Astrocyte Activation and the Calcineurin/NFAT Pathway in Cerebrovascular Disease
- 1Sanders-Brown Center on Aging, University of Kentucky College of Medicine, Lexington, KY, United States
- 2Department of Pharmacology and Nutritional Sciences, University of Kentucky College of Medicine, Lexington, KY, United States
Calcineurin (CN) is a Ca2+/calmodulin-dependent protein phosphatase with high abundance in nervous tissue. Though enriched in neurons, CN can become strongly induced in subsets of activated astrocytes under different pathological conditions where it interacts extensively with the nuclear factor of activated T cells (NFATs). Recent work has shown that regions of small vessel damage are associated with the upregulation of a proteolized, highly active form of CN in nearby astrocytes, suggesting a link between the CN/NFAT pathway and chronic cerebrovascular disease. In this Mini Review article, we discuss CN/NFAT signaling properties in the context of vascular disease and use previous cell type-specific intervention studies in Alzheimer’s disease and traumatic brain injury models as a framework to understand how astrocytic CN/NFATs may couple vascular pathology to neurodegeneration and cognitive loss.
Introduction
Cerebrovascular pathology is one of the leading causes of cognitive loss and mortality. While stroke is usually the most devastating form of cerebrovascular disease, other forms of vascular damage and dysfunction including microinfarcts, microhemorrhages, cerebral amyloid angiopathy and cerebral hypoperfusion are more insidious and can lead to chronic and progressive cognitive loss, especially in aged individuals. These vascular contributions to cognitive impairment and dementia (VCID) are the second leading cause of dementia, behind Alzheimer’s disease, and frequently co-exist with other neurodegenerative conditions (O’Brien et al., 2003). Importantly, VCID comorbidities appear to interfere with the treatment of Alzheimer’s disease-related functional deficits in animal models (Weekman et al., 2016), highlighting the need to understand the cellular mechanisms that link vascular dysfunction to neurodegeneration and impaired cognition (Snyder et al., 2015; Horsburgh et al., 2018).
Brain ischemia results when stroke or other forms of VCID block the blood supply to parts of the brain, resulting in depletion of oxygen and glucose. This depletion rapidly exhausts the energy production of neural cells and their ability to maintain the normal balance of ions across cellular membranes, thus causing excitotoxicity and Ca2+ overload, among other adverse effects (Choi, 1988; Horst and Postigo, 1996; Szydlowska and Tymianskia, 2010). Ca2+ overload originates from a variety of sources and directly affects numerous intracellular signaling cascades, many of which have been explored as potential treatment targets for stroke and other forms of cerebrovascular disease (Harris et al., 1982; Infeld et al., 1999; Ray, 2006; Mattson, 2007; Rostas et al., 2017; Wu and Tymianski, 2018). In most cases, Ca2+-signaling pathways have been investigated in neurons, which are the primary target of excitotoxic damage. In the following Mini Review article, we will discuss the importance of the Ca2+/calmodulin dependent protein phosphatase, calcineurin (CN) and its dysregulation in astrocytes as a pathological mechanism and potential target for neurodegeneration and cognitive loss due to cerebrovascular damage.
CN Dysregulation in Stroke Models
CN, or protein phosphatase 3, is the only phosphatase in mammals that is directly activated by Ca2+/calmodulin. CN consists of a catalytic subunit (PPP3CA) and a Ca2+ binding regulatory subunit (PPP3R1). When cellular Ca2+ levels are low, the phosphatase activity of CN is held in check by an autoinhibitory domain located near the C terminus of the catalytic subunit. The interaction of Ca2+ with the CN regulatory subunit and calmodulin leads to a physical interaction between the CN catalytic subunit and Ca2+/calmodulin, which, in turn, displaces the AID and frees the catalytic core from inhibition. When cellular Ca2+ levels fall, calmodulin is released from the catalytic subunit and AID-mediated inhibition of phosphatase activity is reinstated (Klee et al., 1998; Aramburu et al., 2000). In healthy nervous tissue, CN provides an essential mechanism for bidirectional synaptic plasticity through the induction and maintenance of activity-dependent synaptic depression (Mansuy, 2003). In this capacity, CN is widely thought to link Ca2+ signaling to several forms of learning and memory, including extinction learning (Baumgärtel et al., 2008; de la Fuente et al., 2011; Rivera-Olvera et al., 2018). However, due to its exquisite sensitivity to Ca2+, CN is also frequently identified as a central player in numerous deleterious or maladaptive processes arising from Ca2+ overload and/or dysregulation (Uchino et al., 2008; Mukherjee and Soto, 2011; Reese and Taglialatela, 2011; Furman and Norris, 2014; Sompol and Norris, 2018).
Large and/or sustained surges in Ca2+ can lead to calpain or caspase-mediated proteolytic disruption of the CN AID (Wang et al., 1989; Wu et al., 2004), which partially and irreversibly uncouples CN from Ca2+, resulting in constitutive phosphatase activity. Several acute and chronic neurodegenerative conditions are associated with the generation of high activity CN proteolytic fragments (ΔCN), thus perpetuating de-phosphorylation of the myriad of CN targets (Norris, 2014). Hypoxic/ischemic insults appear to be particularly effective at triggering the proteolysis of CN from its full length highly-regulated form (60 kDa), to high activity fragments (ΔCN) ranging in size from 45 to 57 kDa (Shioda et al., 2006, 2007; Rosenkranz et al., 2012). Conversely, blockade of CN typically provides considerable neuroprotection during ischemia and other adverse consequences of cerebrovascular damage. For instance, the CN inhibiting immunosuppressant drug, tacrolimus (or FK506), has been shown to reduce infarct size (Sharkey and Butcher, 1994; Butcher et al., 1997), suppress neuroinflammation (Zawadzka and Kaminska, 2005) and promote recovery of function (Sharkey et al., 1996) in middle cerebral artery occlusion models of ischemic stroke. More recently, a CN modulatory protein, known as regulator of CN (RCAN), was found to favorably affect the pathogenesis of stroke in vivo and hypoxia in vitro using both gene overexpression and knockout approaches (Brait et al., 2012; Sobrado et al., 2012). Together, these results suggest that CN proteolysis (hyperactivation) is not only a biomarker, but also an important mediator, of neurodegeneration resulting from vascular damage.
