Diffusion Tensor Imaging Tractography Reveals Disrupted White Matter Structural Connectivity Network in Healthy Adults with Insomnia Symptoms
- 1Bioimaging Core, Faculty of Health Sciences, University of Macau, Macau, China
- 2Chengdu Mental Health Center, Chengdu, China
- 3The Clinical Hospital of Chengdu Brain Science Institute, MOE Key Lab for Neuroinformation, University of Electronic Science and Technology of China, Chengdu, China
- 4Beijing Institute of Traditional Chinese Medicine, Beijing Hospital of Traditional Chinese Medicine Affiliated to Capital Medical University, Beijing, China
- 5Department of Radiology, Beijing Anding Hospital, Capital Medical University, Beijing, China
- 6Key Laboratory for NeuroInformation of Ministry of Education, School of Life Science and Technology, University of Electronic Science and Technology of China, Chengdu, China
Neuroimaging studies have revealed that insomnia is characterized by aberrant neuronal connectivity in specific brain regions, but the topological disruptions in the white matter (WM) structural connectivity networks remain largely unknown in insomnia. The current study uses diffusion tensor imaging (DTI) tractography to construct the WM structural networks and graph theory analysis to detect alterations of the brain structural networks. The study participants comprised 30 healthy subjects with insomnia symptoms (IS) and 62 healthy subjects without IS. Both the two groups showed small-world properties regarding their WM structural connectivity networks. By contrast, increased local efficiency and decreased global efficiency were identified in the IS group, indicating an insomnia-related shift in topology away from regular networks. In addition, the IS group exhibited disrupted nodal topological characteristics in regions involving the fronto-limbic and the default-mode systems. To our knowledge, this is the first study to explore the topological organization of WM structural network connectivity in insomnia. More importantly, the dysfunctions of large-scale brain systems including the fronto-limbic pathways, salience network and default-mode network in insomnia were identified, which provides new insights into the insomnia connectome. Topology-based brain network analysis thus could be a potential biomarker for IS.
Introduction
Insomnia is one of the most prevalent sleep disorders that is distinguished by difficulties in falling or maintaining sleep, and/or early morning awakening (Morin and Benca, 2012; Cheung et al., 2013; Morin et al., 2015; Riedner et al., 2016). Insomnia is associated with impaired daytime functioning and affects approximately one-third of the general population (Ohayon, 2002; Moore, 2012; Morin and Benca, 2012; Kronholm et al., 2015). In addition, individuals with insomnia show an increased risk for developing other psychiatric disorders. For example, nearly 40% of the insomnia patients have a comorbid psychiatric disorder, and almost all of depression patients present high risk for insomnia (Taylor et al., 2005; Kaneita et al., 2006; Ohayon and Hong, 2006; Benca and Peterson, 2008; Wulff et al., 2010; Mayer et al., 2011). More importantly, insomnia can lead to feeling fatigued, poor academic performance, working disability, drugs and alcohol abuse, suicidal thoughts and reduced quality of life (Short et al., 2013; Kronholm et al., 2015). Consequently, insomnia can negatively impact personal and public health, incur direct and indirect healthcare costs, and have a huge socio-economic impact on society (Kucharczyk et al., 2012; Moore, 2012; Lian et al., 2015). Although well-documented neuroimaging studies have investigated insomnia, the neurobiological mechanisms underlying this psychiatric disorder remain poorly understood.
Growing functional and structural neuroimaging evidence shows that widespread brain regions are implicated in the pathobiology of insomnia, including the amygdala, hippocampus, anterior cingulate gyrus, caudate nucleus, insula and the frontal areas (Drummond et al., 2004; Nofzinger et al., 2004; Riemann et al., 2007, 2015; Altena et al., 2008, 2010; Spiegelhalder et al., 2013, 2015; Winkelman et al., 2013; Baglioni et al., 2014; Joo et al., 2014; Stoffers et al., 2014; Liu C.-H. et al., 2016; Lu et al., 2017). In particular, the functional and structural networks are strongly correlated with each other, and the structural connectivity works as a physical substrate of the functional connectivity. The functional connectivity can also affect the structural connectivity according to the brain plasticity (van den Heuvel et al., 2008; Greicius et al., 2009; Rubinov et al., 2009; Zhang J. et al., 2011; Long et al., 2015). In addition, it has been widely recognized that functional interactions among different brain regions are effectively constrained by large-scale structural connections (Hagmann et al., 2008; Honey et al., 2009). However, the network-level structural deficits remain largely unknown, especially the topological alterations associated with insomnia. It is therefore essential to examine the structural substrate of interactions among distributed brain regions to understand the functional brain activation patterns in insomnia.
Diffusion tensor imaging (DTI) tractography is a robust, non-invasive method that can be utilized to reconstruct the white matter (WM) tracts of the human brain (Basser et al., 2000; Guo W.-B. et al., 2012; Guo W. et al., 2012). When combined with a graph theoretical approach, this advanced neuroimaging technique can allow us to characterize the structural connection patterns of the human brain in vivo. Graph theoretical analysis can delineate the whole brain as a large-scale network consisting of nodes (brain areas) and edges (functional connectivity between pairs of areas; Bullmore and Sporns, 2009). Both of these methods have been increasingly used for the reconstruction of brain WM structural connectivity networks in psychiatric disorders such as post-traumatic stress disorder (Long et al., 2013), depression (Long et al., 2015), Alzheimer’s disease (Lo et al., 2010), and multiple sclerosis (Shu et al., 2011). So far, there are only two studies exploring the integrity of WM in insomnia, one study demonstrated that insomnia is associated with reduced integrity of WM tracts in the anterior internal capsule by comparing the fractional anisotropy (FA) between 24 primary insomnia (PI) patients and 35 healthy controls (Spiegelhalder et al., 2014) and by performing the between-group comparisons of the WM tracts between 23 PI patients and 30 healthy controls, another study suggested that the insomnia patients had decreased integrity of WM tracts predominantly in the areas of right anterior and posterior limb internal capsule, right anterior and superior corona radiate and right thalamus (Li S. et al., 2016). As such, this pilot work focuses on revealing topology abnormalities in WM structural connectivity networks associated with insomnia.
In this study, we hypothesized that compared to the healthy subjects without insomnia symptoms (NIS) group, the healthy subjects with IS group would exhibit an altered structural topology and disrupted nodal network properties of the brain areas mainly involved in the fronto-limbic system, salience network, and default-mode network. The DTI data from IS group and NIS group were collected first. Then, we constructed the whole brain WM structural connectivity networks with 90 nodes represented by cerebrum brain areas using the automated anatomical labeling (AAL) template and corresponding edges defined as the mean FA using DTI tractography. In addition, we applied graph theoretical analysis to generate the small-world characteristics of these WM networks, which can be used to identify the altered topological properties of brain networks in insomnia. More importantly, we examined the associations between clinical data and the altered network topologies.
Materials and Methods
Participants
The study participants comprised 92 right-handed healthy subjects (female/male: 51/41, age: 20–60 years). All participants were first screened with the Non-Patient Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; SCID) by two independent experienced psychiatrists (LRT and CLT) as described in our previous study (Lu et al., 2017). All the subjects had no neurological or psychiatric disorders, such as depression, anxiety disorders, epilepsy, schizophrenia, mental retardation, or chronic pain. In addition, none of the subjects had taken any psychotropic medication in at least 2 months prior to the MRI scans.
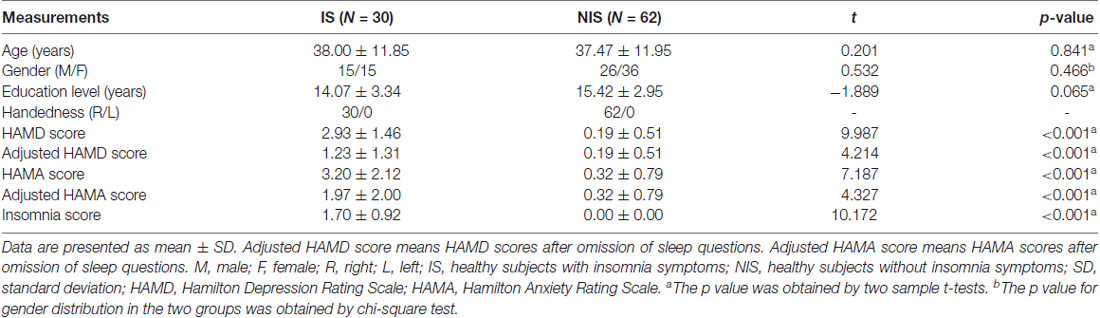
All clinical tests were approved by the Medical Ethics Committee of Beijing Anding Hospital, Capital Medical University, the Imaging Center for Brain Research of Beijing Normal University and the Biomedical Ethics Board of the Faculty of Health Sciences at the University of Macau (Macao SAR, China) in accordance with the approved guidelines. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The study groups included 30 healthy subjects with IS (age: 38.00 ± 11.85 years) and 62 healthy subjects without IS (age: 37.47 ± 11.95 years). We find no significant differences in gender, age, as well as educational level between two groups. Table 1 provides the demographic characteristics of the participants.
