1
Weldon School of Biomedical Engineering, Purdue University, West Lafayette, IN, USA
2
Department of Materials Science and Engineering, University of Michigan, Ann Arbor, MI, USA
3
Biotectix, LCC, Ann Arbor, MI, USA
4
Department of Biomedical Engineering, University of Michigan, Ann Arbor, MI, USA
5
Department of Biological Sciences, Purdue University, West Lafayette, IN, USA
Chronic microstimulation-based devices are being investigated to treat conditions such as blindness, deafness, pain, paralysis, and epilepsy. Small-area electrodes are desired to achieve high selectivity. However, a major trade-off with electrode miniaturization is an increase in impedance and charge density requirements. Thus, the development of novel materials with lower interfacial impedance and enhanced charge storage capacity is essential for the development of micro-neural interface-based neuroprostheses. In this report, we study the use of conducting polymer poly(3,4-ethylenedioxythiophene) (PEDOT) as a neural interface material for microstimulation of small-area iridium electrodes on silicon-substrate arrays. Characterized by electrochemical impedance spectroscopy, electrodeposition of PEDOT results in lower interfacial impedance at physiologically relevant frequencies, with the 1 kHz impedance magnitude being 23.3 ± 0.7 kΩ, compared to 113.6 ± 3.5 kΩ for iridium oxide (IrOx) on 177 μm2 sites. Further, PEDOT exhibits enhanced charge storage capacity at 75.6 ± 5.4 mC/cm2 compared to 28.8 ± 0.3 mC/cm2 for IrOx, characterized by cyclic voltammetry (50 mV/s). These improvements at the electrode interface were corroborated by observation of the voltage excursions that result from constant current pulsing. The PEDOT coatings provide both a lower amplitude voltage and a more ohmic representation of the applied current compared to IrOx. During repetitive pulsing, PEDOT-coated electrodes show stable performance and little change in electrical properties, even at relatively high current densities which cause IrOx instability. These findings support the potential of PEDOT coatings as a micro-neural interface material for electrostimulation.
Chronic electrical stimulation of neural tissue has shown considerable promise in treating movement and sensory impairments, especially with deep brain stimulation and cochlear implants. While many of these treatments involve low-channel count macrostimulation using large surface area (>0.1 mm2) macroelectrodes, the breadth of neural therapies can be increased and improved with more selective microstimulation through small surface area microelectrodes. Chronic microstimulation-based devices have shown potential as auditory (Lim and Anderson, 2006
; McCreery, 2008
; Otto et al., 2005
; Rousche and Normann, 1999
; Rousche et al., 2003
), visual (Bak et al., 1990
; Bradley et al., 2005
; Normann et al., 1999
; Schmidt et al., 1996
; Weiland et al., 2005
), somatosensory (Butovas and Schwarz, 2007
; Romo and Salinas, 2001
), motor control (Mushahwar et al., 2002
), and bladder control (Pikov et al., 2007
) prostheses. While electrode miniaturization results in high selectivity, a negative side-effect is that the charge density requirements of the electrode material are increased. Consequently, performance of chronic microstimulation-based devices is limited due to both the lack of materials that safely and reliably deliver appropriate charge and the insulating effect of tissue reactions.
Platinum (Pt), iridium (Ir), and alloys of the two (PtIr) rate among the highest in Warburg capacitance (Geddes and Roeder, 2003
) and have commonly been used as implantable electrodes for electrostimulation, including deep brain stimulator electrodes and cochlear implants. However, due to low charge injection limits (0.05–0.3 mC/cm2), bare noble metals are often disregarded as small-area neural stimulation electrode materials (Cogan et al., 2005
; Rose and Robblee, 1990
). Iridium oxide (IrOx) films display a large electrochemical surface area and the ability to quickly inject reversible charge through electron exchange that occurs during the reversible oxidation and reduction of Ir3+ and Ir4+ (Mozota and Conway, 1983
). IrOx displays a charge injection limit of 0.9 mC/cm2 which can be increased to 3.3 mC/cm2 with the application of voltage-biased asymmetric waveforms (Cogan et al., 2006
). However, short-term pulsing results in IrOx instability at charge density levels between 2 and 3 mC/cm2, characterized by damaged IrOx films and Ir fragments in the adjacent tissue (Cogan et al., 2004
). Long-term in vivo stimulation studies have shown instability in the IrOx electrode–tissue interface, resulting in adverse tissue reactions (Weiland and Anderson, 2000
).