NFATs
The exact mechanisms through which CN acts are complex and multifaceted. CN has a broad and diverse range of substrates, many of which have been implicated as downstream targets in CN-mediated cellular dysfunction and neurotoxicity (Uchino et al., 2008; Mukherjee and Soto, 2011; Reese and Taglialatela, 2011; Furman and Norris, 2014). Perhaps the best characterized substrate of CN is the nuclear factor of activated T cells (NFATs), a transcription factor related to NFκB/Rel-family proteins (Rao et al., 1997). There are four CN-dependent NFAT family members (NFATs 1–4), all of which are expressed in nervous tissue (Nguyen and Di Giovanni, 2008; Vihma et al., 2008). NFATs reside in the cytosol in their resting state, but upon de-phosphorylation by CN, they translocate to the nucleus where they can activate or suppress numerous gene expression programs linked to immune/inflammatory signaling, Ca2+ regulation, and cell survival, among other things (Im and Rao, 2004). NFAT isoforms have different cellular distributions inside and outside of the nervous system (Horsley and Pavlath, 2002; Abdul et al., 2010) and appear to engage in both overlapping and distinct transcriptional programs through interactions with multiple other transcription factor families (Rao et al., 1997; Im and Rao, 2004; Wu et al., 2006). Of the four isoforms, NFATs 1 and 4 seem to show a greater bias for glial cells where they respond to many different kinds of inflammatory factors and other noxious stimuli, including blood derived factors (Canellada et al., 2008; Sama et al., 2008; Abdul et al., 2009; Nagamoto-Combs and Combs, 2010; Serrano-Pérez et al., 2011; Neria et al., 2013; Furman et al., 2016; Manocha et al., 2017; Sompol et al., 2017).
Hyperactive Astrocytic CN/NFAT Signaling: Biomarker for Vascular Damage?
Astrocytic CN/NFAT signaling may provide, and give rise to, useful biomarkers for cerebrovascular damage. One of the most striking changes in CN/NFAT expression following CNS injury and disease is strong and selective expression in subsets of activated astrocytes (Hashimoto et al., 1998; Norris et al., 2005; Celsi et al., 2007; Serrano-Pérez et al., 2011; Lim et al., 2013; Neria et al., 2013; Furman et al., 2016; Pleiss et al., 2016; Sompol et al., 2017). For instance, the NFAT4 isoform, which is weakly expressed in healthy nervous tissue, appears at elevated levels in many activated astrocytes following kainic acid lesions, cortical stab wounds and controlled cortical contusion injuries (Serrano-Pérez et al., 2011; Neria et al., 2013; Furman et al., 2016). NFAT4 expression in a mouse model of Alzheimer’s disease also exhibited extensive co-localization with activated astrocytes, increasing directly in proportion to the expression of GFAP (Sompol et al., 2017). Using a custom antibody to CN, based on calpain-dependent cleavage sites, our lab recently observed intense labeling of a 45–48 kDa ΔCN fragment in activated astrocytes surrounding microinfarcts in human neocortex (Pleiss et al., 2016). Labeling for ΔCN was very faint throughout most brain areas examined, but increased dramatically in GFAP-positive astrocytes around the periphery of the lesion (Figure 1). These observations suggest considerable molecular heterogeneity in astrocytes depending on distance from vascular injury, consistent with studies in other injury/disease models (Zamanian et al., 2012; Itoh et al., 2018).
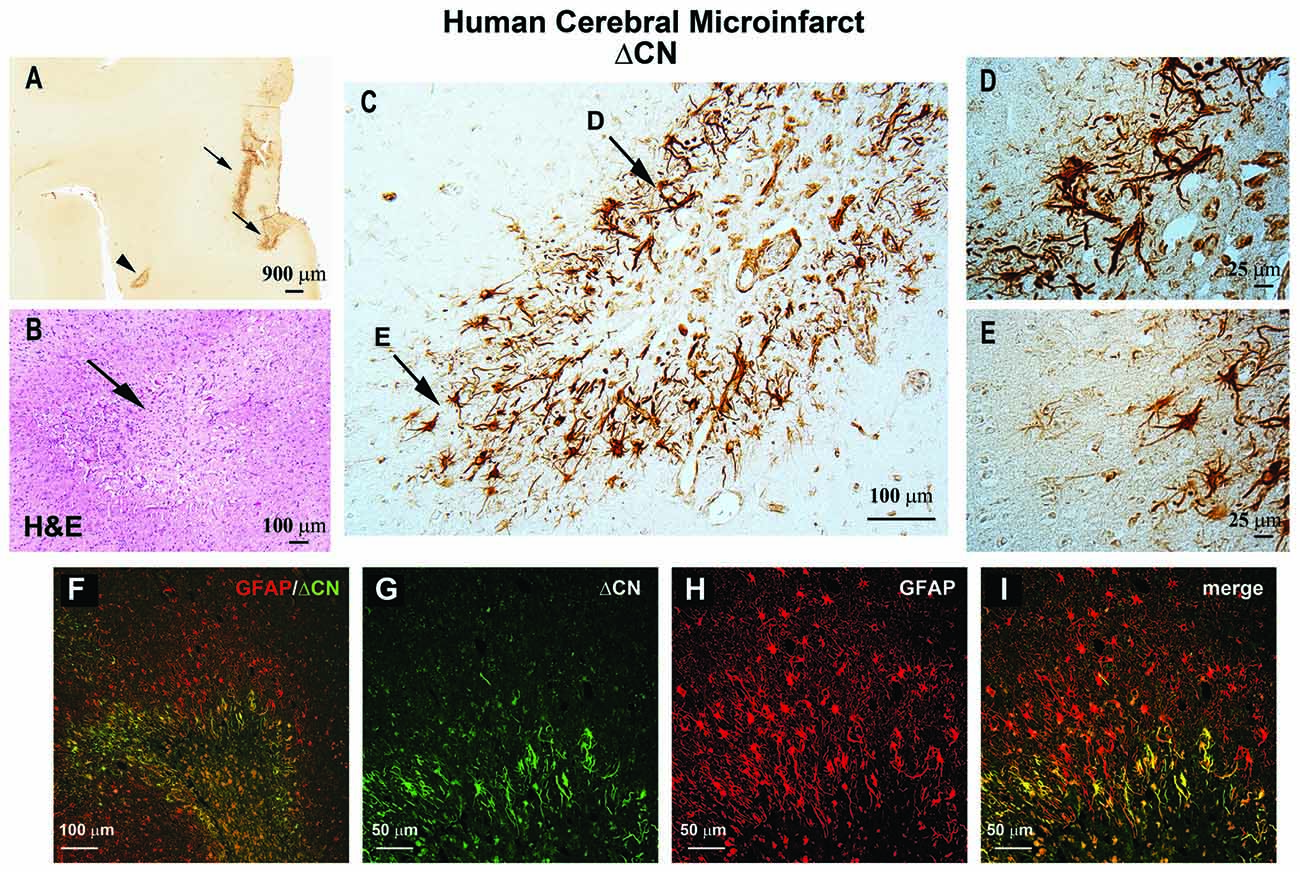
Figure 1. ΔCN is intensely expressed in activated astrocytes surrounding microinfarcts in human neocortex. (A) Representative low magnification photomicrograph from superior and middle temporal gyrus (SMTG) of a 90 year old human subject with multiple microinfarcts, but little-to-no Alzheimer’s pathology (Braak stage II) ΔCN labeling is present around several microinfarcts (arrows and arrowhead). (B) Serial section through STMG stained by H&E to confirm the presence of microinfarcts. The image shown is a high magnification of the region denoted by the arrowhead in Panel (A). (C) High power photomicrograph of the region in (A; arrowhead) showing intense ΔCN antibody labeling of astrocytes. Higher magnification of the areas denoted by arrows are shown in panels (D,E). (F) Merged confocal micrograph showing the colocalization of ΔCN (green) with GFAP around a microinfarct in human SMTG (red). (G–I) High magnification images of the infarct in Panel (F) shown in individual channels (G,H) and merged (I). Co-localization of ΔCN with GFAP was most extensive in the region immediately adjacent to the infarct. From Pleiss et al. (2016) used with permission.
