Dynamic Progression of White Matter Hyperintensities in Alzheimer’s Disease and Normal Aging: Results from the Sunnybrook Dementia Study
- 1LC Campbell Cognitive Neurology Research Unit, Hurvitz Brain Sciences Research Program, Sunnybrook Research Institute, Toronto, ON, Canada
- 2Heart and Stroke Foundation Canadian Partnership for Stroke Recovery, Sunnybrook Health Sciences Centre, Toronto, ON, Canada
- 3Graduate Department of Psychological Clinical Science, University of Toronto Scarborough, Toronto, ON, Canada
- 4Faculty of Medicine, School of Graduate Studies, University of Toronto, Toronto, ON, Canada
- 5Department of Medicine, Neurology, University of Toronto and Sunnybrook Health Sciences Centre, Toronto, ON, Canada
Although white matter hyperintensities (WMH), markers of cerebral small vessel disease (SVD), are believed to generally increase over time, some studies have shown sharp decreases after therapeutic intervention, suggesting that WMH progression may be more dynamic than previously thought. Our primary goal was to examine dynamic progression of WMH in a real-world sample of Alzheimer’s disease (AD) patients and normal elderly (NC), with varying degrees of SVD. WMH volumes from serial magnetic resonance imaging (MRI; mean = 1.8 years) were measured from NC (n = 44) and AD patients (n = 113) with high and low SVD burden. Dynamic progression for each individual was measured using spatial overlap images to assess shrinkage, growth, and stable WMH volumes. Significant group differences were found for shrinkage (p < 0.001), growth (p < 0.001) and stable (p < 0.001) WMH, where the AD high SVD group showed the largest changes relative to low SVD and NC. Our results suggest spatial progression measured at the individual patient level may be more sensitive to the dynamic nature of WMH.
Introduction
Recent advances in neuroimaging techniques have allowed for the volumetric quantification of white matter hyperintensities (WMH) of presumed vascular origin, a radiological biomarker of cerebral small vessel disease (SVD; Wardlaw et al., 2013) commonly visualized on T2-weighted (T2), proton density (PD), and fluid-attenuated inversion recovery (FLAIR) magnetic resonance imaging (MRI; Pantoni, 2010). The longitudinal progression of WMH have been previously associated with vascular risk factors such as hypertension (Wolfson et al., 2013) and diabetes (Taylor et al., 2003), aging (Vannorsdall et al., 2009), decreased gait performance (Silbert et al., 2008), cognitive decline (Longstreth et al., 2005; Kramer et al., 2007; Debette et al., 2011), and brain atrophy (Schmidt et al., 2005), as well as Alzheimer’s disease (AD) pathology at post-mortem (Erten-Lyons et al., 2013).
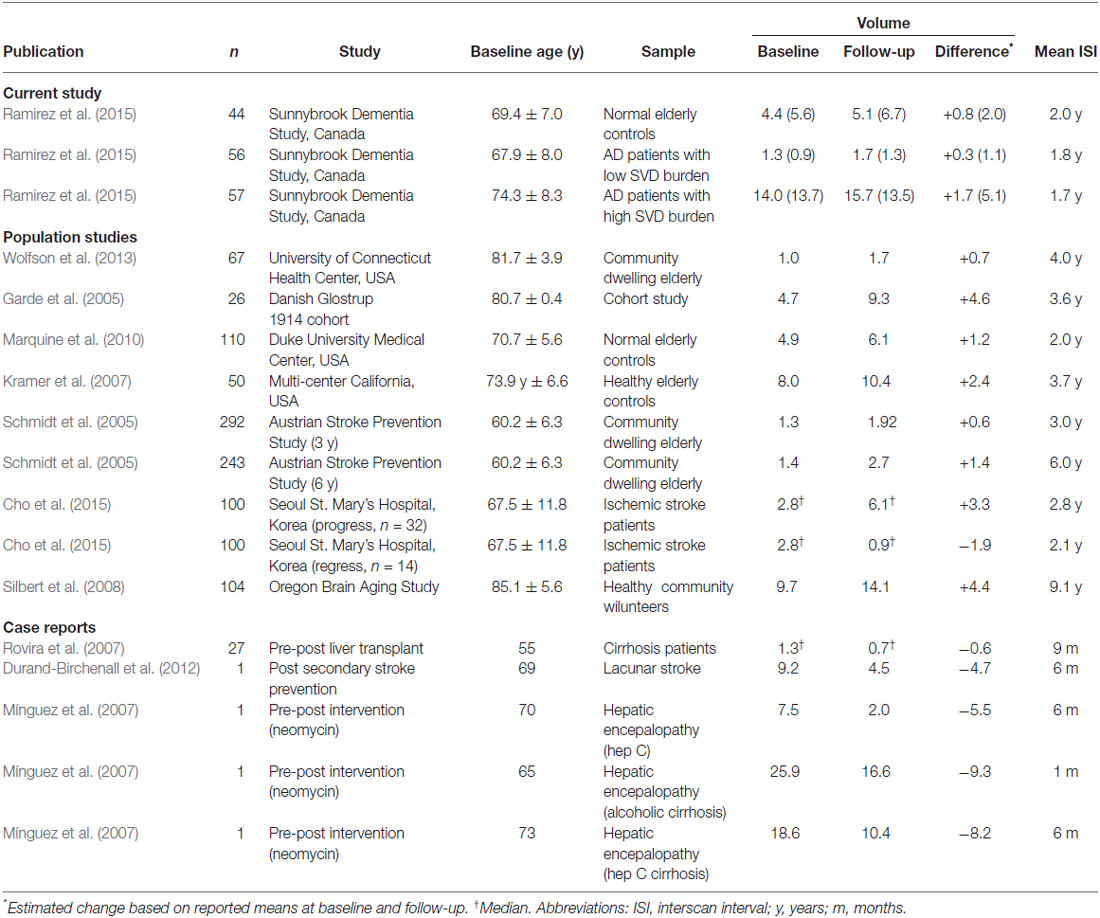
While it is generally accepted that WMH typically increase with aging and cerebrovascular disease progression, the estimated growth rates often vary between studies. Among the progression studies with quantitative WMH volumetrics producing continuous measures, baseline to follow-up imaging show increased WMH mean/median volumes ranging from 0.74 cc (~4 years) to 4.6 cc (~3.6 years) in normal elderly (NC) cohorts (Kloppenborg et al., 2014).