Insomnia Symptoms Measurements
The 17-item Hamilton Depression Rating Scale (HAMD-17) was used to measure the severity of participants’ IS (Hamilton, 1967), which is based on the sum of the three items on the sleep subscale of HAMD-17. A total score greater than or equal to one indicated IS. The severity of depression and anxiety in all the subjects was also assessed using HAMD-17 and the Hamilton Anxiety Rating Scale (HAMA). In the current study, the adjusted HAMD and adjusted HAMA scores were generated by omitting the insomnia-related items to prevent a potential influence of IS from these scales on our findings (Lu et al., 2017). Clinical data for the two groups are given in Table 1.
Image Acquisition
All participants were scanned with a 3T Trio MRI scanner (Siemens Medical Solutions, Erlangen, Germany) located in the National Key Laboratory for Cognitive Neuroscience and Learning, Beijing Normal University. Foam padding and earplugs were used for all subjects in order to reduce the head motion. During the data recording, all participants were instructed to completely relax without thinking of particular things, rest quietly, close their eyes, remain still and keep awake. T1-weighted, sagittal 3D magnetization-prepared rapid gradient-echo (MP-RAGE) sequences were acquired with the following scanning parameters: repetition time (TR) = 2530 ms; echo time (TE) = 3.39 ms; matrix = 256 × 256; slices = 128; flip angle = 7°; field of view (FOV) = 256 × 256 mm2; slice thickness = 1.33 mm; inter-slice gap = 0 mm; voxel size = 1 × 1 × 1.33 mm3; orientation = sagittal. DTI data were collected using a single-shot echo-planar imaging (EPI) sequence: TR = 7200 ms; TE = 104 ms; matrix = 128 × 128; slices = 49; flip angle = 90°; FOV = 128 × 128 mm2; slice thickness = 2.5 mm; inter-slice gap = 0 mm; voxel size = 1.8 × 1.8 × 2.5 mm3; orientation = axial; 64 non-collinear diffusion weighting gradient direction (b = 1000 s/mm2) and one additional image without diffusion weighting (b = 0 s/mm2).
Structural Network Construction
The structural networks were constructed for all participants. The network nodes were characterized by the brain areas divided by the AAL template (Tzourio-Mazoyer et al., 2002), whereas the network edges were defined as fiber tracts that linked with these nodes.
Definition of Network Nodes
The procedure to define network nodes was according to the previous study (Gong et al., 2009). The regions of interest (ROIs) were described in diffusion native space. In brief, each subject’s T1-weighted image was first co-registered to the non-diffusion-weighted (b = 0 s/mm2) images in the diffusion native space through a linear transformation. Then the co-registered T1 images were non-linearly converted to the ICBM-152 T1-template in the Montreal Neurological Institute (MNI) space. The 12 degrees of freedom combined with nonlinear warps were applied in this step. The inverse transformation parameter was used to warp the AAL areas from MNI space to the DTI native space by using a nearest-neighbor interpolation method based on the statistical parametric mapping (SPM8) package. Using this procedure, 90 cortical and subcortical brain areas were generated (45 for each hemisphere, see Supplementary Table S1).
WM Tractography
The following steps were carried out to reconstruct the whole-brain WM tracts. The distortions were corrected for the effects of eddy current with an affine alignment of the diffusion-weighted images to the non-diffusion-weighted images based on the FMRIB Diffusion Toolbox (FSL)1. Subsequently, the diffusion tensor matrix was generated on a voxel-by-voxel analysis, which was further diagonalized to generate three eigenvalues and associated eigenvectors. The diffusion tensor models were calculated using the linear least-squares fitting algorithm at the voxel level by using the Diffusion Toolkit (Wang et al., 2007). The DTI fiber tracking procedures were carried out in diffusion native space based on the Fiber Assignment by Continuous Tracking (FACT) method through the Diffusion Toolkit (Wang et al., 2007). All of the path tracing in the dataset terminated if either the FA of each voxel did not exceed 0.2 or the tracking angle was greater than 45 degrees (Shu et al., 2011).
Definition of Network Edges
To determine the brain network edges in the native diffusion space, an ROI i and ROI j were considered to be linked through an edge where at least one fiber was present between them (Gong et al., 2009). The connections were weighted by the mean FA values of fibers that connected these two ROIs to depict the connectivity strength between ROI i and ROI j.
Network Analysis
Threshold Selection
Each correlation matrix was thresholded into a set of undirected binary networks by applying a threshold of sparsity S, which was computed as the ratio of the total number of existing edges with the all possible total number of edges. Then the number of nodes and edges of these normalized resulting networks were the same which made it possible to explore the between-group differences in regard to network topological organization (Bullmore and Bassett, 2011). Each connectivity matrix was thresholded over a threshold range of 0.1 ≤ S ≤ 0.2 with intervals 0.01 repeatedly.
The minimum threshold (S = 0.1) was determined according to the criterion that the averaged degree of all network nodes at each thresholded should be larger than 2log(N), in which N was 90 here denotes the total number of nodes. All individual networks reached 90% full connections at the minimum threshold. The maximum threshold (S = 0.2) was computed by obtaining the individual network topological cost without being thresholded, and then selected the minimum sparsity threshold (Long et al., 2013).
Small-World Properties
To measure the small-world properties of constructed structural brain networks, we first produced 100 random networks by using a Markov-chain algorithm and each random network has the same number in regard to nodes, edges and degree distribution with a real brain network (Liao et al., 2010). A real brain network can be regarded as a small world network only if it satisfied the conditions of both γ > 1 and λ ≈ 1 (Watts and Strogatz, 1998), or sigma σ = λ/γ > 1, which indicated that a small-world network possess a higher clustering coefficient and a similar path length as compared with a random network (Humphries et al., 2006; Liu et al., 2008). Typically, we scaled the characteristic shortest path length Lp and the clustering coefficient Cp of the constructed structural networks with the averaged Lrandom and Crandom of all 100 random networks (i.e., the normalized characteristic path length, and the normalized clustering coefficient, ), where Lrandom and Crandom denote the averaged characteristic shortest path length and the averaged clustering coefficient of 100 generated random networks, respectively.
Network Metrics
To evaluate the nodal properties of cortical and subcortical brain regions in structural networks, three key measurements were computed: the nodal degree Degi, the nodal efficiency Ei, and the nodal betweenness BCi. Additionally, the global efficiency Eglo and local efficiency Eloc were used to define the network efficiency (Achard and Bullmore, 2007). We first computed the six global network properties Cp, Lp, λ, γ, Eglo, and Eloc, and three regional nodal parameters Degi, Ei and BCi. Furthermore, we computed the area under the curve (AUC) of each parameter, which denoted an integrated index for the topological organization of brain networks (Zhang Z. et al., 2011; Liu F. et al., 2016). Detailed information about the network properties is provided in the Supplementary Materials.
Statistical Analysis
Differences in the Network Properties
To show the differences of the global network topological properties between the two groups, nonparametric permutation tests (5000 iterations, p < 0.05, uncorrected) were carried out for each network topology over the threshold of 0.1 ≤ S ≤ 0.2 with intervals of 0.01 and the AUCs (Bullmore et al., 1999) of each network topology. In addition, nonparametric permutation tests (5000 iterations, p < 0.05, uncorrected) were also carried out on the AUCs of each regional nodal property to determine whether there were significant group distinctions between groups. Before performing the permutation tests, a multiple regression analysis was conducted to regress out the gender, age, educational level, adjusted HAMA score and adjusted HAMD score as dependent variables to exclude the influence of the depression and anxiety and the independent variable is the AUC of each network metric. A value of p < 0.05 was considered uncorrected significant for multiple regression analysis.