Several outstanding issues regarding the relationship between astrocytic CN/NFAT and microinfarcts require further clarification. Presently, it is unknown whether CN/NFAT alterations occur immediately following microinfarct induction, or are more characteristic of chronic changes that arise with the formation of glial scars. The molecular phenotype of ΔCN-positive astrocytes has also yet to be elucidated. In primary neural cultures, forced overexpression of ΔCN in astrocytes induces the expression of numerous transcripts associated with morphogenesis and immune response (Norris et al., 2005). Studies are presently underway in our lab to determine the time course of ΔCN expression in photothrombosis models of microinfarct pathology (Risher et al., 2010; Masuda et al., 2011; Summers et al., 2017; Underly and Shih, 2017) and to determine if endogenous expression of ΔCN is associated with transcriptional changes, reminiscent of forced overexpression studies.
It deserves noting that many of the transcripts induced by CN/NFAT activity in glial cells, and in other cell types, encode releasable factors, such as cytokines and chemokines (Norris et al., 2005; Canellada et al., 2008; Sama et al., 2008; Nagamoto-Combs and Combs, 2010; Neria et al., 2013). Given the intimate structural and functional interactions between astrocytes and cerebral blood vessels, it seems likely that many CN/NFAT-dependent factors released from activated astrocytes could find their way into the bloodstream near regions of vascular damage. Presence of these factors (or ΔCN itself) in blood could then be used as potential biomarkers for the presence of microinfarcts or other forms of vascular pathology. Indeed, given the insidious nature of microinfarcts, the identification of peripheral biomarkers would be most helpful for diagnostic and/or prognostic screening purposes. Of course, additional research will be necessary to assess these possibilities.
Functional Impact of CN Signaling in Activated Astrocytes
Astrocyte activation is a complex process associated with both neuroprotective and deleterious consequences for surrounding nervous tissue (Khakh and Sofroniew, 2015; Pekny et al., 2016; Verkhratsky et al., 2016). The increased expression of CN/NFAT components in astrocytes associated with vascular pathology may offer important targets that could be exploited for determining the functional impact of these cells. Overexpression of ΔCN in hippocampal astrocytes of intact healthy adult rats causes reduced synaptic strength and hyperexcitability in nearby neurons, which is consistent with other studies linking activated astrocytes with impaired neuronal connectivity in acute injury models (Wilhelmsson et al., 2004). In contrast, astrocytic expression of ΔCN has also been found to reduce amyloid pathology and improve cognitive function in mouse models of Alzhieimer’s disease, consistent with other reports that have found protective roles of activated astrocytes in neurodegenerative conditions (Okada et al., 2006; Kraft et al., 2013; Wanner et al., 2013; Tyzack et al., 2014). Whether CN gives rise to beneficial or detrimental processes may depend critically on the presence of different activating factors and/or the recruitment of different transcription factor families (Furman and Norris, 2014). For instance, the pro-inflammatory cytokine TNF was shown to trigger the association of CN with the transcription factors NFκB and FOXO3, which, in turn, induced pro-inflammatory responses for promoting neurodegeneration (Fernandez et al., 2012, 2016). In contrast, CN stimulation by the insulin-like growth factor (IGF-I), has been proposed to mediate neuroprotective responses of activated astrocytes via interactions between NFκB and PPARγ (Fernandez et al., 2012).