However, there is some recent evidence to suggest that in some cases, WMH burden may appear stable, with some studies even demonstrating regression or decreased WMH volume over time. Although early reports of this phenomenon were attributed to measurement error (Schmidt et al., 2003; Sachdev et al., 2007), recent radiological case reports have shown WMH regression in patients after cerebral infarction and ischemic stroke (Moriya et al., 2009; Durand-Birchenall et al., 2012; Cho et al., 2015), liver transplantation and improved hepatic encephalopathy treatment (Mínguez et al., 2007; Rovira et al., 2007), and carotid artery stenting (Yamada et al., 2010). Moreover, there is some evidence to suggest that drug treatments may decrease or slow the progression of WMH (Mok et al., 2009), leading some clinical trials to implement WMH burden as a neuroimaging outcome measure (Dufouil et al., 2005; White et al., 2013).
These previous findings suggest that WMH progression may be more dynamic than previously understood. In this study, we implement an imaging-based method to quantify the dynamic progression of WMH which allows for the spatial estimation of shrinking, growing, and stable WMH, at the individual patient level. We examined dynamic progression of WMH on a sample of NC participants (n = 44), and a real-world sample of AD patients (n = 113) with high and low WMH burden (Ramirez et al., 2015). Additionally, we contrasted these results with those obtained using the more conventional approach of just comparing baseline to follow-up net volumes.
Materials and Methods
Participants
Participants were sampled from the Sunnybrook Dementia Study (ClinicalTrials.gov NCT 01800214), an ongoing longitudinal study conducted at the Linda C. Campbell Cognitive Neurology Research Unit, Hurvitz Brain Sciences Program at the Sunnybrook Research Institute, and the University of Toronto, Canada. NC participants (n = 44) met strict criteria that included pre-screening and neuropsychological test performance that was within normal limits for their age and education levels. All AD participants (n = 113) met National Institute of Neurological and Communicative Disorders and Stroke—AD and Related Disorders Association criteria (McKhann et al., 2011) probable/possible AD. Probable AD included patients with none to mild WMH burden (AD low SVD, WMH < 3.5 cc: n = 56), and possible AD included patients with moderate to severe WMH burden (AD high SVD, WMH > 3.5 cc: n = 57). Although our AD high SVD group included patients with severe WMH burden, this sample had no evidence of any additional clinical criteria such as stroke temporally related to cognitive impairment, multiple or extensive infarcts, features of dementia with Lewy bodies, or other significant co-morbid disease that could substantially affect cognition (McKhann et al., 2011). Exclusion criteria included neurological diseases other than dementia, history of significant head trauma, tumors, normal pressure hydrocephalus, psychotic disorders unrelated to dementia, psychoactive substance abuse, and major depression.
All participants underwent a standardized comprehensive clinical evaluation and consented to neuropsychological testing, standardized brain MRI, and consented to the use of their data for research purposes. Research was ethically conducted and approved by the Sunnybrook Research Ethics Board.
MRI Acquisition
All brain imaging was acquired on a 1.5T GE Signa system (Milwaukee, WI, USA) with the following protocol: a T1-weighted axial 3D spoiled gradient recalled (SPGR): Echo Time (TE) = 5 ms, Repetition Time (TR) = 35 ms, Number of Averages (NEX) = 1, flip angle = 35°, Field Of View (FOV) = 22 cm, in-plane voxel size = 0.86 × 0.86 mm, slice thickness range: 1.2–1.4 mm and an interleaved PD/T2-weighted axial dual-echo spin echo: TE = 30/80 ms, TR = 3000 ms, NEX = 0.5, flip angle = 90°, FOV = 20 cm, in plane voxel size = 0.78 × 0.78 mm, slice thickness = 3 mm. The mean interscan interval (ISI) between baseline and follow-up scan was approximately 2 years (Table 1).
Tissue Quantification
Tissue quantification was accomplished using in-house segmentation tools (www.sabre.brainlab.ca) that yielded an individualized volumetric profile of brain tissue and volumetric imaging markers of SVD. Baseline and follow-up volumes for gray matter (GM), white matter (WM), sulcal cerebrospinal fluid (sCSF), and ventricular CSF (vCSF) were obtained using a local histogram-based automatic segmentation tool (Kovacevic et al., 2002). Baseline and follow-up volumes for SVD markers, such as WMH and cystic fluid-filled lacunar-like infarcts or “black holes” (BH), were obtained using a semi-automatic tri-feature (T1/PD/T2) segmentation algorithm called Lesion Explorer (Ramirez et al., 2011, 2014). Baseline head size volumes were measured by the total intracranial capacity (TIC), which included all supratentorial brain matter and CSF voxels. Baseline global brain atrophy was assessed by the Brain Parenchymal Fraction (BPF) which was calculated on an individual basis as follows: BPF = (GM + WM + WMH)/TIC.
WMH Progression Volumetrics
Volumetric data was generated using the conventional method by calculating the net change in total WMH volume from baseline to follow-up (total volume change = follow-up − baseline) for comparison with our dynamic progression method.
For our dynamic progression method, we used spatial location to gain more information about the potential dynamic nature of WMH. Baseline and follow-up T1 MRIs were transformed into each individual’s intermediate space using linear registration (FSL’s “FLIRT”, 6° of freedom; Jenkinson et al., 2012). This was accomplished by registering each baseline T1 image to follow-up using “FLIRT” and then using the “avscale” command to generate forward and backward halfway transformations. This half transformation was then applied to each baseline and follow-up image to create an intermediate image for both time points, which was then averaged to create one image that represented both baseline and follow-up in an intermediate space.
Once the intermediate T1 was generated, we registered WMH and vCSF segmentations for baseline and follow-up to each individual’s intermediate space. We then used “fslmaths” to create spatial difference/overlap masks between baseline and follow-up. Spatial change in WMH included: shrinking (WMH present in baseline but not in follow-up), growing (WMH not present in baseline but present in follow-up), as well as stable WMH (present in both baseline and follow-up; Figure 1). Additionally, WMH voxels that fell within the ventricles were masked out to account for ventricular expansion into pre-existing WMH voxels (Figure 2).