Correlations between the Network Properties and Insomnia Scores
We also examined the relationships between the global and nodal network metrics with the insomnia scores in the IS group. Pearson’s correlation analysis was performed with age, gender, educational level, adjusted HAMA score and adjusted HAMD score as unconcerned confounding factors, the AUCs of each network property as an independent variables and the insomnia scores in the IS group as dependent variables. An exploratory threshold matching value of one divided by the number of nodes (1/90 = 0.011) was adopted as a significant threshold for false-positive correction for all analyses.
Results
Demographic Data and Clinical Variables
Demographic information and clinical variables from both the IS and NIS groups are provided in Table 1. We found no significant differences between the two groups regarding age (t(91) = 0.201, p = 0.841), gender ( = 0.532, p = 0.466), and educational level (t(91) = −1.889, p = 0.065). However, both the original HAMD scores and the adjusted HAMD scores exhibited significant differences between the IS and NIS groups (p < 0.001, Table 1). The original HAMA scores and the adjusted HAMA scores also differed significantly between groups (p < 0.001, Table 1). In addition, the original and adjusted HAMD and HAMA scores in IS group are higher than that in NIS group.
Group Differences in Global Network Properties
Statistical analysis was performed to detect distinctions in the global organization of brain structural networks between the IS and NIS groups. Both groups showed prominent small-world properties for all threshold values from 0.1 to 0.2 (Figure 1), suggesting that the small-world architecture of the human brain is robust to brain aberrations or disorders (Achard et al., 2006). Importantly, the IS group exhibited an increased local efficiency and a decreased global efficiency in the anatomic brain networks as compared with NIS group (Supplementary Table S2), indicating an insomnia-related shift in the topology toward regular networks. However, no statistically significant differences between groups were revealed in regard to the measures of global properties of structural networks (Figure 1 and Supplementary Figure S1, Supplementary Table S2).
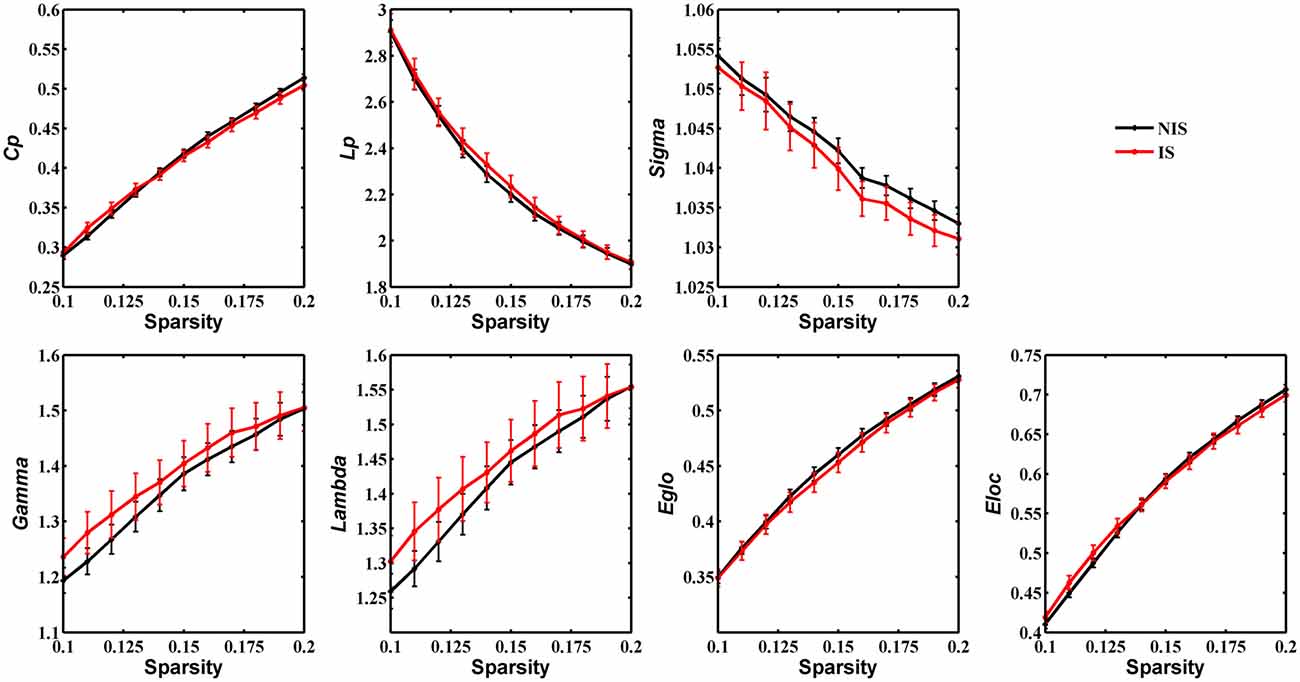
Figure 1. Group comparison of global network topological properties (Cp, Lp, Sigma, Gamma, Lambda, Eglo and Eloc) between the IS and NIS groups (5000 permutations, p < 0.05, uncorrected). The small-worldness suggests a small-world topology for brain networks of both the IS and NIS groups. The error bar represents the standard deviation (SD). Cp, clustering coefficient; Lp, characteristic path length; Eglo, global efficiency; Eloc, local efficiency; IS, healthy participants with insomnia symptoms; NIS, healthy participants without insomnia symptoms.
Alterations in Nodal Network Properties in Healthy Participants with Insomnia Symptoms
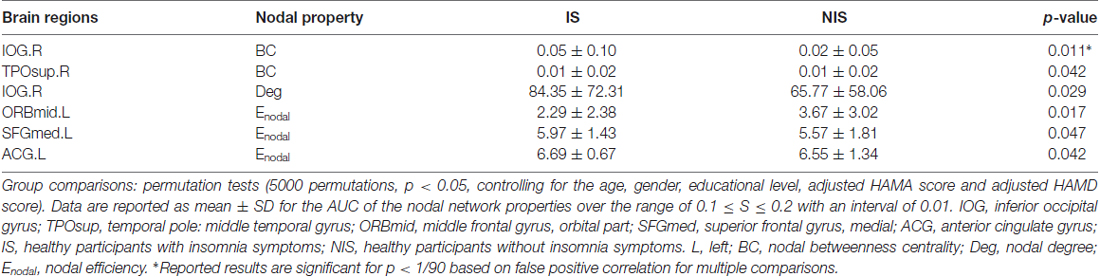
Table 2 provides the results of statistical comparisons of the nodal properties (nodal betweenness centrality, nodal degree and nodal efficiency) between the IS and NIS groups (p < 0.05, uncorrected). In comparison with the NIS group, the IS group showed significantly stronger nodal betweenness centrality over two brain regions (the right inferior occipital gyrus (IOG.R) and the right temporal pole: middle temporal gyrus (TPOsup.R)), as well as significantly larger nodal degree in one region (the IOG.R) and significantly higher nodal efficiency over two regions (the left anterior cingulate gyrus (ACG.L) and left superior frontal gyrus, medial (SFGmed.L); Figure 2 and Table 2). In addition, the subjects in the IS group exhibited decreased nodal efficiency in the orbital part of left middle frontal gyrus (ORBmid.L; Figure 2 and Table 2).