Conductive polymers are emerging as neural stimulation materials and exhibit both fast and high charge delivery capacities due to high ionic conductivity and a large electroactive surface area (Ghosh and Inganas, 2000
). These properties lead to very low impedance and highly effective charge transfer (Abidian and Martin, 2008
). Poly(3,4-ethylenedioxythiophene) (PEDOT) coatings on neural electrodes have been reported to display charge injection limits similar to IrOx at 2.3–3.6 mC/cm2 (Cui and Zhou, 2007
; Nyberg et al., 2007
) and much higher than IrOx at approximately 15 mC/cm2 (Cogan, 2008
). Thin PEDOT coatings have demonstrated electrochemical stability during long-term stimulation at charge densities of 0.35 mC/cm2 in phosphate buffered saline (PBS) (Cui and Zhou, 2007
). Further, bioactive molecules can be incorporated into PEDOT films (Abidian et al., 2006
; Cui and Martin, 2003
; Kim et al., 2007
) and PEDOT can be polymerized in and around living neural tissue, signifying the possibility of a hybrid polymer-live cell electrode (Richardson-Burns et al., 2007a
,b
). This report examines PEDOT as a micro-neural interface material for electrical stimulation. Electrochemically deposited PEDOT and activated IrOx coatings on small-area Ir electrode arrays were used. The coatings were characterized by scanning electron microscopy (SEM), electrochemical impedance spectroscopy (EIS), cyclic voltammetry (CV), and voltage excursion measurements. The effects of short-term application of relevant charge densities on coating stability were also characterized.
Microelectrode Arrays
Microfabricated silicon-substrate electrode arrays used in this study were provided by the University of Michigan Center for Neural Communication Technology (CNCT). The arrays consisted of four 15-μm thick silicon shanks spaced 125 μm apart with four thin-film Ir sites on each shank. Sites diameters were either 15 or 23 μm, resulting in a site area of 177 or 413 μm2. The electrodes were activated to form IrOx or electrochemically deposited with PEDOT as described below. An FEI Quanta 3D FEG field emission SEM (FEI Company Inc, Hillsboro, OR) was used to study the surface morphology of IrOx- and PEDOT-coated sites. SEM images were taken using a LVED detector in low vacuum mode (0.9 T) with a voltage of 15 kV and spot size of 6.
Electrochemical Measurements
Electrochemical measurements were made using an Autolab Potentiostat/Galvanostat PGSTAT12 (Eco Chemie, Utrecht, Netherlands) with a built-in frequency response analyzer (Brinkmann, Westbury, NY, NY). A three-electrode cell configuration in 1× PBS having a concentration of 154 mM NaCl, 5.8 mM NaH2PO4, and 1.1 mM KH2PO4 was used. The microelectrode site functioned as the working electrode (WE), a large-area Pt wire functioned as the counter electrode (CE), and an Accumet, gel-filled, KCl saturated calomel electrode (Thermo Fischer Scientific, Fair Lawn, NJ, USA) functioned as the reference electrode.
EIS measurements were collected by configuring the Autolab to sequentially inject overpotentials of 5 mVRMS sine waves at 36 frequencies logarithmically spaced from 1 Hz to 1 MHz. CV measurements were collected by configuring the Autolab to sweep the voltage of the WE at a constant rate within the potential limits of hydrolysis and monitor the resulting current flow between the WE and CE. The potential range used for Ir and IrOx was +0.8 to −0.6 V while that for PEDOT was +0.6 to −0.8 V. Both slow (50 mV/s) and fast (1 V/s) sweep rates were used to assess the static equilibrium at each voltage point and the charge available at the exposed area of the microelectrode site. The CV measurements were used to evaluate electrochemical reactions at the interface, double layer charging, and the cathodal charge storage capacity (Qcap). The location of peaks in the CV indicate potentials where oxidation and reduction reactions are occurring; peak amplitudes indicate the amount of charge carriers at the interface, and the Qcap is a measure of the charge available at the electrode interface. Qcap was calculated by integrating the cathodal current density enclosed by the CV and dividing by the sweep rate.