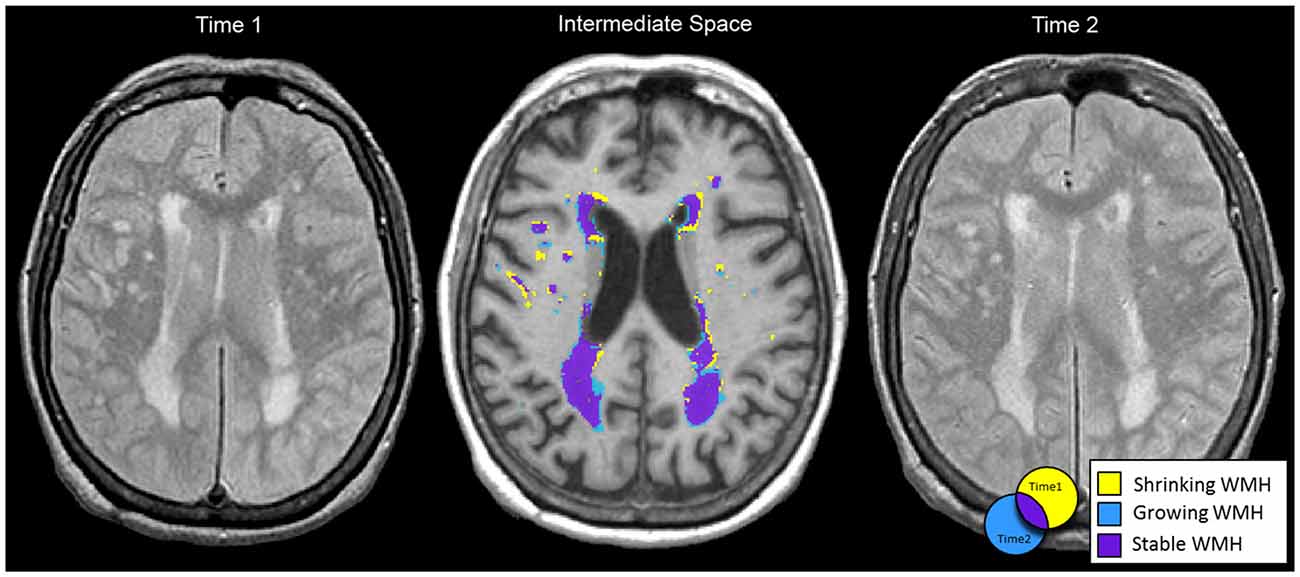
Figure 1. Baseline (left) and follow-up (right) MRIs were half transformed into intermediate space (middle) using linear registration (FLIRT). Shrinking (yellow), growing (blue) and stable (purple) white matter hyperintensities (WMH) spatial segmentations are displayed in intermediate space for a 71 year old female with Alzheimer’s disease (AD). Net change in WMH total volume from baseline to follow-up was −1.5 cc. However, when considering dynamic progression based on spatial information: shrinking WMH = −8.2 cc, growing WMH = +6.3 cc, and stable WMH = 32.4 cc.
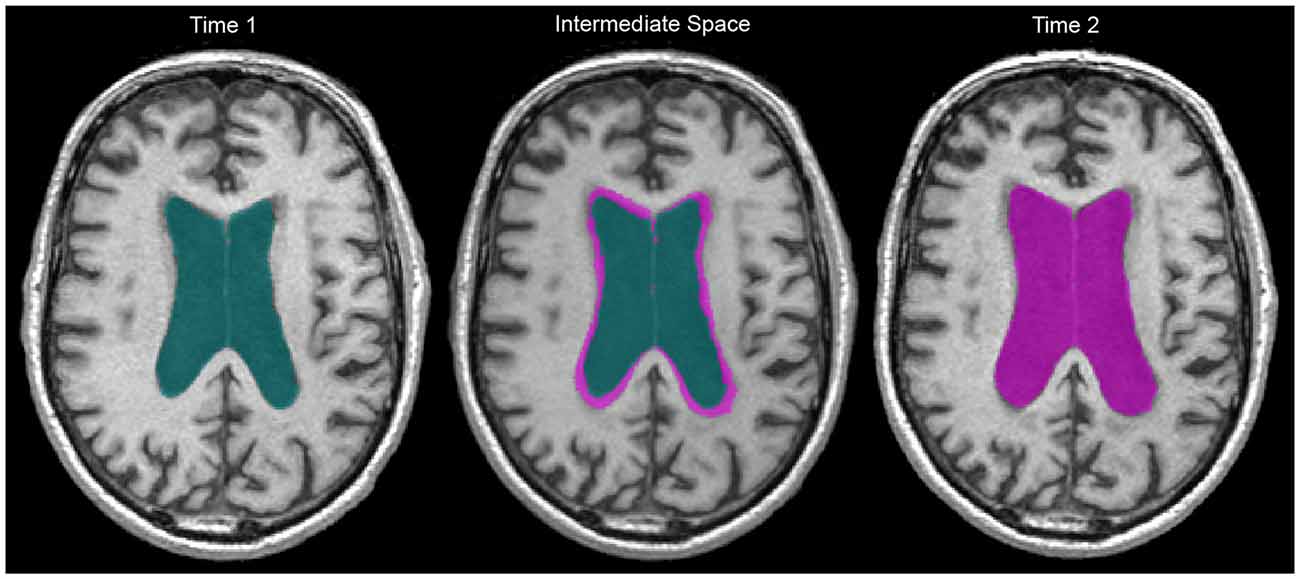
Figure 2. Two year ventricular expansion in a 60 year old man living with AD. Baseline vCSF = 83.6 cc, follow-up vCSF = 119.0 cc. Green indicates baseline vCSF voxels, pink indicates follow-up (right) and growth (middle). WMH within vCSF growth regions were subsequently removed to account for ventricular expansion.
Statistical Analysis
Statistical analyses were performed on a PC using IBM SPSS Statistics (version 22). AD patients were sub-divided into AD low SVD (n = 56) and AD high SVD (n = 57) groups based on the median split of WMH at baseline (Behl et al., 2007; McNeely et al., 2015). Multivariate analysis of covariance (MANCOVA) were performed to examine potential differences in basic demographics and baseline characteristics (age, sex, education, ISI, Mini Mental State Exam (MMSE), TIC, vCSF difference and BH difference), secondary variables of interest (BPF, GM difference and WM difference), and the primary between group analyses for dynamic progression of WMH (i.e., shrink, grow, stable, and total volume). Bonferroni corrected pairwise comparisons were conducted post hoc. Due to the commonly observed skewed distribution of SVD imaging markers (DeCarli et al., 2005; Barnes et al., 2013), WMH change volumes were log transformed to allow for parametric testing. Baseline TIC was used to account for variations in head size, baseline vCSF and BPF were included to account for brain atrophy, and baseline MMSE (Folstein et al., 1975) scores were included to account for disease severity.