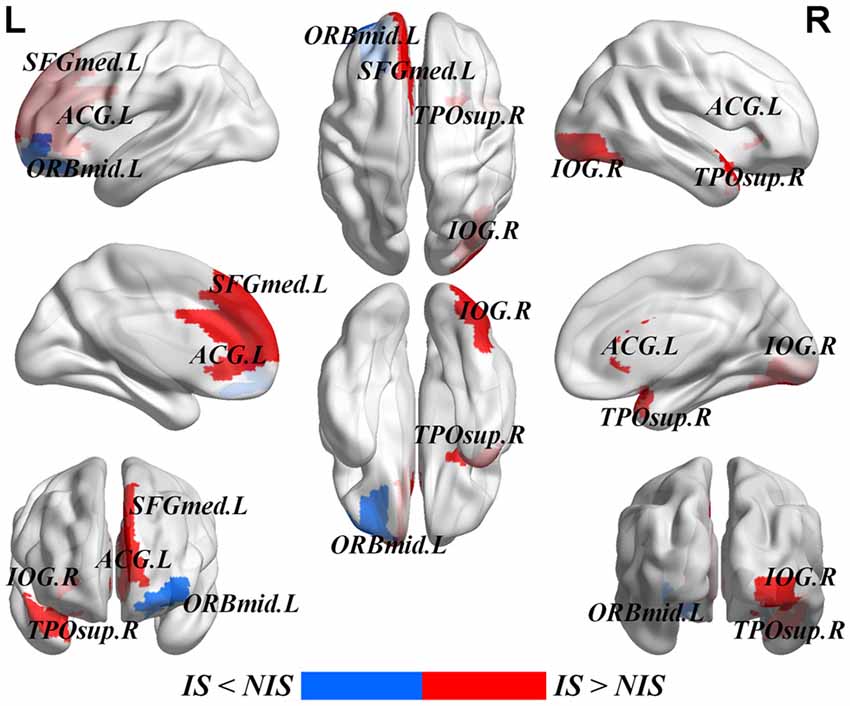
Figure 2. Brain areas with altered nodal betweenness centrality, nodal degree and nodal efficiency in IS group. Group comparisons were based on permutation tests (5000 permutations, p < 0.05, uncorrected, controlling for the age, gender, educational level, adjusted HAMA score and adjusted HAMD score). Colored brain areas displayed the significantly aberrant nodal network properties in IS group. The red and blue represent significantly increased and decreased nodal topology in IS group as compared with NIS group, respectively. The more detailed information were showed in Table 2. L, left; R, right; IS, healthy participants with insomnia symptoms; NIS, healthy participants without insomnia symptoms; IOG, inferior occipital gyrus; TPOsup, temporal pole: middle temporal gyrus; ORBmid, middle frontal gyrus, orbital part; SFGmed, superior frontal gyrus, medial; ACG, anterior cingulate gyrus. Figures were visualized based on the BrainNet Viewer software (https://www.nitrc.org/projects/bnv/).
Insomnia Was Associated with Nodal Structural Connectivity Topology of Areas Involved in Salience and Default-Mode Networks
Multiple linear regression analyses revealed no significant correlations between the global network topology and insomnia scores in the IS group. However, the nodal efficiency in the left insula (INS.L) of the salience network showed a negative correlation with insomnia scores (p < 0.011, false positive correction; Figure 3 and Table 3). In addition, the nodal betweenness centrality and the nodal degree of the right postcentral gyrus (PoCG.R) exhibited significant negative correlations with insomnia scores (p < 0.011, false positive correction; Table 3). Furthermore, the nodal betweenness of the right precuneus (PCUN.R) in the default-mode network also showed significant negative correlations with insomnia scores (p < 0.05, uncorrected; Figure 3 and Table 3). Importantly, the nodal betweenness centrality of the left heschl gyrus (HES.L; Table 3) and the nodal degree of the right insula (INS.R; Figure 3 and Table 3) showed significant positive relationships with insomnia scores (p < 0.011, false positive correction). In particular, the nodal network properties from several brain regions were also significantly related with insomnia scores, including the right middle temporal gyrus (MTG.R), IOG.R, left superior parietal gyrus (SPG.L), and right HES (HES.R; p < 0.05, uncorrected; Table 3).
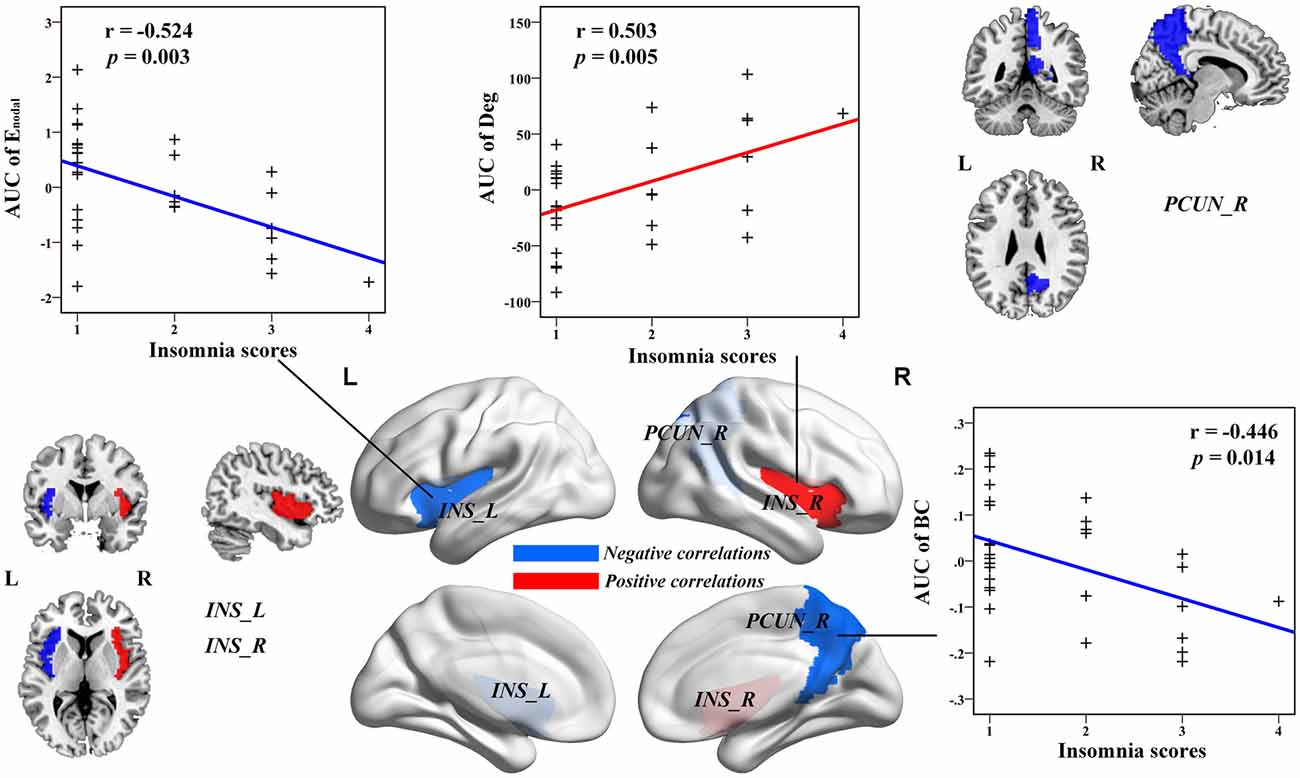
Figure 3. The Pearson correlation between the AUC of the nodal network properties with insomnia scores in healthy participants with insomnia symptoms (p < 0.05, uncorrected, controlling for the age, gender, educational level, adjusted HAMA scores and adjusted HAMD score). The AUC of each nodal topology was computed over the range of 0.1 ≤ S ≤ 0.2 with an interval of 0.01. The red color stands for the positive correlation while the blue color reveals the negative correlation. For more detailed information see Table 3. L, left; R, right; AUC, area under the curve; BC, nodal betweenness centrality; Enodal, nodal efficiency; INS, insula; PCUN, precuneus.
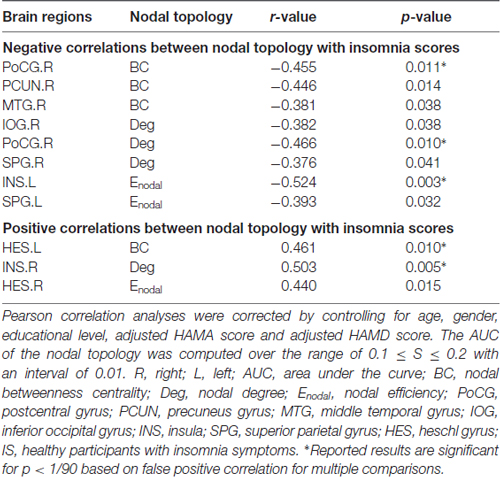
Table 3. Significant correlations between nodal network properties and the insomnia scores in IS group.