IrOx and Pedot Coatings
IrOx was activated on bare Ir sites using the Autolab Potentiostat/Galvanostat and three-electrode setup described previously. The activation performed in this report is a well established technique that has been described in detail elsewhere (Robblee et al., 1983
). In brief, activation involved applying 1-Hz voltage pulses with a 50% duty cycle, no interpulse delay, and an anodic potential of +0.8 V followed by a cathodic potential of −0.9 V. This was done for 1600 s in 1× PBS to result in a Qcap of approximately 30 mC/cm2.
PEDOT coatings used in this study were provided by Biotectix, LCC. PEDOT was deposited on the microelectrode sites by electrochemical polymerization. The electrode was placed in an electrically connected reservoir containing the aqueous monomer solution comprised of 0.01 M EDOT (Bayer/HC Starck) in deionized water with 2.5 mg/ml polyanionic poly(sodium styrene sulfonate) (PSS; Sigma-Aldrich). A platinum foil served as the CE and an electrode site of the neural probe was the WE. Galvanostatic electrodeposition was used to form the polymer on electrode sites using a current amplitude of 6 nA. Current was passed between the WE and the CE in the monomer solution using an Autolab Potentiostat/Galvanostat PGSTAT12 for a duration of 900 s.
Electrical Stimulation
Electrical stimulation was delivered via a programmable multichannel microstimulator system (Tucker-Davis Technologies, Alachua, FL, USA) in 1× PBS. Cathodal-first, charge-balanced, biphasic current pulses were applied between the microelectrode site and a large-area Pt wire. The current amplitude was chosen as the maximum current that could be safely applied without inducing neural damage, determined by the following equation
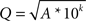
where Q is charge per phase in μC, A is the electrode surface area in cm2, and k is a constant of 1.7 (Shannon, 1992
). Using 200 μs phase durations, the maximum amplitude is approximately 45 μA for 177 μm2 surface area electrodes (charge density of 5 mC/cm2) and 70 μA for 413 μm2 surface area electrodes (charge density of 3.4 mC/cm2). In this study, pulses were delivered at these maximum amplitudes continuously at 100-Hz for 2 h (720,000 pulses). The resulting voltage excursions were measured with respect to the Pt CE during current pulsing in order to assess the safety and efficacy of the interface during charge transfer.
Statistics
Student’s t-tests were used to evaluate the statistical significance in all datasets, P < 0.05. All error-bar regions represent standard error mean from n = 9 sites (177 μm2) from three different arrays for IrOx and n = 4 sites (177 μm2) from one array for PEDOT.
Electrode Surface Morphology
Figures 1
A,C show SEM images of IrOx and PEDOT coatings on the Ir electrode sites (413 μm2). The IrOx coatings display a slightly rough surface (Figure 1
B) with noticeable cracks along the edge of the electrode site. The PEDOT coatings are much thicker and a dense arrangement of submicron sized clumps appear to uniformly cover the entire electrode site (Figure 1
D).
Figure 1. Scanning electron microscopy (SEM) images of electrode surfaces (413 μm2). (A) An overall coating and (B) microscale surface morphology of IrOx which was activated by biasing bare Ir between +0.8 and −0.9 V at 1 Hz for 1600 s is shown. (C) An overall coating and (D) microscale surface morphology of PEDOT which was galvanostatically deposited at 6 nA for 900 s is shown.
Electrochemical Measurements
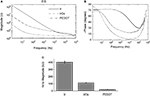
The complex impedance magnitude for the Ir microelectrodes greatly decreased after IrOx and PEDOT deposition. Mean impedance magnitude for IrOx was significantly less than Ir for all frequencies below 10 kHz and PEDOT displayed a lower impedance magnitude than both Ir and IrOx for all frequencies (Figure 2
A). Low phase values near 0 indicate highly resistive impedance and high phase values near −90 indicate highly capacitive impedance. Phase values for IrOx were significantly lower than Ir at all frequencies below 100 kHz and PEDOT displayed a lower phase value than both Ir and IrOx for all frequencies above 10 Hz, representing a more resistive interface (Figure 2
B). One kilohertz impedance magnitudes are particularly important due to the biological relevance of action potentials having a fundamental frequency of 1 kHz. Bare Ir, IrOx, and PEDOT surfaces displayed 1 kHz impedance values of 404.5 ± 15.2, 113.6 ± 3.5, and 23.3 ± 0.7 kΩ (Figure 2
C).