Results
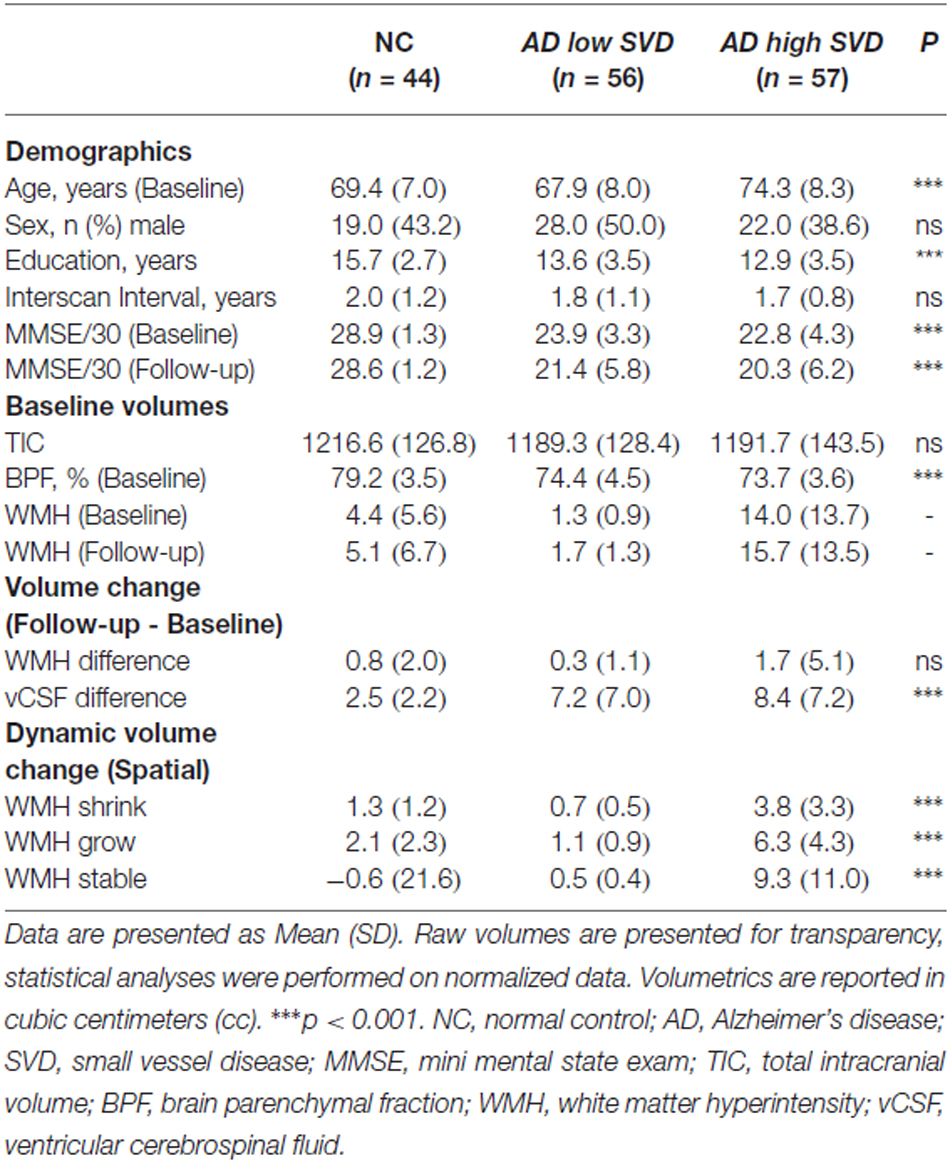
Demographic and volumetric data are summarized on Table 1. Age (p < 0.001), education (p < 0.001), and MMSE at baseline and follow-up (p < 0.001) were found to be significantly different between groups. Head-size (TIC), sex, and ISI were not significantly different between groups and were subsequently removed as covariates in further analyses. All subsequent analyses were adjusted for baseline age, education, disease severity, atrophy, and incidental infarcts. Significant between group differences were demonstrated in baseline BPF measures (p < 0.001), GM change (p < 0.001), WM change (p < 0.001), and vCSF change (p < 0.001). Interestingly, the conventional method yielded baseline to follow-up total net WMH volume changes that were not significantly different between groups (Table 1: “Volume change”, Figure 3: “Δ Total Volume”).
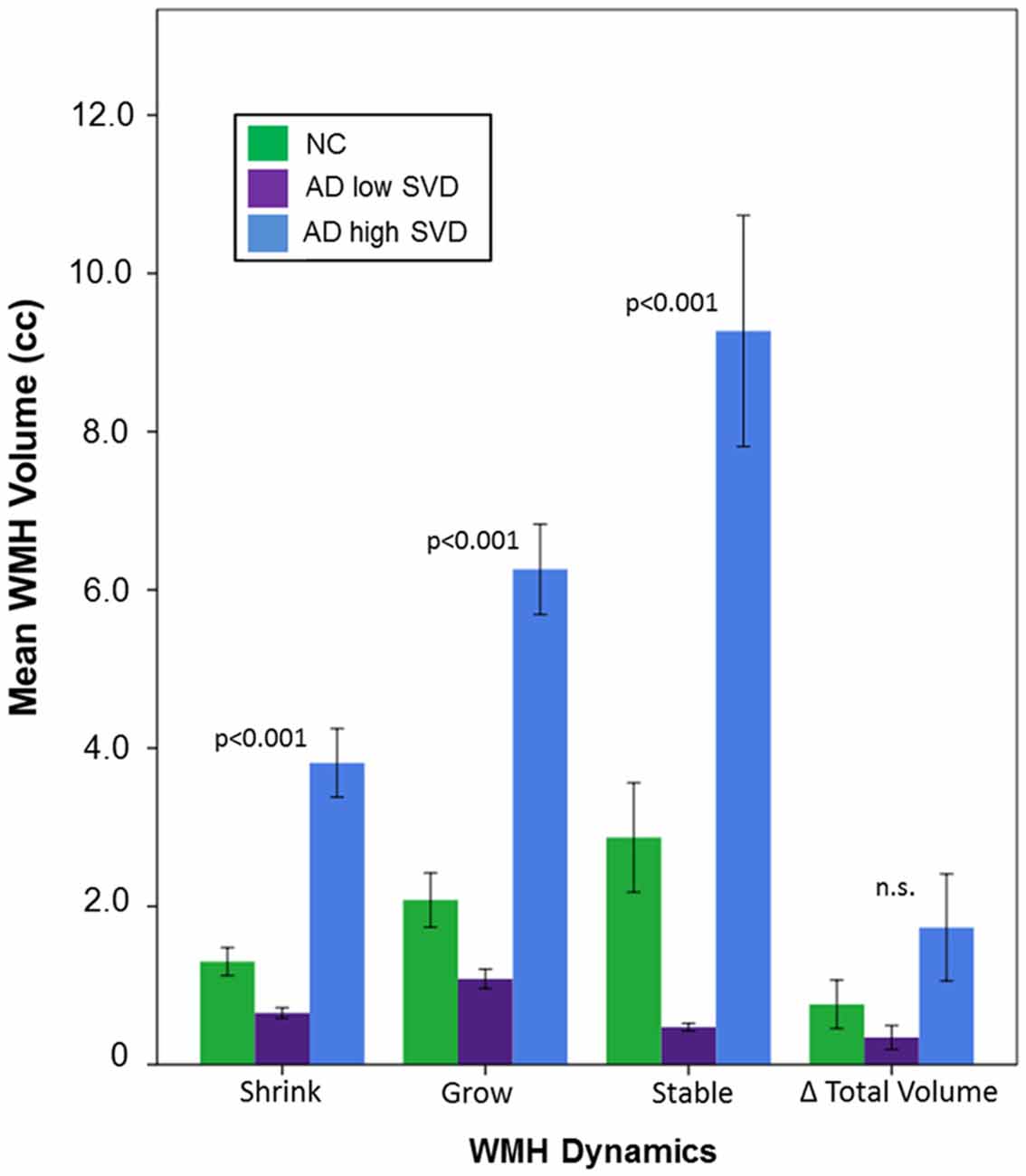
Figure 3. Dynamic changes in WMH volume over a mean interscan interval (ISI) of 2 years for NC, AD low small vessel disease (SVD), and AD high SVD groups. P values represent significant differences between all diagnostic groups, based on estimated marginal means, accounting for baseline age, years of education, mini mental state exam (MMSE), and vCSF.