Discussion
This study examined the topology organization of WM networks in insomnia using DTI tractography and graph theory analysis. We discovered that: (1) both the two groups exhibited optimized small-world organization with respect to their WM structural networks; (2) the IS group showed a lower global efficiency and a higher local efficiency than that of the NIS group, illustrating an insomnia-related shift of the topology towards regular networks; (3) the IS group manifested altered nodal brain structural network properties (nodal betweenness centrality, nodal degree and nodal efficiency) in fronto-limbic pathways including the SFGmed.L, the ORBmid.L and the ACG.L; and (4) the salience and default-mode networks showed correlations with insomnia scores. The insomnia scores were negatively associated with the nodal efficiency of the INS.L of the salience network and the nodal betweenness centrality of the PCUN.R of the default-mode network. These findings revealed large-scale topological organization substrates of the structural network, which could provide novel tools for better understanding of the neural circuitry that underlies insomnia. Our results were not explained by gender, age or educational level, which were controlled for during the group comparison analysis. The current results also removed the influence of adjusted HAMD scores and adjusted HAMA scores, suggesting that insomnia rather than depression or anxiety is associated with an altered topology of the fronto-limbic and salience network and default-mode network structural connectivity.
The small-worldness is an important topological property that demonstrates two fundamental organizations of functional segregation as well as functional integration. Functional segregation is associated with specialized processing within densely interconnected brain regions, and functional integration characterizes the ability of information communication between distributed brain areas (Rubinov and Sporns, 2010; Bullmore and Bassett, 2011). Interestingly, we demonstrated that both the healthy participants with IS and those without IS showed economic small-world topology with respect to their large-scale brain WM structural connectivity networks.
However, despite the common small-world topology organizations, the IS and NIS groups exhibited significant differences in nodal topological characteristics. Specifically, increased nodal properties in the IS group were identified in mainly the frontal and temporal lobes (e.g., the SFGmed.L and the TPOsup.R) compared with the NIS group (Table 2). In addition, the nodal network properties in MTG.R in the IS group also showed significant correlations with insomnia scores. The frontal regions are known to play important roles in memory, executive functions and emotion processing (Stuss and Alexander, 2000; Baddeley, 2003). A whole-brain voxel-based morphometry (VBM) study on 24 insomnia patients revealed reduced volume in the left orbitofrontal cortex (Altena et al., 2010). Drummond et al. (2013) demonstrated that individuals with insomnia exhibited decreased activations in the frontal regions when performing working memory tasks. In addition, Li C. et al. (2016) suggested that the insomnia patients had decreased amplitude of low-frequency fluctuation (ALFF) values in the left orbitofrontal cortex and right middle frontal gyrus during resting-state.
Another ALFF study showed significantly decreased amplitudes in the prefrontal cortex and default-mode network sub-regions (Zhou et al., 2016). The temporal lobe structures were recognized to be responsible for disturbed sleep or dyssomnia (Van Sweden, 1996). A recent study highlighted that individuals with poor sleep quality showed increased rates of atrophy within the frontal, temporal and parietal regions (Sexton et al., 2014). Another study showed decreased local coherence in the right temporal, parietal and frontal lobe regions in obstructive sleep apnea (OSA) patients (Santarnecchi et al., 2013). More importantly, a DTI study observed widespread WM integrity alteration, which includes axons linking brain structures within the limbic system, frontal and temporal cortices in OSA (Macey et al., 2008). Furthermore, studies based on VBM approaches demonstrated that the gray matter concentration was significantly decreased in both the cortical and subcortical brain regions, including the fronto-parietal cortices, temporal lobe and anterior cingulate cortex in OSA patients compared to healthy volunteers (Joo et al., 2010; Torelli et al., 2011). However, these discrepancies in frontal and temporal lobe analyses could be caused by the different subjects and imaging methods used (e.g., fMRI, EEG, structural MRI and DTI).
The present method was the first to use the DTI tractography to inspect the small-world changes in WM network in insomnia. Our findings indicated that the alterations of WM structural connectivity in frontal and temporal regions could influence information communication and functional integration for insomnia. In addition, increased nodal properties in the IS group were found in the default-mode network region, including the ACG.L. The ACG is the important part of the limbic system, which is implicated in regulating cognitive and emotional processing (Bush et al., 2000). The ventral ACG is also part of the default-mode network (Margulies et al., 2007). The anterior cingulate areas have extensive connections with the INS, prefrontal cortex, amygdala, hypothalamus and brainstem (Margulies et al., 2007; Cersosimo and Benarroch, 2013). Recently, there has been increasing evidence obtained using positron emission tomography and glucose metabolism from fludeoxyglucose shows that the ACG plays an essential role in the regulation of normal sleep, including between sleep and wake, during sleep deprivation, as well as across sleep stages in humans (Braun et al., 1997; Nofzinger et al., 1997, 2002; Thomas et al., 2000). In addition, using VBM, Winkelman et al. (2013) have found increased rostral ACG in PI patients compared to good-sleeper controls. Using graph theoretical analysis, our findings indicated WM alterations of the structural connections in the ACG. The increased nodal efficiency in the ACG in insomnia may reflect a compensatory response to repetitive sleep disturbance.
The INS is thought to be a key hub of the salience network (Kelly et al., 2012; Tahmasian et al., 2016) and is responsible for the detection of salience, making decision, emotion judgment, attention modulation, motor/sensory processes and cognition regulation (Menon and Uddin, 2010; Cauda et al., 2012; Uddin, 2015). It can be divided into two core parts: one is the anterior insula which is linked with the frontal and parietal cortex, ACG, and limbic regions. It is mainly responsible for salience detection and other emotional processes. Another is the posterior insula which is associated with the sensorimotor, temporal, premotor and posterior cingulate regions. It plays a critical role in perception processing, emotion regulation, interoception and sensorimotor integration (Cauda et al., 2011, 2012). Previous studies have observed insula abnormalities in insomnia patients. For example, Liu C.-H. et al. (2016) observed a decreased fractional ALFF in the INS, indicating the misperception and hyperarousal during sleep state in insomnia patients. Further, Huang et al. (2012) reported decreased functional connections mainly between the INS and the amygdala as well as between the thalamus and striatum in PI.
Our study also showed that decreased nodal efficiency of the left INS was associated with increased insomnia scores. However, Chen et al. (2014) showed an increased activation in the anterior INS with the salience network in female insomnia patients. Recently, Li et al. (2014) found that the PI group exhibited strong connectivity between the right INS and the bilateral superior parietal lobe. In the current study, we discovered that the increased right INS in the nodal degree was related with increased insomnia scores. These discrepant results may due to a result of different sample size of the insomnia patients, gender distinction, potential confounding variables that were controlled for in different studies, or methodological differences. Our findings regarding different changes in the left INS and right INS may provide new evidence that they play different roles in information processing in insomnia. We demonstrated that the INS could be an important neural marker for the hyperarousal pathophysiology underlying insomnia. Taken together, our findings suggest that IS may disrupt the role of the INS in maintaining the functions of alertness and cognitive processing.
Some limitations need to be considered. First, we did not use the Pittsburgh Sleep Quality Index or Duke structured interview to measure the IS. We applied the three-item sleep subscale based on HAMD-17 instead since it is better associated with sleep diaries (Manber et al., 2005). Second, the false-positive correction (1/number of regions) was used in this study, which was not as conservative as the false discovery rate (FDR) correction. Third, we divided the whole brain into 90 sub-regions based on the AAL atlas to construct the brain large-scale structural network. However, previous studies suggest that different parcellation strategies may result in distinct network topological properties (Fornito et al., 2010; Sanabria-Diaz et al., 2010). Therefore, it is necessary to apply a more precise parcellation strategy to provide the information for the brain network topology alterations in insomnia. Fourth, the voxel size of DTI data was not isotropic in the present study which may cause underestimate FA values in brain regions with crossing fibers (Oouchi et al., 2007), which may influence the structural connectivity network. Finally, future studies should employ a high b-value diffusion-weighted acquisition sequence and streamline tractography to estimate structural connectivity and model WM architecture.
Conclusion
We applied DTI tractography combined with graph theory approaches to explore the abnormalities of topological organization in WM structural networks of subjects with IS. Both the healthy subjects with IS and those without IS showed small-world organization. However, the insomnia group showed altered regional network properties in the fronto-limbic system. The salience and default-mode networks were also strongly linked with insomnia. Our results demonstrated a disrupted WM network integrity and thus provided structural insights into the insomnia connectome. Importantly, certain structural networks can provide important implications for understanding the brain structural connectome in insomnia.