Blockade of CN interactions with NFAT transcription factors, using the peptide VIVIT, has been associated with many beneficial effects in cell culture and intact animal models of neurodegeneration. VIVIT mimics the CN-binding PxIxIT motif found in the regulatory region of NFATs 1–4 (Aramburu et al., 1999). When delivered to numerous cell types, VIVIT prevents CN from binding to NFATs and therefore inhibits NFAT nuclear localization, without inhibiting CN catalytic activity per se. Expression of VIVIT in hippocampal astrocytes, using adeno-associated virus (AAV) vectors equipped with the human GFAP promoter Gfa2 (Lee et al., 2008), improved synaptic strength and/or normalized synaptic plasticity in animal models of Alzheimer’s disease and traumatic brain injury (Furman et al., 2012, 2016; Sompol et al., 2017). Where tested, AAV-Gfa2-VIVIT delivery to the hippocampus also improved hippocampal-dependent cognitive function (Furman et al., 2012; Sompol et al., 2017). In primary neural cultures, VIVIT prevented the loss of astrocyte-enriched glutamate transporters, primarily GLT1, in response to pro-inflammatory cytokines and oligomeric Aβ, leading to reduced extracellular glutamate levels, reduced neuronal excitability and greater neuronal survival (Sama et al., 2008; Abdul et al., 2009). VIVIT similarly restored GLT1 levels in intact 5xFAD mice—an aggressive mouse model for Alzheimer’s disease (Sompol et al., 2017). Mice treated with AAV-Gfa2-VIVIT showed greater GLT1 expression, measured via immunofluorescent microscopy and Western blot. VIVIT-treated 5xFAD mice also exhibited fewer and shorter-duration spontaneous glutamate transients (measured in vivo), healthier neurite morphology, reduced synaptic hyperexcitability, and normalized NMDA-to-AMPA receptor activity ratios (Sompol et al., 2017). Together, these observations suggest that hyperactive CN/NFAT signaling underlies a neurotoxic activated astrocyte phenotype characterized by glutamate dysregulation and excitotoxicity.
Interestingly, many of the same telltale signs of glutamate toxicity, including a loss of GLT1 and neuronal hyperactivity, have been noted in experimental models of ischemia and stroke (Maragakis and Rothstein, 2004; Soni et al., 2014). Moreover, glutamate dysregulation would not only influence the behavior and viability of surrounding neurons, but may also be expected to negatively affect the cerebrovascular unit as well. For instance, functional knockdown of GLT1 in otherwise healthy animals can lead to reduced cerebral blood flow and/or impaired neurovascular coupling (Petzold et al., 2008). Other work has shown that hyperexcitable neural networks and/or excitotoxic insults compromise the structural integrity of vascular endothelial cells and perivascular astrocyte endfeet, and precipitate blood brain barrier (BBB) leakage (Bolton and Perry, 1998; Parathath et al., 2006; Alvestad et al., 2013; Gondo et al., 2014; Ryu and McLarnon, 2016) leading to perivascular and parenchymal neuroinflammation.
Summary and Future Directions
Cerebrovascular pathology is one of the leading causes of dementia and a frequently identified comorbid factor in many neurologic diseases, such as Alzheimer’s disease. Numerous studies have reported a role for CN hyperactivity in the pathophysiologic sequelae coupling vascular disruption and damage to neuronal death and cognitive loss. Mounting evidence suggests that CN/NFAT signaling may play a particularly important role in neural changes that arise with astrocyte activation in many different neurodegenerative diseases, including cerebrovascular disease. However, no studies to date have tested the specific involvement of astrocytic CN/NFAT signaling in either global ischemia models, models characterized by localized damage to microvessels, or in models that develop chronic vascular inflammation and microhemhorrages. Based on the observations discussed above, we hypothesize that acutely and chronically developing vascular damage will lead to the activation of astrocytes and hyperactivation of CN/NFAT signaling (Figure 2). In this scenario, increased CN/NFAT activity would lead to the induction and release of numerous immune/inflammatory factors and/or to the dysregulation of astrocytic glutamate uptake, resulting in impaired synaptic function, excitotoxicity, impaired neuronal viability and neuroinflammation. These deleterious actions, could, in turn, promote further vascular damage and inflammation and hasten neurodegeneration and cognitive loss as part of vicious positive feedback cycle. Of course, this hypothesis will require extensive testing using astrocyte-specific targeting strategies in experimental models of stroke and/or VCID.
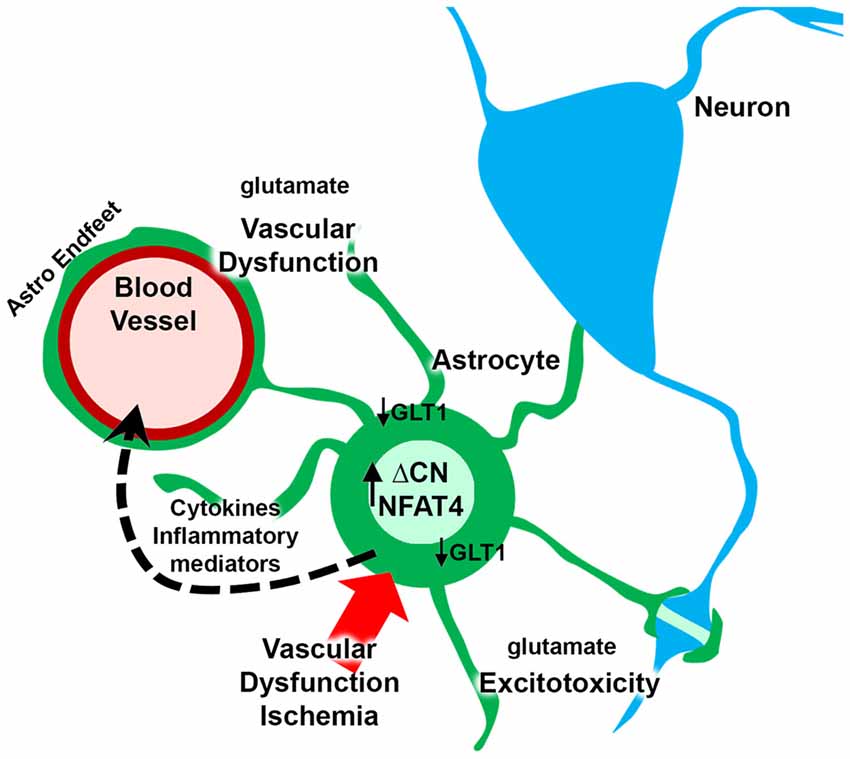
Figure 2. Putative role for astrocytic CN/nuclear factor of activated T cell (NFAT) in vascular dysfunction and neurodegeneration. Ischemia arising from vascular degeneration or disruption leads to increased expression of ΔCN and hyperactivation of NFAT4 in astrocytes. The CN/NFAT pathway induces numerous cytokines and other inflammatory mediators linked to neuroinflammation. Some of these factors may target blood vessels, leading to perivascular inflammation. CN/NFAT signaling also leads to the downregulation of GLT1 glutamate transporters resulting in elevated extracellular glutamate levels. Glutamate causes excitotoxicity at synaptic connections and disrupts astrocyte endfeet and/or blood brain barrier (BBB) integrity, leading to further vascular dysfunction and/or degeneration.