However, significant between group differences were demonstrated using dynamic progression of WMH metrics: WMH shrinkage (p < 0.001), WMH growth (p < 0.001), and stable WMH (p < 0.001). Bonferroni corrected post hoc tests revealed that dynamic progression of WMH was significantly different between all groups, where the AD high SVD group had significantly more shrinkage, growth, and stable WMH volumes than NC (all, p < 0.001) and AD low SVD groups (all, p < 0.001; Figure 3).
Discussion
Our main findings suggest that WMH progression may be more dynamic than previously thought. Specifically, AD patients with significant radiological signs of cerebral SVD pathology at baseline exhibited more dynamic progression of WMH over a 2 year period, when compared to AD patients with minimal SVD burden and their NC counterparts.
Progression rates of WMH volumes from serial MRI studies vary considerably, however, the overall net volume change results in our study were within the range of values previously reported in the literature (see Table 2; Garde et al., 2005; Schmidt et al., 2005; Kramer et al., 2007; Silbert et al., 2008; Marquine et al., 2010; Wolfson et al., 2013). Interestingly, the largest net volume increase of WMH in our study was observed in AD patients with high SVD burden at baseline, which likely reflects previous reports that demonstrate how baseline WMH burden can significantly predict higher progression rates (Prins and Scheltens, 2015).
Despite these reports of overall net increases of WMH volume over time, some studies have demonstrated differential progression rates spatially across various brain regions, and even regression or decreases, providing early support for the potential dynamic nature of WMH. When the spatial distribution of WMH have been considered, differential progression rates have been shown in the periventricular compared to deep white brain regions (van den Heuvel et al., 2004; Sabayan et al., 2015). Additionally, significant WMH regression and signal attenuation has also been previously demonstrated in radiological case reports with pre- and post-therapeutic intervention imaging (Mínguez et al., 2007; Rovira et al., 2007; Durand-Birchenall et al., 2012).
Moreover, a recent serial MRI study performed on ischemic stroke patients demonstrated both progression and regression, with WMH regression observed in 21.5% of stroke patients (Cho et al., 2015). These findings are particularly important in individual cases where there are signs of simultaneous progression (growing), regression (shrinking), and stable WMH, as demonstrated in our study. The loss of information from baseline to follow-up from volumetric averaging can further be reflected in our group analysis, where the conventional WMH net volume change between groups was non-significant but dynamic progression rates were.
In light of the commonly accepted view that the pathogenesis and clinical characteristics of “WMH of presumed vascular origin” can be attributed to a set of heterogeneous and complex etiologies associated with cerebral SVD (Gouw et al., 2011), our findings provide further support for the dynamic nature of the underlying pathology that is reflected by these radiological entities. Although poorly understood, there is some evidence to suggest that a common substrate of diffuse WMH in the periventricular regions of the brain may reflect a form of venous stenosis (Moody et al., 1995; Black et al., 2009; Pantoni, 2010). Early findings from imaging-pathology case studies suggest that the confluent periventricular WMH may reflect edema related to collagenosis of the periventricular deep medullary veins (Gao et al., 2014).
Fluid from chronic edema resulting from increased blood-brain barrier permeability (Farrall and Wardlaw, 2009), intraparenchymal venous abnormalities (Brown et al., 2009), and/or disturbances in interstitial fluid circulation (Weller et al., 2009), may also explain the dynamic shrinkage and growth of WMH that was demonstrated in our study, as edematous fluid has been known to partially resolve after time has elapsed (or therapeutic intervention is performed). Thus, edema visualized as WMH on MRI may be an indicator of complex multiple underlying pathologies that include venous collagenosis and/or arteriolosclerosis. These chronic SVD pathologies may result in increased blood-brain barrier leakage and interstitial fluid accumulation due to increased venous pressure and decreased fluid drainage along the perivascular channels as a consequence of vascular rigidity and reduced vascular pulsation. These proposed multi-factorial etiologies make it challenging to attribute regression to “recent” vs. “older” lesions. However, collagenosis and/or arteriolosclerosis are insidious chronic processes, which could gradually lead to increased fluid accumulation, but also allows time for fluid resolution, where WMH regression may reflect the rate of resolution (clearance or drainage of fluid) when it is temporarily greater than the rate of fluid accumulation. Moreover, this dynamic process of fluid accumulation and clearance may also be modulated by other factors, such as blood pressure (Hauser et al., 1988), during image acquisition.
Additionally, there is some evidence to suggest that a proportion of focal infarcts may resemble and/or distort WMH volumes due to their similarity in signal intensity (Wang et al., 2012). As incomplete and partially cavitated lacunar infarcts and acute small subcortical strokes tend to resemble WMH, when measured longitudinally, many of these lesions (94%) have been shown to completely or incompletely cavitate, reduce in diameter, and/or disappear (5%) on follow-up imaging (Lammie et al., 1998; Potter et al., 2010; Loos et al., 2012). This process may also partially account for the dynamic progression findings in our study.
One limitation of our study is the lack of additional time points for neuroimaging measures. As the Sunnybrook Dementia Study is a longitudinal observational study, additional follow-up scans for each individual will become available for future analysis to provide a more accurate picture regarding the dynamic changes of WMH in a real-world sample of dementia patients with varying degrees of comorbid SVD pathology (Ramirez et al., 2015). Additionally, as both a benefit and a caveat, the ability of our method to spatially account for vCSF expansion is particularly relevant in studies on aging and dementia since ventricle size is an important indicator of brain atrophy, commonly observed in AD-related neurodegeneration and normal aging (Nestor et al., 2008; Apostolova et al., 2012; Madsen et al., 2015). Unfortunately, while this dynamic feature in our method accounts for ventricular expansion, it may also introduce potential error, as baseline WMH voxels which overlay onto newly labeled vCSF voxels are removed due to ventricular expansion at follow-up. Should the baseline volumes of periventricular WMH that eventually become engulfed into the ventricular compartment be quantified as a separate unique volume? To our knowledge, previous studies have not considered this, although many studies (including this one) include ventricular volume as a covariate to account for medial atrophy.