Author Contributions
F-ML, JD, C-HL, H-FC, M-XH and ZY conceived and designed the experiments. C-HL, S-LL, L-RT and C-LT acquired the data, which F-ML, HC and ZY analyzed. F-ML, JD, TAC, Y-TX and ZY wrote the article, which all authors reviewed and approved for submission.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors gratefully appreciate the volunteers for participating in this study, the staffs from the Beijing Normal University Imaging Center for Brain Research and Prof. Yu-Feng Zang for their contributions in recruitment and data acquisition. This work was supported by MYRG2015-00036-FHS and MYRG2016-00110-FHS grants from the University of Macau and FDCT 026/2014/A1 and FDCT 025/2015/A1 grants from Macao government in Macau. This work was also supported by the National Natural Science Foundation of China (Grant No. 81471389), and the High Level Personnel Health System in Beijing City (Grant No. 2014-3-095).
Footnotes
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2017.00583/full#supplementary-material
References
Achard, S., and Bullmore, E. (2007). Efficiency and cost of economical brain functional networks. PLoS Comput. Biol. 3:e17. doi: 10.1371/journal.pcbi.0030017
Achard, S., Salvador, R., Whitcher, B., Suckling, J., and Bullmore, E. (2006). A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J. Neurosci. 26, 63–72. doi: 10.1523/jneurosci.3874-05.2006
Altena, E., Van Der Werf, Y. D., Sanz-Arigita, E. J., Voorn, T. A., Rombouts, S., Kuijer, J., et al. (2008). Prefrontal hypoactivation and recovery in insomnia. Sleep 31, 1271–1276.
Altena, E., Vrenken, H., Van Der Werf, Y. D., van den Heuvel, O. A., and Van Someren, E. J. (2010). Reduced orbitofrontal and parietal gray matter in chronic insomnia: a voxel-based morphometric study. Biol. Psychiatry 67, 182–185. doi: 10.1016/j.biopsych.2009.08.003
Baddeley, A. (2003). Working memory: looking back and looking forward. Nat. Rev. Neurosci. 4, 829–839. doi: 10.1038/nrn1201
Baglioni, C., Spiegelhalder, K., Regen, W., Feige, B., Nissen, C., Lombardo, C., et al. (2014). Insomnia disorder is associated with increased amygdala reactivity to insomnia-related stimuli. Sleep 37, 1907–1917. doi: 10.5665/sleep.4240
Basser, P. J., Pajevic, S., Pierpaoli, C., Duda, J., and Aldroubi, A. (2000). in vivo fiber tractography using DT-MRI data. Magn. Reson. Med. 44, 625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o
Benca, R. M., and Peterson, M. J. (2008). Insomnia and depression. Sleep Med. 9, S3–S9. doi: 10.1016/S1389-9457(08)70010-8
Braun, A., Balkin, T., Wesenten, N., Carson, R., Varga, M., Baldwin, P., et al. (1997). Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain 120, 1173–1197. doi: 10.1093/brain/120.7.1173
Bullmore, E. T., and Bassett, D. S. (2011). Brain graphs: graphical models of the human brain connectome. Annu. Rev. Clin. Psychol. 7, 113–140. doi: 10.1146/annurev-clinpsy-040510-143934
Bullmore, E., and Sporns, O. (2009). Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 10, 186–198. doi: 10.1038/nrn2575
Bullmore, E. T., Suckling, J., Overmeyer, S., Rabe-Hesketh, S., Taylor, E., and Brammer, M. J. (1999). Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans. Med. Imaging 18, 32–42. doi: 10.1109/42.750253
Bush, G., Luu, P., and Posner, M. I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 4, 215–222. doi: 10.1016/s1364-6613(00)01483-2
Cauda, F., Costa, T., Torta, D. M. E., Sacco, K., D’Agata, F., Duca, S., et al. (2012). Meta-analytic clustering of the insular cortex: characterizing the meta-analytic connectivity of the insula when involved in active tasks. Neuroimage 62, 343–355. doi: 10.1016/j.neuroimage.2012.04.012
Cauda, F., D’Agata, F., Sacco, K., Duca, S., Geminiani, G., and Vercelli, A. (2011). Functional connectivity of the insula in the resting brain. Neuroimage 55, 8–23. doi: 10.1016/j.neuroimage.2010.11.049
Cersosimo, M. G., and Benarroch, E. E. (2013). “Chapter 5—central control of autonomic function and involvement in neurodegenerative disorders,” in Handbook of Clinical Neurology, eds M. B. Ruud and F. S. Dick (Amsterdam: Elsevier), 45–57.
Chen, M. C., Chang, C., Glover, G. H., and Gotlib, I. H. (2014). Increased insula coactivation with salience networks in insomnia. Biol. Psychol. 97, 1–8. doi: 10.1016/j.biopsycho.2013.12.016
Cheung, J. M., Bartlett, D. J., Armour, C. L., and Saini, B. (2013). The insomnia patient perspective, a narrative review. Behav. Sleep Med. 11, 369–389. doi: 10.1080/15402002.2012.694382
Drummond, S. P., Smith, M. T., Orff, H. J., Chengazi, V., and Perlis, M. L. (2004). Functional imaging of the sleeping brain: review of findings and implications for the study of insomnia. Sleep Med. Rev. 8, 227–242. doi: 10.1016/j.smrv.2003.10.005
Drummond, S., Walker, M., Almklov, E., Campos, M., Anderson, D. E., and Straus, L. D. (2013). Neural correlates of working memory performance in primary insomnia. Sleep 36, 1307–1316. doi: 10.5665/sleep.2952
Fornito, A., Zalesky, A., and Bullmore, E. T. (2010). Network scaling effects in graph analytic studies of human resting-state FMRI data. Front. Syst. Neurosci. 4:22. doi: 10.3389/fnsys.2010.00022
Gong, G., He, Y., Concha, L., Lebel, C., Gross, D. W., Evans, A. C., et al. (2009). Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography. Cereb. Cortex 19, 524–536. doi: 10.1093/cercor/bhn102
Greicius, M. D., Supekar, K., Menon, V., and Dougherty, R. F. (2009). Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb. Cortex 19, 72–78. doi: 10.1093/cercor/bhn059
Guo, W.-B., Liu, F., Chen, J.-D., Xu, X.-J., Wu, R.-R., Ma, C.-Q., et al. (2012). Altered white matter integrity of forebrain in treatment-resistant depression: a diffusion tensor imaging study with tract-based spatial statistics. Prog. Neuropsychopharmacol. Biol. Psychiatry 38, 201–206. doi: 10.1016/j.pnpbp.2012.03.012
Guo, W., Liu, F., Liu, Z., Gao, K., Xiao, C., Chen, H., et al. (2012). Right lateralized white matter abnormalities in first-episode, drug-naive paranoid schizophrenia. Neurosci. Lett. 531, 5–9. doi: 10.1016/j.neulet.2012.09.033
Hagmann, P., Cammoun, L., Gigandet, X., Meuli, R., Honey, C. J., Wedeen, V. J., et al. (2008). Mapping the structural core of human cerebral cortex. PLoS Biol. 6:e159. doi: 10.1371/journal.pbio.0060159
Hamilton, M. (1967). Development of a rating scale for primary depressive illness. Br. J. Soc. Clin. Psychol. 6, 278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x
Honey, C. J., Sporns, O., Cammoun, L., Gigandet, X., Thiran, J. P., Meuli, R., et al. (2009). Predicting human resting-state functional connectivity from structural connectivity. Proc. Natl. Acad. Sci. U S A 106, 2035–2040. doi: 10.1073/pnas.0811168106
Huang, Z., Liang, P., Jia, X., Zhan, S., Li, N., Ding, Y., et al. (2012). Abnormal amygdala connectivity in patients with primary insomnia: evidence from resting state fMRI. Eur. J. Radiol. 81, 1288–1295. doi: 10.1016/j.ejrad.2011.03.029
Humphries, M. D., Gurney, K., and Prescott, T. J. (2006). The brainstem reticular formation is a small-world, not scale-free, network. Proc. Biol. Sci. 273, 503–511. doi: 10.1098/rspb.2005.3354
