NK cells from elderly people. Exp Gerontol. (1999) 34:253–65. doi: 10.1016/S0531-5565(98)00076-X
116. Targan S, Britvan L, Dorey F. Activation of human NKCC by moderate exercise: increased frequency of NK cells with enhanced capability of effector–target lytic interactions. Clin Exp Immunol. (1981) 45:352–60.
117. Brahmi Z, Thomas JE, Park M, Park M, Dowdeswell IR. The effect of acute exercise on natural killer-cell activity of trained and sedentary human subjects. J Clin Immunol. (1985) 5:321–8. doi: 10.1007/BF00918251
118. Pedersen BK, Ullum H. NK cell response to physical activity: possible mechanisms of action. Med Sci Sports Exerc. (1994) 26:140–6. doi: 10.1249/00005768-199402000-00003
119. Evans W. NK cell recruitment and exercise: Potential immunotherapeutic role of shear stress and endothelial health. Med Hypotheses. (2017) 109:170–3. doi: 10.1016/j.mehy.2017.10.015
120. Zimmer P, Bloch W, Kieven M, Lövenich L, Lehmann J, Holthaus M, et al. Serotonin shapes the migratory potential of NK Cells - an in vitro approach. Int J Sports Med. (2017) 38:857–63. doi: 10.1055/s-0043-113042
121. Bigley AB, Rezvani K, Chew C, Sekine T, Pistillo M, Crucian B, et al. Acute exercise preferentially redeploys NK-cells with a highly-differentiated phenotype and augments cytotoxicity against lymphoma and multiple myeloma target cells. Brain Behav Immun. (2014) 39:160–71. doi: 10.1016/j.bbi.2013.10.030
122. Shephard RJ, Shek PN. Potential impact of physical activity and sport on the immune system - a brief review. Br J Sports Med. (1994) 28:247–55. doi: 10.1136/bjsm.28.4.247
123. Shephard RJ, Shek PN. Effects of exercise and training on natural killer cell counts and cytolytic activity: a meta-analysis. Sports Med. (1999) 28:177–95. doi: 10.2165/00007256-199928030-00003
124. Tomasi TB, Trudeau FB, Czerwinski D, Erredge S. Immune parameters in athletes before and after strenuous exercise. J Clin Immunol. (1982) 2:173–8. doi: 10.1007/BF00915219
125. Morgado JM, Rama L, Silva I, de Jesus Inácio M, Henriques A, Laranjeira P, et al. Cytokine production by monocytes, neutrophils, and dendritic cells is hampered by long-term intensive training in elite swimmers. Eur J Appl Physiol. (2012) 112:471–82. doi: 10.1007/s00421-011-1966-4
126. Gannon GA, Shek PN, Shephard RJ. Natural killer cells: modulation by intensity and duration of exercise. Exerc Immunol Rev. (1995) 1:26–48.
127. Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. (2011) 11:607–15. doi: 10.1038/nri3041
128. Castelo-Branco C, Soveral I. The immune system and aging: a review. Gynecol Endocrinol. (2014) 30:16–22. doi: 10.3109/09513590.2013.852531
129. Della Bella S, Bierti L, Presicce P, Arienti R, Valenti M, Saresella M, et al. Peripheral blood dendritic cells and monocytes are differently regulated in the elderly. Clin Immunol. (2007) 122:220–8. doi: 10.1016/j.clim.2006.09.012
130. Chiang J, Chen YY, Akiko T, Huang YC, Hsu ML, Jang TR, et al. Tai Chi Chuan increases circulating myeloid dendritic cells. Immunol Invest. (2010) 39:863–73. doi: 10.3109/08820139.2010.503766
131. Thijssen DH, Vos JB, Verseyden C, van Zonneveld AJ, Smits P, Sweep FC, et al. Haematopoietic stem cells and endothelial progenitor cells in healthy men: effect of aging and training. Aging Cell (2006) 5:495–503. doi: 10.1111/j.1474-9726.2006.00242.x
132. Ortega E, Collazos ME, Maynar M, Barriga C, De la Fuente M. Stimulation of the phagocytic function of neutrophils in sedentary men after acute moderate exercise. Eur J Appl Physiol Occup Physiol. (1993) 66:60–4. doi: 10.1007/BF00863401
133. Haaland DA, Sabljic TF, Baribeau DA, Mukovozov IM, Hart LE. Is regular exercise a friend or foe of the aging immune system? A systematic review. Clin J Sport Med. (2008) 18:539–48. doi: 10.1097/JSM.0b013e3181865eec
134. Yan H, Kuroiwa A, Tanaka H, Shindo M, Kiyonaga A, Nagayama A. Effect of moderate exercise on immune senescence in men. Eur J Appl Physiol. (2001) 86:105–11. doi: 10.1007/s004210100521
136. Shimizu K, Kimura F, Akimoto T, Akama T, Tanabe K, Nishijima T, et al. Effect of moderate exercise training on T-helper cell subpopulations in elderly people. Exerc Immunol Rev. (2008) 14:24–37.