Future work examining dynamic progression with spatial information from both WMH and ventricular expansion would be beneficial when examining vascular co-pathology in AD and neurodegenerative populations. Future therapeutic strategies that target cerebrovascular contributions to AD dementia, such as the use of anti-hypertensive treatments (Dufouil et al., 2009; Jochemsen et al., 2012, 2015; White et al., 2013), may be particularly interested in the shrinkage, growth, and stable metrics examined in our study. Although several complex statistical modeling approaches have been proposed to analyze longitudinal structural data (McArdle et al., 2004; Grimm et al., 2012), our approach is relatively simple by comparison and can be implemented into any serial imaging study in parallel with the conventional baseline to follow-up net volume change approaches.
Conclusion
The findings from this study suggest that individual WMH volume changes are more dynamic than previously thought. The simultaneous shrinkage and growth of WMH was particularly evident in AD patients with significant signs of cerebral SVD. Given that our dynamic progression measures provided information that was above and beyond the standard net volume change, our results suggest that the measurement of dynamic volumetrics for WMH may be particularly valuable in clinical trials that use imaging outcome measures and may further elucidate the complex pathogenesis and longitudinal progression of this radiological entity we commonly refer to as WMH of presumed vascular origin.
Author Contributions
JR: literature review, manuscript writing, statistical analysis, project development. AAM: neuroimaging analysis, literature review, manuscript writing. CB: neuroimaging analysis, manuscript editing, statistical analysis. FG: project development, radiological consultation, manuscript editing. SEB: patient assessment, project development, principal investigator, manuscript writing.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors gratefully acknowledge financial support from the Canadian Institutes of Health Research (MOP#13129), the Alzheimer Society of Canada and Alzheimer Association (US), the Heart and Stroke Foundation Canadian Partnership for Stroke Recovery (HSFCPSR), Hurvitz Brain Sciences Research program at Sunnybrook Research Institute, and the Linda C. Campbell Foundation. JR received partial funding from the Canadian Vascular Network. JR, AAM, CB, FG received salary support from HSFCPSR and SEB from the Brill Chair in the Department of Medicine (Neurology), Sunnybrook Health Sciences Centre and University of Toronto.
References
Apostolova, L. G., Green, A. E., Babakchanian, S., Hwang, K. S., Chou, Y. Y., Toga, A. W., et al. (2012). Hippocampal atrophy and ventricular enlargement in normal aging, mild cognitive impairment (MCI) and Alzheimer Disease. Alzheimer Dis. Assoc. Disord. 26, 17–27. doi: 10.1097/WAD.0b013e3182163b62
Barnes, J., Carmichael, O. T., Leung, K. K., Schwarz, C., Ridgway, G. R., Bartlett, J. W., et al. (2013). Vascular and Alzheimer’s disease markers independently predict brain atrophy rate in Alzheimer’s Disease Neuroimaging Initiative controls. Neurobiol. Aging 34, 1996–2002. doi: 10.1016/j.neurobiolaging.2013.02.003
Behl, P., Bocti, C., Swartz, R. H., Gao, F., Sahlas, D. J., Lanctot, K. L., et al. (2007). Strategic subcortical hyperintensities in cholinergic pathways and executive function decline in treated Alzheimer patients. Arch. Neurol. 64, 266–272. doi: 10.1001/archneur.64.2.266
Black, S. E., Gao, F. Q., and Bilbao, J. (2009). Understanding white matter disease: imaging-pathological correlations in vascular cognitive impairment. Stroke 40, S48–S52. doi: 10.1161/STROKEAHA.108.537704
Brown, W. R., Moody, D. M., Thore, C. R., Anstrom, J. A., and Challa, V. R. (2009). Microvascular changes in the white mater in dementia. J. Neurol. Sci. 283, 28–31. doi: 10.1016/j.jns.2009.02.328
Cho, A., Kim, H., Kim, W., and Yang, W. (2015). White matter hyperintensity in ischemic stroke patients: it may regress over time. J. Stroke 17, 60–66. doi: 10.5853/jos.2015.17.1.60
Debette, S., Seshadri, S., Beiser, A., Au, R., Himali, J. J., Palumbo, C., et al. (2011). Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology 77, 461–468. doi: 10.1212/WNL.0b013e318227b227
DeCarli, C., Massaro, J., Harvey, D., Hald, J., Tullberg, M., Au, R., et al. (2005). Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiol. Aging 26, 491–510. doi: 10.1016/j.neurobiolaging.2004.05.004
Dufouil, C., Chalmers, J., Coskun, O., Besançon, V., Bousser, M. G., Guillon, P., et al. (2005). Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging Substudy. Circulation 112, 1644–1650. doi: 10.1161/circulationaha.104.501163
Dufouil, C., Godin, O., Chalmers, J., Coskun, O., MacMahon, S., Tzourio-Mazoyer, N., et al. (2009). Severe cerebral white matter hyperintensities predict severe cognitive decline in patients with cerebrovascular disease history. Stroke 40, 2219–2221. doi: 10.1161/STROKEAHA.108.540633
Durand-Birchenall, J., Leclercq, C., Daouk, J., Monet, P., Godefroy, O., and Bugnicourt, J. M. (2012). Attenuation of brain white matter lesions after lacunar stroke. Int. J. Prev. Med. 3, 134–138.