Joo, E. Y., Kim, H., Suh, S., and Hong, S. B. (2014). Hippocampal substructural vulnerability to sleep disturbance and cognitive impairment in patients with chronic primary insomnia: magnetic resonance imaging morphometry. Sleep 37, 1189–1198. doi: 10.5665/sleep.3836
Joo, E. Y., Tae, W. S., Lee, M. J., Kang, J. W., Park, H. S., Lee, J. Y., et al. (2010). Reduced brain gray matter concentration in patients with obstructive sleep apnea syndrome. Sleep 33, 235–241. doi: 10.1093/sleep/33.2.235
Kaneita, Y., Ohida, T., Uchiyama, M., Takemura, S., Kawahara, K., Yokoyama, E., et al. (2006). The relationship between depression and sleep disturbances: a Japanese nationwide general population survey. J. Clin. Psychiatry 67, 196–203. doi: 10.4088/JCP.v67n0204
Kelly, C., Toro, R., Di Martino, A., Cox, C. L., Bellec, P., Castellanos, F. X., et al. (2012). A convergent functional architecture of the insula emerges across imaging modalities. Neuroimage 61, 1129–1142. doi: 10.1016/j.neuroimage.2012.03.021
Kronholm, E., Puusniekka, R., Jokela, J., Villberg, J., Urrila, A. S., Paunio, T., et al. (2015). Trends in self-reported sleep problems, tiredness and related school performance among Finnish adolescents from 1984 to 2011. J. Sleep Res. 24, 3–10. doi: 10.1111/jsr.12258
Kucharczyk, E. R., Morgan, K., and Hall, A. P. (2012). The occupational impact of sleep quality and insomnia symptoms. Sleep Med. Rev. 16, 547–559. doi: 10.1016/j.smrv.2012.01.005
Li, C., Ma, X., Dong, M., Yin, Y., Hua, K., Li, M., et al. (2016). Abnormal spontaneous regional brain activity in primary insomnia: a resting-state functional magnetic resonance imaging study. Neuropsychiatr. Dis. Treat. 12, 1371–1378. doi: 10.2147/NDT.s109633
Li, S., Tian, J., Bauer, A., Huang, R., Wen, H., Li, M., et al. (2016). Reduced integrity of right lateralized white matter in patients with primary Insomnia: a diffusion-tensor imaging study. Radiology 280, 520–528. doi: 10.1148/radiol.2016152038
Li, Y., Wang, E., Zhang, H., Dou, S., Liu, L., Tong, L., et al. (2014). Functional connectivity changes between parietal and prefrontal cortices in primary insomnia patients: evidence from resting-state fMRI. Eur. J. Med. Res. 19:32. doi: 10.1186/2047-783x-19-32
Lian, Y., Xiao, J., Liu, Y., Ning, L., Guan, S., Ge, H., et al. (2015). Associations between insomnia, sleep duration and poor work ability. J. Psychosom. Res. 78, 45–51. doi: 10.1016/j.jpsychores.2014.09.009
Liao, W., Chen, H., Feng, Y., Mantini, D., Gentili, C., Pan, Z., et al. (2010). Selective aberrant functional connectivity of resting state networks in social anxiety disorder. Neuroimage 52, 1549–1558. doi: 10.1016/j.neuroimage.2010.05.010
Liu, Y., Liang, M., Zhou, Y., He, Y., Hao, Y., Song, M., et al. (2008). Disrupted small-world networks in schizophrenia. Brain 131, 945–961. doi: 10.1093/brain/awn018
Liu, C.-H., Liu, C.-Z., Zhang, J., Yuan, Z., Tang, L.-R., Tie, C.-L., et al. (2016). Reduced spontaneous neuronal activity in the insular cortex and thalamus in healthy adults with insomnia symptoms. Brain Res. 1648, 317–324. doi: 10.1016/j.brainres.2016.07.024
Liu, F., Zhuo, C., and Yu, C. (2016). Altered cerebral blood flow covariance network in schizophrenia. Front. Neurosci. 10:308. doi: 10.3389/fnins.2016.00308
Lo, C. Y., Wang, P. N., Chou, K. H., Wang, J., He, Y., and Lin, C. P. (2010). Diffusion tensor tractography reveals abnormal topological organization in structural cortical networks in Alzheimer’s disease. J. Neurosci. 30, 16876–16885. doi: 10.1523/JNEUROSCI.4136-10.2010
Long, Z., Duan, X., Wang, Y., Liu, F., Zeng, L., Zhao, J.-P., et al. (2015). Disrupted structural connectivity network in treatment-naive depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 56, 18–26. doi: 10.1016/j.pnpbp.2014.07.007
Long, Z., Duan, X., Xie, B., Du, H., Li, R., Xu, Q., et al. (2013). Altered brain structural connectivity in post-traumatic stress disorder: a diffusion tensor imaging tractography study. J. Affect. Disord. 150, 798–806. doi: 10.1016/j.jad.2013.03.004
Lu, F.-M., Liu, C.-H., Lu, S.-L., Tang, L.-R., Tie, C.-L., Zhang, J., et al. (2017). Disrupted topology of frontostriatal circuits is linked to the severity of insomnia. Front. Neurosci. 11:214. doi: 10.3389/fnins.2017.00214
Macey, P. M., Kumar, R., Woo, M. A., Valladares, E. M., Yan-Go, F. L., and Harper, R. M. (2008). Brain structural changes in obstructive sleep apnea. Sleep 31, 967–977. doi: 10.5665/sleep/31.7.967
Manber, R., Blasey, C., Arnow, B., Markowitz, J. C., Thase, M. E., Rush, A. J., et al. (2005). Assessing insomnia severity in depression: comparison of depression rating scales and sleep diaries. J. Psychiatr. Res. 39, 481–488. doi: 10.1016/j.jpsychires.2004.12.003
Margulies, D. S., Kelly, A. C., Uddin, L. Q., Biswal, B. B., Castellanos, F. X., and Milham, M. P. (2007). Mapping the functional connectivity of anterior cingulate cortex. Neuroimage 37, 579–588. doi: 10.1016/j.neuroimage.2007.05.019
Mayer, G., Jennum, P., Riemann, D., and Dauvilliers, Y. (2011). Insomnia in central neurologic diseases—occurrence and management. Sleep Med. Rev. 15, 369–378. doi: 10.1016/j.smrv.2011.01.005
Menon, V., and Uddin, L. Q. (2010). Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 214, 655–667. doi: 10.1007/s00429-010-0262-0
Moore, M. (2012). Looking for a good night’s sleep. Lancet 380, 322–323. doi: 10.1016/s0140-6736(12)61220-3
Morin, C. M., and Benca, R. (2012). Chronic insomnia. Lancet 379, 1129–1141. doi: 10.1016/S0140-6736(11)60750-2
Morin, C. M., Drake, C. L., Harvey, A. G., Krystal, A. D., Manber, R., Riemann, D., et al. (2015). Insomnia disorder. Nat. Rev. Dis. Primers 1:15026. doi: 10.1038/nrdp.2015.37
Nofzinger, E. A., Buysse, D. J., Germain, A., Price, J. C., Miewald, J. M., and Kupfer, D. J. (2004). Functional neuroimaging evidence for hyperarousal in insomnia. Am. J. Psychiatry 161, 2126–2128. doi: 10.1176/appi.ajp.161.11.2126
Nofzinger, E. A., Buysse, D. J., Miewald, J. M., Meltzer, C. C., Price, J. C., Sembrat, R. C., et al. (2002). Human regional cerebral glucose metabolism during non-rapid eye movement sleep in relation to waking. Brain 125, 1105–1115. doi: 10.1093/brain/awf103
Nofzinger, E. A., Mintun, M. A., Wiseman, M., Kupfer, D. J., and Moore, R. Y. (1997). Forebrain activation in REM sleep: an FDG PET study. Brain Res. 770, 192–201. doi: 10.1016/s0006-8993(97)00807-x
Ohayon, M. M. (2002). Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med. Rev. 6, 97–111. doi: 10.1053/smrv.2002.0186
Ohayon, M. M., and Hong, S.-C. (2006). Prevalence of major depressive disorder in the general population of South Korea. J. Psychiatr. Res. 40, 30–36. doi: 10.1016/j.jpsychires.2005.02.003
Oouchi, H., Yamada, K., Sakai, K., Kizu, O., Kubota, T., Ito, H., et al. (2007). Diffusion anisotropy measurement of brain white matter is affected by voxel size: underestimation occurs in areas with crossing fibers. Am. J. Neuroradiol. 28, 1102–1106. doi: 10.3174/ajnr.a0488