Author Contributions
SK and CN researched and wrote this manuscript.
Funding
This work was supported by National Institutes of Health Grants AG027297, AG056998, AG051945 and a gift from the Hazel Embry Research Trust.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abdul, H. M., Furman, J. L., Sama, M. A., Mathis, D. M., and Norris, C. M. (2010). NFATs and Alzheimer’s disease. Mol. Cell. Pharmacol. 2, 7–14.
Abdul, H. M., Sama, M. A., Furman, J. L., Mathis, D. M., Beckett, T. L., Weidner, A. M., et al. (2009). Cognitive decline in Alzheimer’s disease is associated with selective changes in calcineurin/NFAT signaling. J. Neurosci. 29, 12957–12969. doi: 10.1523/jneurosci.1064-09.2009
Alvestad, S., Hammer, J., Hoddevik, E. H., Skare, Ø., Sonnewald, U., Amiry-Moghaddam, M., et al. (2013). Mislocalization of AQP4 precedes chronic seizures in the kainate model of temporal lobe epilepsy. Epilepsy Res. 105, 30–41. doi: 10.1016/j.eplepsyres.2013.01.006
Aramburu, J., Rao, A., and Klee, C. B. (2000). Calcineurin: from structure to function. Curr. Top. Cell. Regul. 36, 237–295. doi: 10.1016/s0070-2137(01)80011-x
Aramburu, J., Yaffe, M. B., López-Rodriguez, C., Cantley, L. C., Hogan, P. G., and Rao, A. (1999). Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science 285, 2129–2133. doi: 10.1126/science.285.5436.2129
Baumgärtel, K., Genoux, D., Welzl, H., Tweedie-Cullen, R. Y., Koshibu, K., Livingstone-Zatchej, M., et al. (2008). Control of the establishment of aversive memory by calcineurin and Zif268. Nat. Neurosci. 11, 572–578. doi: 10.1038/nn.2113
Bolton, S. J., and Perry, V. H. (1998). Differential blood-brain barrier breakdown and leucocyte recruitment following excitotoxic lesions in juvenile and adult rats. Exp. Neurol. 154, 231–240. doi: 10.1006/exnr.1998.6927
Brait, V. H., Martin, K. R., Corlett, A., Broughton, B. R., Kim, H. A., Thundyil, J., et al. (2012). Over-expression of DSCR1 protects against post-ischemic neuronal injury. PLoS One 7:e47841. doi: 10.1371/journal.pone.0047841
Butcher, S. P., Henshall, D. C., Teramura, Y., Iwasaki, K., and Sharkey, J. (1997). Neuroprotective actions of FK506 in experimental stroke: in vivo evidence against an antiexcitotoxic mechanism. J. Neurosci. 17, 6939–6946. doi: 10.1523/jneurosci.17-18-06939.1997
Canellada, A., Ramirez, B. G., Minami, T., Redondo, J. M., and Cano, E. (2008). Calcium/calcineurin signaling in primary cortical astrocyte cultures: Rcan1–4 and cyclooxygenase-2 as NFAT target genes. Glia 56, 709–722. doi: 10.1002/glia.20647
Celsi, F., Svedberg, M., Unger, C., Cotman, C. W., Carri, M. T., Ottersen, O. P., et al. (2007). β-amyloid causes downregulation of calcineurin in neurons through induction of oxidative stress. Neurobiol. Dis. 26, 342–352. doi: 10.1016/j.nbd.2006.12.022
Choi, D. W. (1988). Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci. 11, 465–469. doi: 10.1016/0166-2236(88)90200-7
de la Fuente, V., Freudenthal, R., and Romano, A. (2011). Reconsolidation or extinction: transcription factor switch in the determination of memory course after retrieval. J. Neurosci. 31, 5562–5573. doi: 10.1523/JNEUROSCI.6066-10.2011
Fernandez, A. M., Hervas, R., Dominguez-Fraile, M., Garrido, V. N., Gomez-Gutierrez, P., Vega, M., et al. (2016). Blockade of the interaction of calcineurin with FOXO in astrocytes protects against amyloid-β-induced neuronal death. J. Alzheimers Dis. 52, 1471–1478. doi: 10.3233/jad-160149
Fernandez, A. M., Jimenez, S., Mecha, M., Davila, D., Guaza, C., Vitorica, J., et al. (2012). Regulation of the phosphatase calcineurin by insulin-like growth factor I unveils a key role of astrocytes in Alzheimer’s pathology. Mol. Psychiatry 17, 705–718. doi: 10.1038/mp.2011.128
Furman, J. L., and Norris, C. M. (2014). Calcineurin and glial signaling: neuroinflammation and beyond. J. Neuroinflammation 11:158. doi: 10.1186/s12974-014-0158-7
Furman, J. L., Sama, D. M., Gant, J. C., Beckett, T. L., Murphy, M. P., Bachstetter, A. D., et al. (2012). Targeting astrocytes ameliorates neurologic changes in a mouse model of Alzheimer’s disease. J. Neurosci. 32, 16129–16140. doi: 10.1523/jneurosci.2323-12.2012
Furman, J. L., Sompol, P., Kraner, S. D., Pleiss, M. M., Putman, E. J., Dunkerson, J., et al. (2016). Blockade of astrocytic calcineurin/NFAT signaling helps to normalize hippocampal synaptic function and plasticity in a rat model of traumatic brain injury. J. Neurosci. 36, 1502–1515. doi: 10.1523/jneurosci.1930-15.2016
Gondo, A., Shinotsuka, T., Morita, A., Abe, Y., Yasui, M., and Nuriya, M. (2014). Sustained down-regulation of β-dystroglycan and associated dysfunctions of astrocytic endfeet in epileptic cerebral cortex. J. Biol. Chem. 289, 30279–30288. doi: 10.1074/jbc.m114.588384
Harris, R. J., Branston, N. M., Symon, L., Bayhan, M., and Watson, A. (1982). The effects of a calcium antagonist, nimodipine, upon physiological responses of the cerebral vasculature and its possible influence upon focal cerebral ischaemia. Stroke 13, 759–766. doi: 10.1161/01.str.13.6.759
Hashimoto, T., Kawamata, T., Saito, N., Sasaki, M., Nakai, M., Niu, S., et al. (1998). Isoform-specific redistribution of calcineurin Aα and Aβ in the hippocampal CA1 region of gerbils after transient ischemia. J. Neurochem. 70, 1289–1298. doi: 10.1046/j.1471-4159.1998.70031289.x
Horsburgh, K., Wardlaw, J. M., Van Agtmael, T., Allan, S. M., Ashford, M. L. J., Bath, P. M., et al. (2018). Small vessels, dementia and chronic diseases—molecular mechanisms and pathophysiology. Clin. Sci. 132, 851–868. doi: 10.1042/CS20171620
Horsley, V., and Pavlath, G. K. (2002). NFAT: ubiquitous regulator of cell differentiation and adaptation. J. Cell Biol. 156, 771–774. doi: 10.1083/jcb.200111073
Horst, G. J. T., and Postigo, A. (1996). “Stroke: prevalence and mechanism of cell death,” in Clinical Pharmacology of Cerebral Ischemia, eds G. J. Ter Horst and J. Korf (New York, NY: Springer Science and Business Media), 1–30.