Erten-Lyons, D., Woltjer, R., Kaye, J., Mattek, N., Dodge, H. H., Green, S., et al. (2013). Neuropathologic basis of white matter hyperintensity accumulation with advanced age. Neurology 81, 977–983. doi: 10.1212/WNL.0b013e3182a43e45
Farrall, A. J., and Wardlaw, J. M. (2009). Blood-brain barrier: ageing and microvascular disease–systematic review and meta-analysis. Neurobiol. Aging 30, 337–352. doi: 10.1016/j.neurobiolaging.2007.07.015
Folstein, M. F., Folstein, S. E., and McHugh, P. R. (1975). “Mini Mental State” A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198. doi: 10.1016/0022-3956(75)90026-6
Gao, F., Keith, J., Noor, R., Kiss, A., and Black, S. (2014). Collagenosis of the deep medullary veins correlates with periventricular white matter changes in Alzheimer’s disease. Alzheimers Dement. 10:P424. doi: 10.1016/j.jalz.2014.05.549
Garde, E., Lykke Mortensen, E., Rostrup, E., and Paulson, O. B. (2005). Decline in intelligence is associated with progression in white matter hyperintensity volume. J. Neurol. Neurosurg. Psychiatry 76, 1289–1291. doi: 10.1136/jnnp.2004.055905
Gouw, A. A., Seewann, A., van der Flier, W. M., Barkhof, F., Rozemuller, A. M., Scheltens, P., et al. (2011). Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J. Neurol. Neurosurg. Psychiatry 82, 126–135. doi: 10.1136/jnnp.2009.204685
Grimm, K. J., An, Y., McArdle, J. J., Zonderman, A. B., and Resnick, S. M. (2012). Recent changes leading to subsequent changes: extensions of multivariate latent difference score models. Struct. Equ. Modeling 19, 268–292. doi: 10.1080/10705511.2012.659627
Hauser, R. A., Lacey, D. M., and Knight, M. R. (1988). Hypertensive encephalopathy. Magnetic resonance imaging demonstration of reversible cortical and white matter lesions. Arch. Neurol. 45, 1078–1083. doi: 10.1001/archneur.1988.00520340032007
Jenkinson, M., Beckmann, C. F., Behrens, T. E. J., Woolrich, M. W., and Smith, S. M. (2012). FSL. Neuroimage 62, 782–790. doi: 10.1016/j.neuroimage.2011.09.015
Jochemsen, H. M., Geerlings, M. I., Grool, A. M., Vincken, K. L., Mali, W. P., van der Graaf, Y., et al. (2012). Angiotensin-converting enzyme and progression of white matter lesions and brain atrophy–the SMART-MR study. J. Alzheimers Dis. 29, 39–49. doi: 10.3233/JAD-2012-111772
Jochemsen, H. M., van der Flier, W. M., Ashby, E. L., Teunissen, C. E., Jones, R. E., Wattjes, M. P., et al. (2015). Angiotensin-converting enzyme in cerebrospinal fluid and risk of brain atrophy. J. Alzheimers Dis. 44, 153–162. doi: 10.3233/JAD-131496
Kloppenborg, R. P., Nederkoorn, P. J., Geerlings, M. I., and van den Berg, E. (2014). Presence and progression of white matter hyperintensities and cognition: a meta-analysis. Neurology 82, 2127–2138. doi: 10.1212/WNL.0000000000000505
Kovacevic, N., Lobaugh, N. J., Bronskill, M. J., Levine, B., Feinstein, A., and Black, S. E. (2002). A robust method for extraction and automatic segmentation of brain images. Neuroimage 17, 1087–1100. doi: 10.1006/nimg.2002.1221
Kramer, J. H., Mungas, D., Reed, B. R., Wetzel, M. E., Burnett, M. M., Miller, B. L., et al. (2007). Longitudinal MRI and cognitive change in healthy elderly. Neuropsychology 21, 412–418. doi: 10.1037/0894-4105.21.4.412
Lammie, G. A., Brannan, F., and Wardlaw, J. M. (1998). Incomplete lacunar infarction (Type Ib lacunes). Acta Neuropathol. 96, 163–171. doi: 10.1007/s004010050877
Longstreth, W. T., Jr., Arnold, A. M., Beauchamp, N. J., Jr., Manolio, T. A., Lefkowitz, D., Jungreis, C., et al. (2005). Incidence, manifestations and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the cardiovascular health study. Stroke 36, 56–61. doi: 10.1161/01.str.0000149625.99732.69
Loos, C. M. J., Staals, J., Wardlaw, J. M., and van Oostenbrugge, R. J. (2012). Cavitation of deep lacunar infarcts in patients with first-ever lacunar stroke: a 2-year follow-up study with MR. Stroke 43, 2245–2247. doi: 10.1161/STROKEAHA.112.660076
Madsen, S. K., Gutman, B. A., Joshi, S. H., Toga, A. W., Jack, C. R. Jr., Weiner, M. W., et al. (2015). Mapping ventricular expansion onto cortical gray matter in older adults. Neurobiol. Aging 36, S32–S41. doi: 10.1016/j.neurobiolaging.2014.03.044
Marquine, M. J., Attix, D. K., Goldstein, L. B., Samsa, G. P., Payne, M. E., Chelune, G. J., et al. (2010). Differential patterns of cognitive decline in anterior and posterior white matter hyperintensity progression. Stroke 41, 1946–1950. doi: 10.1161/STROKEAHA.110.587717
McArdle, J. J., Hamgami, F., Jones, K., Jolesz, F., Kikinis, R., Spiro, A., et al. (2004). Structural modeling of dynamic changes in memory and brain structure using longitudinal data from the normative aging study. J. Gerontol. B Psychol. Sci. Soc. Sci. 59, P294–P304. doi: 10.1093/geronb/59.6.p294
McKhann, G. M., Knopman, D. S., Chertkow, H., Hyman, B. T., Jack, C. R., Jr., Kawas, C. H., et al. (2011). The diagnosis of dementia due to Alzheimer’s disease: recommendations from the national institute on aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 263–269. doi: 10.1016/j.jalz.2011.03.005
McNeely, A. A., Ramirez, J., Nestor, S. M., Zhao, J., Gao, F., Kiss, A., et al. (2015). Cholinergic subcortical hyperintensities in Alzheimer’s disease patients from the sunnybrook dementia study: relationships with cognitive dysfunction and hippocampal atrophy. J. Alzheimers Dis. 43, 785–796. doi: 10.3233/JAD-140588
Mínguez, B., Rovira, A., Alonso, J., and Córdoba, J. (2007). Decrease in the volume of white matter lesions with improvement of hepatic encephalopathy. AJNR Am. J. Neuroradiol. 28, 1499–1500. doi: 10.3174/ajnr.a0611
Mok, V. C., Lam, W. W., Fan, Y. H., Wong, A., Ng, P. W., Tsoi, T. H., et al. (2009). Effects of statins on the progression of cerebral white matter lesion: post hoc analysis of the rocas (regression of cerebral artery stenosis) study. J. Neurol. 256, 750–757. doi: 10.1007/s00415-009-5008-7
Moody, D. M., Brown, W. R., Challa, V. R., and Anderson, R. L. (1995). Periventricular venous collagenosis: association with leukoaraiosis. Radiology 194, 469–476. doi: 10.1148/radiology.194.2.7824728
Moriya, Y., Kozaki, K., Nagai, K., and Toba, K. (2009). Attenuation of brain white matter hyperintensities after cerebral infarction. Am. J. Neuroradiol. 30:E43. doi: 10.3174/ajnr.A1340