Riedner, B. A., Goldstein, M. R., Plante, D. T., Rumble, M. E., Ferrarelli, F., Tononi, G., et al. (2016). Regional patterns of elevated α and high-frequency electroencephalographic activity during nonrapid eye movement sleep in chronic insomnia: a pilot study. Sleep 39, 801–812. doi: 10.5665/sleep.5632
Riemann, D., Nissen, C., Palagini, L., Otte, A., Perlis, M. L., and Spiegelhalder, K. (2015). The neurobiology, investigation, and treatment of chronic insomnia. Lancet Neurol. 14, 547–558. doi: 10.1016/s1474-4422(15)00021-6
Riemann, D., Voderholzer, U., Spiegelhalder, K., Hornyak, M., Buysse, D. J., Nissen, C., et al. (2007). Chronic insomnia and MRI-measured hippocampal volumes: a pilot study. Sleep 30, 955–958. doi: 10.1093/sleep/30.8.955
Rubinov, M., and Sporns, O. (2010). Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52, 1059–1069. doi: 10.1016/j.neuroimage.2009.10.003
Rubinov, M., Sporns, O., van Leeuwen, C., and Breakspear, M. (2009). Symbiotic relationship between brain structure and dynamics. BMC Neurosci. 10:55. doi: 10.1186/1471-2202-10-55
Sanabria-Diaz, G., Melie-García, L., Iturria-Medina, Y., Alemán-Gómez, Y., Hernández-González, G., Valdés-Urrutia, L., et al. (2010). Surface area and cortical thickness descriptors reveal different attributes of the structural human brain networks. Neuroimage 50, 1497–1510. doi: 10.1016/j.neuroimage.2010.01.028
Santarnecchi, E., Sicilia, I., Richiardi, J., Vatti, G., Polizzotto, N. R., Marino, D., et al. (2013). Altered cortical and subcortical local coherence in obstructive sleep apnea: a functional magnetic resonance imaging study. J. Sleep Res. 22, 337–347. doi: 10.1111/jsr.12006
Sexton, C. E., Storsve, A. B., Walhovd, K. B., Johansen-Berg, H., and Fjell, A. M. (2014). Poor sleep quality is associated with increased cortical atrophy in community-dwelling adults. Neurology 83, 967–973. doi: 10.1212/WNL.0000000000000774
Short, M. A., Gradisar, M., Lack, L. C., and Wright, H. R. (2013). The impact of sleep on adolescent depressed mood, alertness and academic performance. J. Adolesc. 36, 1025–1033. doi: 10.1016/j.adolescence.2013.08.007
Shu, N., Liu, Y., Li, K., Duan, Y., Wang, J., Yu, C., et al. (2011). Diffusion tensor tractography reveals disrupted topological efficiency in white matter structural networks in multiple sclerosis. Cereb. Cortex 21, 2565–2577. doi: 10.1093/cercor/bhr039
Spiegelhalder, K., Regen, W., Baglioni, C., Nissen, C., Riemann, D., and Kyle, S. D. (2015). Neuroimaging insights into insomnia. Curr. Neurol. Neurosci. Rep. 15:9. doi: 10.1007/s11910-015-0527-3
Spiegelhalder, K., Regen, W., Baglioni, C., Riemann, D., and Winkelman, J. W. (2013). Neuroimaging studies in insomnia. Curr. Psychiatry Rep. 15:405. doi: 10.1007/s11920-013-0405-0
Spiegelhalder, K., Regen, W., Prem, M., Baglioni, C., Nissen, C., Feige, B., et al. (2014). Reduced anterior internal capsule white matter integrity in primary insomnia. Hum. Brain Mapp. 35, 3431–3438. doi: 10.1002/hbm.22412
Stoffers, D., Altena, E., van der Werf, Y. D., Sanz-Arigita, E. J., Voorn, T. A., Astill, R. G., et al. (2014). The caudate: a key node in the neuronal network imbalance of insomnia? Brain 137, 610–620. doi: 10.1093/brain/awt329
Stuss, D. T., and Alexander, M. P. (2000). Executive functions and the frontal lobes: a conceptual view. Psychol. Res. 63, 289–298. doi: 10.1007/s004269900007
Tahmasian, M., Rosenzweig, I., Eickhoff, S. B., Sepehry, A. A., Laird, A. R., Fox, P. T., et al. (2016). Structural and functional neural adaptations in obstructive sleep apnea: an activation likelihood estimation meta-analysis. Neurosci. Biobehav. Rev. 65, 142–156. doi: 10.1016/j.neubiorev.2016.03.026
Taylor, D. J., Lichstein, K. L., Durrence, H. H., Reidel, B. W., and Bush, A. J. (2005). Epidemiology of insomnia, depression, and anxiety. Sleep 28, 1457–1464. doi: 10.1093/sleep/28.11.1457
Thomas, M., Sing, H., Belenky, G., Holcomb, H., Mayberg, H., Dannals, R., et al. (2000). Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J. Sleep Res. 9, 335–352. doi: 10.1046/j.1365-2869.2000.00225.x
Torelli, F., Moscufo, N., Garreffa, G., Placidi, F., Romigi, A., Zannino, S., et al. (2011). Cognitive profile and brain morphological changes in obstructive sleep apnea. Neuroimage 54, 787–793. doi: 10.1016/j.neuroimage.2010.09.065
Tzourio-Mazoyer, N., Landeau, B., Papathanassiou, D., Crivello, F., Etard, O., Delcroix, N., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289. doi: 10.1006/nimg.2001.0978
Uddin, L. Q. (2015). Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 16, 55–61. doi: 10.1038/nrn3857
van den Heuvel, M., Mandl, R., Luigjes, J., and Hulshoff Pol, H. (2008). Microstructural organization of the cingulum tract and the level of default mode functional connectivity. J. Neurosci. 28, 10844–10851. doi: 10.1523/JNEUROSCI.2964-08.2008
Wang, R., Beener, T., Sorensen, A., and Weeden, V. (2007). Diffusion toolkit: a software package for diffusion imaging data processing and tractography. Proc. Int. Soc. Magn. Reson. Med. 15:3720. Available online at: http://www.trackvis.org/faq/2007_ISMRM_diffusion_toolkit.pdf
Watts, D. J., and Strogatz, S. H. (1998). Collective dynamics of ‘small-world’ networks. Nature 393, 440–442. doi: 10.1038/30918
Winkelman, J. W., Plante, D. T., Schoerning, L., Benson, K., Buxton, O. M., O’Connor, S. P., et al. (2013). Increased rostral anterior cingulate cortex volume in chronic primary insomnia. Sleep 36, 991–998. doi: 10.5665/sleep.2794
Wulff, K., Gatti, S., Wettstein, J. G., and Foster, R. G. (2010). Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat. Rev. Neurosci. 11, 589–599. doi: 10.1038/nrn2868
Zhang, Z., Liao, W., Chen, H., Mantini, D., Ding, J. R., Xu, Q., et al. (2011). Altered functional-structural coupling of large-scale brain networks in idiopathic generalized epilepsy. Brain 134, 2912–2928. doi: 10.1093/brain/awr223
Zhang, J., Wang, J., Wu, Q., Kuang, W., Huang, X., He, Y., et al. (2011). Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biol. Psychiatry 70, 334–342. doi: 10.1016/j.biopsych.2011.05.018
Keywords: insomnia, diffusion tensor imaging tractography, white matter, graph theoretical analysis, small-world network
Citation: Lu F-M, Dai J, Couto TA, Liu C-H, Chen H, Lu S-L, Tang L-R, Tie C-L, Chen H-F, He M-X, Xiang Y-T and Yuan Z (2017) Diffusion Tensor Imaging Tractography Reveals Disrupted White Matter Structural Connectivity Network in Healthy Adults with Insomnia Symptoms. Front. Hum. Neurosci. 11:583. doi: 10.3389/fnhum.2017.00583
Received: 06 September 2017; Accepted: 17 November 2017;
Published: 30 November 2017.
Edited by:
Xiaolin Zhou, Peking University, ChinaReviewed by:
Feng Liu, Tianjin Medical University General Hospital, ChinaMarc Seal, Murdoch Childrens Research Institute, Australia
Copyright © 2017 Lu, Dai, Couto, Liu, Chen, Lu, Tang, Tie, Chen, He, Xiang and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhen Yuan, zhenyuan@umac.mo
† These authors have contributed equally to this work.
 Feng-Mei Lu
Feng-Mei Lu Jing Dai2,3†
Jing Dai2,3†  Chun-Hong Liu
Chun-Hong Liu Heng Chen
Heng Chen Hua-Fu Chen
Hua-Fu Chen Zhen Yuan
Zhen Yuan