Im, S. H., and Rao, A. (2004). Activation and deactivation of gene expression by Ca2+/calcineurin-NFAT-mediated signaling. Mol. Cells 18, 1–9.
Infeld, B., Davis, S. M., Donnan, G. A., Yasaka, M., Lichtenstein, M., Mitchell, P. J., et al. (1999). Nimodipine and perfusion changes after stroke. Stroke 30, 1417–1423. doi: 10.1161/01.str.30.7.1417
Itoh, N., Itoh, Y., Tassoni, A., Ren, E., Kaito, M., Ohno, A., et al. (2018). Cell-specific and region-specific transcriptomics in the multiple sclerosis model: focus on astrocytes. Proc. Natl. Acad. Sci. U S A 115, E302–E309. doi: 10.1073/pnas.1716032115
Khakh, B. S., and Sofroniew, M. V. (2015). Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 18, 942–952. doi: 10.1038/nn.4043
Klee, C. B., Ren, H., and Wang, X. (1998). Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J. Biol. Chem. 273, 13367–13370. doi: 10.1074/jbc.273.22.13367
Kraft, A. W., Hu, X., Yoon, H., Yan, P., Xiao, Q., Wang, Y., et al. (2013). Attenuating astrocyte activation accelerates plaque pathogenesis in APP/PS1 mice. FASEB J. 27, 187–198. doi: 10.1096/fj.12-208660
Lee, Y., Messing, A., Su, M., and Brenner, M. (2008). GFAP promoter elements required for region-specific and astrocyte-specific expression. Glia 56, 481–493. doi: 10.1002/glia.20622
Lim, D., Iyer, A., Ronco, V., Grolla, A. A., Canonico, P. L., Aronica, E., et al. (2013). Amyloid β deregulates astroglial mGluR5-mediated calcium signaling via calcineurin and Nf-kB. Glia 61, 1134–1145. doi: 10.1002/glia.22502
Manocha, G. D., Ghatak, A., Puig, K. L., Kraner, S. D., Norris, C. M., and Combs, C. K. (2017). NFATc2 modulates microglial activation in the AβPP/PS1 mouse model of Alzheimer’s disease. J. Alzheimers Dis. 58, 775–787. doi: 10.3233/jad-151203
Mansuy, I. M. (2003). Calcineurin in memory and bidirectional plasticity. Biochem. Biophys. Res. Commun. 311, 1195–1208. doi: 10.1016/j.bbrc.2003.10.046
Maragakis, N. J., and Rothstein, J. D. (2004). Glutamate transporters: animal models to neurologic disease. Neurobiol. Dis. 15, 461–473. doi: 10.1016/j.nbd.2003.12.007
Masuda, T., Croom, D., Hida, H., and Kirov, S. A. (2011). Capillary blood flow around microglial somata determines dynamics of microglial processes in ischemic conditions. Glia 59, 1744–1753. doi: 10.1002/glia.21220
Mattson, M. P. (2007). Calcium and neurodegeneration. Aging Cell 6, 337–350. doi: 10.1111/j.1474-9726.2007.00275.x
Mukherjee, A., and Soto, C. (2011). Role of calcineurin in neurodegeneration produced by misfolded proteins and endoplasmic reticulum stress. Curr. Opin. Cell Biol. 23, 223–230. doi: 10.1016/j.ceb.2010.12.006
Nagamoto-Combs, K., and Combs, C. K. (2010). Microglial phenotype is regulated by activity of the transcription factor, NFAT (nuclear factor of activated T cells). J. Neurosci. 30, 9641–9646. doi: 10.1523/jneurosci.0828-10.2010
Neria, F., Del Carmen Serrano-Perez, M., Velasco, P., Urso, K., Tranque, P., and Cano, E. (2013). NFATc3 promotes Ca2+-dependent MMP3 expression in astroglial cells. Glia 61, 1052–1066. doi: 10.1002/glia.22494
Nguyen, T., and Di Giovanni, S. (2008). NFAT signaling in neural development and axon growth. Int. J. Dev. Neurosci. 26, 141–145. doi: 10.1016/j.ijdevneu.2007.10.004
Norris, C. M. (2014). Calpain interactions with the protein phosphatase calcineurin in neurodegeneration. Adv. Biochem. Health Dis. 8, 17–45. doi: 10.1007/978-1-4614-9099-9_2
Norris, C. M., Kadish, I., Blalock, E. M., Chen, K. C., Thibault, V., Porter, N. M., et al. (2005). Calcineurin triggers reactive/inflammatory processes in astrocytes and is upregulated in aging and Alzheimer’s models. J. Neurosci. 25, 4649–4658. doi: 10.1523/JNEUROSCI.0365-05.2005
O’Brien, J. T., Erkinjuntti, T., Reisberg, B., Roman, G., Sawada, T., Pantoni, L., et al. (2003). Vascular cognitive impairment. Lancet Neurol. 2, 89–98. doi: 10.1016/S1474-4422(03)00305-3
Okada, S., Nakamura, M., Katoh, H., Miyao, T., Shimazaki, T., Ishii, K., et al. (2006). Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat. Med. 12, 829–834. doi: 10.1038/nm1425
Parathath, S. R., Parathath, S., and Tsirka, S. E. (2006). Nitric oxide mediates neurodegeneration and breakdown of the blood-brain barrier in tPA-dependent excitotoxic injury in mice. J. Cell Sci. 119, 339–349. doi: 10.1242/jcs.02734
Pekny, M., Pekna, M., Messing, A., Steinhäuser, C., Lee, J. M., Parpura, V., et al. (2016). Astrocytes: a central element in neurological diseases. Acta Neuropathol. 131, 323–345. doi: 10.1007/s00401-015-1513-1