Nestor, S. M., Rupsingh, R., Borrie, M., Smith, M., Accomazzi, V., Wells, J. L., et al. (2008). Ventricular enlargement as a possible measure of Alzheimer’s disease progression validated using the Alzheimer’s disease neuroimaging initiative database. Brain 131, 2443–2454. doi: 10.1093/brain/awn146
Pantoni, L. (2010). Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 9, 689–701. doi: 10.1016/s1474-4422(10)70104-6
Potter, G. M., Doubal, F. N., Jackson, C. A., Chappell, F. M., Sudlow, C. L., Dennis, M. S., et al. (2010). Counting cavitating lacunes underestimates the burden of lacunar infarction. Stroke 41, 267–272. doi: 10.1161/STROKEAHA.109.566307
Prins, N. D., and Scheltens, P. (2015). White matter hyperintensities, cognitive impairment and dementia: an update. Nat. Rev. Neurol. 11, 157–165. doi: 10.1038/nrneurol.2015.10
Ramirez, J., Gibson, E., Quddus, A., Lobaugh, N. J., Feinstein, A., Levine, B., et al. (2011). Lesion explorer: a comprehensive segmentation and parcellation package to obtain regional volumetrics for subcortical hyperintensities and intracranial tissue. Neuroimage 54, 963–973. doi: 10.1016/j.neuroimage.2010.09.013
Ramirez, J., McNeely, A. A., Scott, C. J. M., Masellis, M., Black, S. E., and Alzheimer’s Disease Neuroimaging Initiative. (2015). White matter hyperintensity burden in elderly cohort studies: the sunnybrook dementia study, Alzheimer disease neuroimaging initiative, and three-city study. Alzheimers Dement. 12, 203–210. doi: 10.1016/j.jalz.2015.06.1886
Ramirez, J., Scott, C., McNeely, A., Berezuk, C., Gao, F., Szilagyi, G., et al. (2014). Lesion explorer: a video-guided, standardized protocol for accurate and reliable MRI-derived Volumetrics in Alzheimer’s disease and normal elderly. J. Vis. Exp. 86:e50887. doi: 10.3791/50887
Rovira, A., Mínguez, B., Aymerich, F. X., Jacas, C., Huerga, E., Córdoba, J., et al. (2007). Decreased white matter lesion volume and improved cognitive function after liver transplantation. Hepatology 46, 1485–1490. doi: 10.1002/hep.21911
Sabayan, B., van der Grond, J., Westendorp, R. G., van Buchem, M. A., and de Craen, A. J. M. (2015). Accelerated progression of white matter hyperintensities and subsequent risk of mortality: a 12-year follow-up study. Neurobiol. Aging 36, 2130–2135. doi: 10.1016/j.neurobiolaging.2015.03.003
Sachdev, P., Wen, W., Chen, X., and Brodaty, H. (2007). Progression of white matter hyperintensities in elderly individuals over 3 years. Neurology 68, 214–222. doi: 10.1212/01.wnl.0000251302.55202.73
Schmidt, R., Enzinger, C., Ropele, S., Schmidt, H., and Fazekas, F. (2003). Progression of cerebral white matter lesions: 6-year results of the Austrian Stroke Prevention Study. Lancet 361, 2046–2048. doi: 10.1016/s0140-6736(03)13616-1
Schmidt, R., Ropele, S., Enzinger, C., Petrovic, K., Smith, S., Schmidt, H., et al. (2005). White matter lesion progression, brain atrophy and cognitive decline: the Austrian stroke prevention study. Ann. Neurol. 58, 610–616. doi: 10.1002/ana.20630
Silbert, L. C., Nelson, C., Howieson, D. B., Moore, M. M., and Kaye, J. A. (2008). Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology 71, 108–113. doi: 10.1212/01.wnl.0000316799.86917.37
Taylor, W. D., MacFall, J. R., Provenzale, J. M., Payne, M. E., McQuoid, D. R., Steffens, D. C., et al. (2003). Serial MR imaging of volumes of in elderly patients: correlation with vascular risk factors. Am. J. Roentgenol. 181, 571–576. doi: 10.2214/ajr.181.2.1810571
van den Heuvel, D. M., Admiraal-Behloul, F., ten Dam, V. H., Olofsen, H., Bollen, E. L., Murray, H. M., et al. (2004). Different progression rates for deep white matter hyperintensities in elderly men and women. Neurology 63, 1699–1701. doi: 10.1212/01.wnl.0000143058.40388.44
Vannorsdall, T. D., Waldstein, S. R., Kraut, M., Pearlson, G. D., and Schretlen, D. J. (2009). White matter abnormalities and cognition in a community sample. Arch. Clin. Neuropsychol. 24, 209–217. doi: 10.1093/arclin/acp037
Wang, X., Valdés Hernández, M. C., Doubal, F., Chappell, F. M., and Wardlaw, J. M. (2012). How much do focal infarcts distort white matter lesions and global cerebral atrophy measures? Cerebrovasc. Dis. 34, 336–342. doi: 10.1159/000343226
Wardlaw, J. M., Smith, E. E., Biessels, G. J., Cordonnier, C., Fazekas, F., Frayne, R., et al. (2013). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 12, 822–838. doi: 10.1016/s1474-4422(13)70124-8
Weller, R. O., Djuanda, E., Yow, H.-Y., and Carare, R. O. (2009). Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 117, 1–14. doi: 10.1007/s00401-008-0457-0
White, W. B., Marfatia, R., Schmidt, J., Wakefield, D. B., Kaplan, R. F., Bohannon, R. W., et al. (2013). Intensive versus standard ambulatory blood pressure lowering to prevent functional decline in the elderly (INFINITY). Am. Heart J. 165, 258–265. doi: 10.1016/j.ahj.2012.11.008
Wolfson, L., Wakefield, D. B., Moscufo, N., Kaplan, R. F., Hall, C. B., Schmidt, J. A., et al. (2013). Rapid buildup of brain white matter hyperintensities over 4 years linked to ambulatory blood pressure, mobility, cognition and depression in old persons. J. Gerontol. A Biol. Sci. Med. Sci. 68, 1387–1394. doi: 10.1093/gerona/glt072
Keywords: Alzheimer’s disease, white matter hyperintensities, small vessel disease, aging, dementia, longitudinal progression
Citation: Ramirez J, McNeely AA, Berezuk C, Gao F and Black SE (2016) Dynamic Progression of White Matter Hyperintensities in Alzheimer’s Disease and Normal Aging: Results from the Sunnybrook Dementia Study. Front. Aging Neurosci. 8:62. doi: 10.3389/fnagi.2016.00062
Received: 14 January 2016; Accepted: 10 March 2016;
Published: 24 March 2016.
Edited by:
Ying Xu, The State University of New York at Buffalo, USAReviewed by:
Bogdan O. Popescu, Colentina Clinical Hospital, RomaniaRamesh Kandimalla, Emory University, USA
Copyright © 2016 Ramirez, McNeely, Berezuk, Gao and Black. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joel Ramirez, joelr@sri.utoronto.ca
† These authors have contributed equally to this work.
 Joel Ramirez
Joel Ramirez Alicia A. McNeely1,2†
Alicia A. McNeely1,2†  Courtney Berezuk
Courtney Berezuk Sandra E. Black
Sandra E. Black