137. Kapasi ZF, Ouslander JG, Schnelle JF, Kutner M, Fahey JL. Effects of an exercise intervention on immunologic parameters in frail elderly nursing home residents. J Gerontol A Biol Sci Med Sci. (2003) 58:636–43. doi: 10.1093/gerona/58.7.M636
138. Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Mackey JR. Randomized controlled trial of exercise and blood immune function in postmenopausal breast cancer survivors. J Appl Physiol. (2005) 98:1534–40. doi: 10.1152/japplphysiol.00566.2004
139. van der Geest KSM, Wang Q, Eijsvogels TMH, Koenen HJP, Joosten I, Brouwer E, et al. Changes in peripheral immune cell numbers and functions in octogenarian walkers - an acute exercise study. Immun Ageing. (2017) 14:5. doi: 10.1186/s12979-017-0087-2
140. Ogawa K, Oka J, Yamakawa J, Higuchi M. Habitual exercise did not affect the balance of type 1 and type 2 cytokines in elderly people. Mech Ageing Dev. (2003) 124:951–6. doi: 10.1016/S0047-6374(03)00167-2
141. Shinkai S, Kohno H, Kimura K, Komura T, Asai H, Inai R, et al. Physical activity and immune senescence in men. Med Sci Sports Exerc. (1995) 27:1516–26. doi: 10.1249/00005768-199511000-00008
142. Drela N, Kozdron E, Szczypiorski P. Moderate exercise may attenuate some aspects of immunosenescence. BMC Geriatr (2004) 4:8. doi: 10.1186/1471-2318-4-8
143. Kohut ML, Arntson BA, Lee W, Rozeboom K, Yoon KJ, Cunnick JE, et al. Moderate exercise improves antibody response to influenza immunization in older adults. Vaccine (2004) 22:2298–306. doi: 10.1016/j.vaccine.2003.11.023
144. Middleton D, Curran M, Maxwell L. Natural killer cells and their receptors. Transpl Immunol. (2002) 10:147–64. doi: 10.1016/S0966-3274(02)00062-X
145. Hayhoe RP, Henson SM, Akbar AN, Palmer DB. Variation of human natural killer cell phenotypes with age: identification of a unique KLRG1-negative subset. Hum Immunol. (2010) 71:676–81. doi: 10.1016/j.humimm.2010.03.014
146. Campbell PT, Wener MH, Sorensen B, Wood B, Chen-Levy Z, Potter JD, et al. Effect of exercise on in vitro immune function: a 12-month randomized, controlled trial among postmenopausal women. J Appl Physiol. (2008) 104:1648–55. doi: 10.1152/japplphysiol.01349.2007
147. Flynn MG, Fahlman M, Braun WA, Lambert CP, Bouillon LE, Brolinson PG, et al. Effects of resistance training on selected indexes of immune function in elderly women. J Appl Physiol (1999) 86:1905–13. doi: 10.1152/jappl.1999.86.6.1905
148. Raso V, Benard GDA, Silva Duarte AJ, Natale VM. Effect of resistance training on immunological parameters of healthy elderly women. Med Sci Sports Exerc. (2007) 39:2152–9. doi: 10.1249/mss.0b013e318156e9fa
149. Bobeuf F, Labonté M, Khalil A, Dionne IJ. Effect of resistance training on hematological blood markers in older men and women: a pilot study. Curr Gerontol Geriatr Res (2009) 2009:156820. doi: 10.1155/2009/156820
150. Rall LC, Roubenoff R, Cannon JG, Abad LW, Dinarello CA, Meydani SN. Effects of progressive resistance training on immune response in aging and chronic inflammation. Med Sci Sports Exerc. (1996) 28:1356–65. doi: 10.1097/00005768-199611000-00003
151. Serra MC, Ryan AS, Ortmeyer HK, Addison O, Goldberg AP. Resistance training reduces inflammation and fatigue and improves physical function in older breast cancer survivors. Menopause (2018) 25:211–6. doi: 10.1097/GME.0000000000000969
152. Tomeleri CM, Ribeiro AS, Souza MF, Schiavoni D, Schoenfeld BJ, Venturini D, et al. Resistance training improves inflammatory level, lipid and glycemic profiles in obese older women: a randomized controlled trial. Exp Gerontol. (2016) 84:80–7. doi: 10.1016/j.exger.2016.09.005
153. Mejías-Peña Y, Estébanez B, Rodriguez-Miguelez P, Fernandez-Gonzalo R, Almar M, de Paz JA, et al. Impact of resistance training on the autophagy-inflammation-apoptosis crosstalk in elderly subjects. Aging (2017) 9:408–18. doi: 10.18632/aging.101167
154. Calle MC, Fernandez ML. Effects of resistance training on the inflammatory response. Nutr Res Pract. (2010) 4:259–69. doi: 10.4162/nrp.2010.4.4.259