Petzold, G. C., Albeanu, D. F., Sato, T. F., and Murthy, V. N. (2008). Coupling of neural activity to blood flow in olfactory glomeruli is mediated by astrocytic pathways. Neuron 58, 897–910. doi: 10.1016/j.neuron.2008.04.029
Pleiss, M. M., Sompol, P., Kraner, S. D., Abdul, H. M., Furman, J. L., Guttmann, R. P., et al. (2016). Calcineurin proteolysis in astrocytes: implications for impaired synaptic function. Biochim. Biophys. Acta 1862, 1521–1532. doi: 10.1016/j.bbadis.2016.05.007
Rao, A., Luo, C., and Hogan, P. G. (1997). Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol. 15, 707–747. doi: 10.1146/annurev.immunol.15.1.707
Ray, S. K. (2006). Currently evaluated calpain and caspase inhibitors for neuroprotection in experimental brain ischemia. Curr. Med. Chem. 13, 3425–3440. doi: 10.2174/092986706779010342
Reese, L. C., and Taglialatela, G. (2011). A role for calcineurin in Alzheimer’s disease. Curr. Neuropharmacol. 9, 685–692. doi: 10.2174/157015911798376316
Risher, W. C., Ard, D., Yuan, J., and Kirov, S. A. (2010). Recurrent spontaneous spreading depolarizations facilitate acute dendritic injury in the ischemic penumbra. J. Neurosci. 30, 9859–9868. doi: 10.1523/JNEUROSCI.1917-10.2010
Rivera-Olvera, A., Nelson-Mora, J., Gonsebatt, M. E., and Escobar, M. L. (2018). Extinction of aversive taste memory homeostatically prevents the maintenance of in vivo insular cortex LTP: calcineurin participation. Neurobiol. Learn. Mem. doi: 10.1016/j.nlm.2018.04.005 [Epub ahead of print].
Rosenkranz, K., May, C., Meier, C., and Marcus, K. (2012). Proteomic analysis of alterations induced by perinatal hypoxic-ischemic brain injury. J. Proteome Res. 11, 5794–5803. doi: 10.1021/pr3005869
Rostas, J. A. P., Spratt, N. J., Dickson, P. W., and Skelding, K. A. (2017). The role of Ca2+-calmodulin stimulated protein kinase II in ischaemic stroke—A potential target for neuroprotective therapies. Neurochem. Int. 107, 33–42. doi: 10.1016/j.neuint.2017.01.012
Ryu, J. K., and McLarnon, J. G. (2016). Pyruvate blocks blood-brain barrier disruption, lymphocyte infiltration and immune response in excitotoxic brain injury. Am. J. Neurodegener. Dis. 5, 69–73.
Sama, M. A., Mathis, D. M., Furman, J. L., Abdul, H. M., Artiushin, I. A., Kraner, S. D., et al. (2008). Interleukin-1β-dependent signaling between astrocytes and neurons depends critically on astrocytic calcineurin/NFAT activity. J. Biol. Chem. 283, 21953–21964. doi: 10.1074/jbc.M800148200
Serrano-Pérez, M. C., Martín, E. D., Vaquero, C. F., Azcoitia, I., Calvo, S., Cano, E., et al. (2011). Response of transcription factor NFATc3 to excitotoxic and traumatic brain insults: identification of a subpopulation of reactive astrocytes. Glia 59, 94–107. doi: 10.1002/glia.21079
Sharkey, J., and Butcher, S. P. (1994). Immunophilins mediate the neuroprotective effects of FK506 in focal cerebral ischaemia. Nature 371, 336–339. doi: 10.1038/371336a0
Sharkey, J., Crawford, J. H., Butcher, S. P., and Marston, H. M. (1996). Tacrolimus (FK506) ameliorates skilled motor deficits produced by middle cerebral artery occlusion in rats. Stroke 27, 2282–2286. doi: 10.1161/01.str.27.12.2282
Shioda, N., Han, F., Moriguchi, S., and Fukunaga, K. (2007). Constitutively active calcineurin mediates delayed neuronal death through Fas-ligand expression via activation of NFAT and FKHR transcriptional activities in mouse brain ischemia. J. Neurochem. 102, 1506–1517. doi: 10.1111/j.1471-4159.2007.04600.x
Shioda, N., Moriguchi, S., Shirasaki, Y., and Fukunaga, K. (2006). Generation of constitutively active calcineurin by calpain contributes to delayed neuronal death following mouse brain ischemia. J. Neurochem. 98, 310–320. doi: 10.1111/j.1471-4159.2006.03874.x
Snyder, H. M., Corriveau, R. A., Craft, S., Faber, J. E., Greenberg, S. M., Knopman, D., et al. (2015). Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimers Dement. 11, 710–717. doi: 10.1016/j.jalz.2014.10.008
Sobrado, M., Ramirez, B. G., Neria, F., Lizasoain, I., Arbones, M. L., Minami, T., et al. (2012). Regulator of calcineurin 1 (Rcan1) has a protective role in brain ischemia/reperfusion injury. J. Neuroinflammation 9:48. doi: 10.1186/1742-2094-9-48
Sompol, P., Furman, J. L., Pleiss, M. M., Kraner, S. D., Artiushin, I. A., Batten, S. R., et al. (2017). Calcineurin/NFAT signaling in activated astrocytes drives network hyperexcitability in Aβ-bearing mice. J. Neurosci. 37, 6132–6148. doi: 10.1523/JNEUROSCI.0877-17.2017
Sompol, P., and Norris, C. M. (2018). Ca2+, astrocyte activation and calcineurin/NFAT signaling in age-related neurodegenerative diseases. Front. Aging Neurosci. 10:199. doi: 10.3389/fnagi.2018.00199
Soni, N., Reddy, B. V., and Kumar, P. (2014). GLT-1 transporter: an effective pharmacological target for various neurological disorders. Pharmacol. Biochem. Behav. 127, 70–81. doi: 10.1016/j.pbb.2014.10.001
Summers, P. M., Hartmann, D. A., Hui, E. S., Nie, X., Deardorff, R. L., Mckinnon, E. T., et al. (2017). Functional deficits induced by cortical microinfarcts. J. Cereb. Blood Flow Metab. 37, 3599–3614. doi: 10.1177/0271678x16685573
Szydlowska, K., and Tymianskia, M. (2010). Calcium, ischemia and excitotoxicity. Cell Calcium 47, 122–129. doi: 10.1016/j.ceca.2010.01.003
Tyzack, G. E., Sitnikov, S., Barson, D., Adams-Carr, K. L., Lau, N. K., Kwok, J. C., et al. (2014). Astrocyte response to motor neuron injury promotes structural synaptic plasticity via STAT3-regulated TSP-1 expression. Nat. Commun. 5:4294. doi: 10.1038/ncomms5294
Uchino, H., Kuroda, Y., Morota, S., Hirabayashi, G., Ishii, N., Shibasaki, F., et al. (2008). Probing the molecular mechanisms of neuronal degeneration: importance of mitochondrial dysfunction and calcineurin activation. J. Anesth. 22, 253–262. doi: 10.1007/s00540-008-0617-3
Underly, R. G., and Shih, A. Y. (2017). Photothrombotic induction of capillary ischemia in the mouse cortex during in vivo two-photon imaging. Bio. Protoc. 7:e2378. doi: 10.21769/bioprotoc.2378
Verkhratsky, A., Steardo, L., Parpura, V., and Montana, V. (2016). Translational potential of astrocytes in brain disorders. Prog. Neurobiol. 144, 188–205. doi: 10.1016/j.pneurobio.2015.09.003
Vihma, H., Pruunsild, P., and Timmusk, T. (2008). Alternative splicing and expression of human and mouse NFAT genes. Genomics 92, 279–291. doi: 10.1016/j.ygeno.2008.06.011
Wang, K. K., Roufogalis, B. D., and Villalobo, A. (1989). Characterization of the fragmented forms of calcineurin produced by calpain I. Biochem. Cell Biol. 67, 703–711. doi: 10.1139/o89-105
Wanner, I. B., Anderson, M. A., Song, B., Levine, J., Fernandez, A., Gray-Thompson, Z., et al. (2013). Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J. Neurosci. 33, 12870–12886. doi: 10.1523/JNEUROSCI.2121-13.2013
Weekman, E. M., Sudduth, T. L., Caverly, C. N., Kopper, T. J., Phillips, O. W., Powell, D. K., et al. (2016). Reduced Efficacy of anti-aβ immunotherapy in a mouse model of amyloid deposition and vascular cognitive impairment comorbidity. J. Neurosci. 36, 9896–9907. doi: 10.1523/JNEUROSCI.1762-16.2016
Wilhelmsson, U., Li, L., Pekna, M., Berthold, C. H., Blom, S., Eliasson, C., et al. (2004). Absence of glial fibrillary acidic protein and vimentin prevents hypertrophy of astrocytic processes and improves post-traumatic regeneration. J. Neurosci. 24, 5016–5021. doi: 10.1523/JNEUROSCI.0820-04.2004
Wu, Y., Borde, M., Heissmeyer, V., Feuerer, M., Lapan, A. D., Stroud, J. C., et al. (2006). FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell 126, 375–387. doi: 10.1016/j.cell.2006.05.042
Wu, H. Y., Tomizawa, K., Oda, Y., Wei, F. Y., Lu, Y. F., Matsushita, M., et al. (2004). Critical role of calpain-mediated cleavage of calcineurin in excitotoxic neurodegeneration. J. Biol. Chem. 279, 4929–4940. doi: 10.1074/jbc.M309767200
Wu, Q. J., and Tymianski, M. (2018). Targeting NMDA receptors in stroke: new hope in neuroprotection. Mol. Brain 11:15. doi: 10.1186/s13041-018-0357-8
Zamanian, J. L., Xu, L., Foo, L. C., Nouri, N., Zhou, L., Giffard, R. G., et al. (2012). Genomic analysis of reactive astrogliosis. J. Neurosci. 32, 6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012
Keywords: vascular contributions to cognitive impairment and dementia, Ca2+, glia, excitotoxicity, Alzheimer’s disease
Citation: Kraner SD and Norris CM (2018) Astrocyte Activation and the Calcineurin/NFAT Pathway in Cerebrovascular Disease. Front. Aging Neurosci. 10:287. doi: 10.3389/fnagi.2018.00287
Received: 26 June 2018; Accepted: 03 September 2018;
Published: 21 September 2018.
Edited by:
Albert Gjedde, University of Southern Denmark, DenmarkReviewed by:
Valentina Echeverria Moran, Bay Pines VA Healthcare System, United StatesIgnacio Torres-Aleman, Consejo Superior de Investigaciones Científicas (CSIC), Spain
Copyright © 2018 Kraner and Norris. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christopher M. Norris, cnorr2@uky.edu
 Susan D. Kraner
Susan D. Kraner Christopher M. Norris
Christopher M. Norris