155. Bermon S, Philip P, Ferrari P, Candito M, Dolisi C. Effects of a short-term strength training programme on lymphocyte subsets at rest in elderly humans. Eur J Appl Physiol Occup Physiol. (1999) 79:336–40. doi: 10.1007/s004210050517
156. Bartholomeu-Neto J, Brito CJ, Nóbrega OT, Sousa VC, Oliveira Toledo J, Silva Paula R, et al. Adaptation to resistance training is associated with higher phagocytic (but Not Oxidative) activity in neutrophils of older women. J Immunol Res. (2015) 2015:724982. doi: 10.1155/2015/724982
157. McFarlin BK, Flynn MG, Campbell WW, Stewart LK, Timmerman KL. TLR4 is lower in resistance-trained older women and related to inflammatory cytokines. Med Sci Sports Exerc. (2004) 36:1876–83. doi: 10.1249/01.MSS.0000145465.71269.10
158. Bomfim GF, Dos Santos RA, Oliveira MA, Giachini FR, Akamine EH, Tostes RC, et al. Toll-like receptor 4 contributes to blood pressure regulation and vascular contraction in spontaneously hypertensive rats. Clin Sci. (2012) 122:535–43. doi: 10.1042/CS20110523
159. McCarthy CG, Goulopoulou S, Wenceslau CF, Spitler K, Matsumoto T, Webb RC. Toll-like receptors and damage-associated molecular patterns: novel links between inflammation and hypertension. Am J Physiol Heart Circ Physiol. (2014) 306:H184–96. doi: 10.1152/ajpheart.00328.2013
160. Bermon S, Philip P, Candito M, Ferrari P, Dolisi C. Effects of strength exercise and training on the natural killer cell counts in elderly humans. J Sports Med Phys Fitness (2001) 41:196–202.
161. Burgomaster KA, Howarth KR, Phillips SM, Rakobowchuk M, Macdonald MJ, McGee SL, et al. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol. (2008) 586:151–60. doi: 10.1113/jphysiol.2007.142109
162. Gillen JB, Martin BJ, MacInnis MJ, Skelly LE, Tarnopolsky MA, Gibala MJ. Twelve weeks of sprint interval training improves indices of cardiometabolic health similar to traditional endurance training despite a five-fold lower exercise volume and time commitment. PLoS ONE (2016) 11:e0154075. doi: 10.1371/journal.pone.0154075
163. Allen NG, Higham SM, Mendham AE, Kastelein TE, Larsen PS, Duffield R. The effect of high-intensity aerobic interval training on markers of systemic inflammation in sedentary populations. Eur J Appl Physiol. (2017) 117:1249–56. doi: 10.1007/s00421-017-3613-1
164. Harnish CR, Sabo RT. Comparison of two different sprint interval training work-to-rest ratios on acute inflammatory responses. Sports Med Open. (2016) 2:20. doi: 10.1186/s40798-016-0044-1
165. Davison G. Innate immune responses to a single session of sprint interval training. Appl Physiol Nutr Metab. (2011) 36:395–404. doi: 10.1139/h11-033
166. Polakowski IJ, Skopinska-Rózewska E, Nazar K, Kaciuba-Uściółko H, Langfort J. Effect of endurance and “sprint” physical training on lymphocyte-induced angiogenesis in rats. Folia Biol (1989) 35:45–9.
167. Saghiv M, Sherve C, Sagiv M, Ben-Sira D. Effect of different exercise lactic acid levels on the immune system response. J Aller Immuno. (2017) 4:24.
168. Hack V, Weiss C, Friedmann B, Suttner S, Schykowski M, Erbe N, et al. Decreased plasma glutamine level and CD4+ T cell number in response to 8 wk of anaerobic training. Am J Physiol. (1997) 272(5 Pt 1):E788–95. doi: 10.1152/ajpendo.1997.272.5.E788
Keywords: physical activity, age, immunosenescence, innate immune system, adaptive immune system
Citation: Sellami M, Gasmi M, Denham J, Hayes LD, Stratton D, Padulo J and Bragazzi N (2018) Effects of Acute and Chronic Exercise on Immunological Parameters in the Elderly Aged: Can Physical Activity Counteract the Effects of Aging? Front. Immunol. 9:2187. doi: 10.3389/fimmu.2018.02187
Received: 03 April 2018; Accepted: 04 September 2018;
Published: 10 October 2018.
Edited by:
Silvia Della Bella, Università degli Studi di Milano, ItalyReviewed by:
Adriano Taddeo, Universitätsspital Bern, SwitzerlandAlejandra Pera, Universidad de Córdoba, Spain
Copyright © 2018 Sellami, Gasmi, Denham, Hayes, Stratton, Padulo and Bragazzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicola Bragazzi, robertobragazzi@gmail.com
 Maha Sellami
Maha Sellami Maha Gasmi2
Maha Gasmi2 Nicola Bragazzi
Nicola Bragazzi