Figure 2. Electrochemical impedance spectroscopy (EIS) measurements. (A) Mean impedance magnitude and (B) phase measurements for 36 frequencies logarithmically spaced between 1 Hz and 1 MHz are shown. (C) Mean 1 kHz impedance magnitudes were significantly less for IrOx- and PEDOT-coated sites.
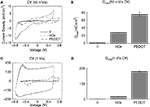
The CV data provide information on the charge transfer properties of the electrode interface. IrOx charge transfer is highly faradaic in behavior corresponding to the distinct peaks associated with the reversible oxidation and reduction of Ir (Figures 3
A,C). PEDOT charge transfer is also faradaic and additionally displays a large rectangular shape for the fast sweep rate (1 V/s) CV, indicating high capacitive charge transfer. Mean Qcap values were calculated from these CV data to quantify the charge available at each material interface and resulted in the following fashion: Ir < IrOx < PEDOT (Figures 3
B,D). The Qcap for IrOx was 28.8 ± 0.3 mC/cm2 for the slow sweep rate CV and 17.6 ± 0.2 mC/cm2 for the fast sweep rate CV while that for PEDOT was 75.6 ± 5.4 and 178.5 ± 5.3 mC/cm2. Interestingly, the Qcap for PEDOT measured from the fast sweep rate CV was much higher than that calculated from the slow sweep rate CV, which could be beneficial for charge transfer during physiologically relevant electrical stimulation.
Figure 3. Cyclic voltammetry (CV) measurements. Both (A) slow (50 mV/s) and (C) fast (1 V/s) sweep rates show PEDOT-coated electrodes with much larger CV areas than IrOx and bare Ir. (B,D) Mean charge storage capacities (Qcap) calculated by integrating the cathodal current area enclosed by the CV divided by the sweep rate show that PEDOT has more charge available at the interface.
Electrical Stimulation
Cathodal-first, charge-balanced, biphasic current pulses are frequently used for effective and safe stimulation of neural tissue (Brummer and Turner, 1975
; Lilly et al., 1955
; Merrill et al., 2005
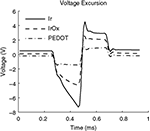
). Measuring the corresponding voltage excursion is commonly employed to calculate the safe charge injection limits of the interface. Ir and IrOx voltage excursions display an initial ohmic voltage drop followed by a more gradual polarization of the interface (Figure 4
). Due to increased charge carriers at the interface, less polarization is seen at the IrOx interface compared to Ir. PEDOT displayed a much lower initial ohmic voltage drop which was not followed by additional polarization. The PEDOT voltage excursion displayed a very ohmic representation of the current delivered which corresponded closely to its 1 kHz impedance value. By employing Ohm’s law, a current of 45 μA delivered across a 23.3 kΩ resistor results in a voltage of 1.05 V and the mean cathodal voltage drop for PEDOT with respect to its zero current potential was 1.14 V. The relatively low voltage amplitude and ohmic representation of the PEDOT voltage excursion is indicative of improved charge injection limits compared to IrOx.
Figure 4. Voltage excursion data taken from biphasic current pulses with 200 μs phase durations, and 45 μA amplitudes resulting in a charge density of 5 mC/cm2. These data are taken from 177 μm2 surface area electrodes.
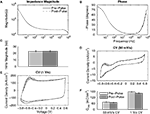
IrOx- and PEDOT-coated electrodes were pulsed at charge densities of approximately 5 mC/cm2 (177 μm2) and 3.4 mC/cm2 (413 μm2) which has been previously reported to cause IrOx delamination (Cogan et al., 2004
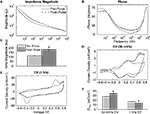
). Pulsing IrOx resulted in a significant increase in the impedance magnitude at all frequencies below 200 kHz (Figure 5
A) and a significant increase in phase at all frequencies above 30 Hz (Figure 5
B). The 1 kHz impedance magnitude increased from 113.6 ± 3.5 to 183.9 ± 12.5 kΩ (Figure 5
C). Slow sweep rate CVs displayed large anodic peaks around 0.6–0.7 V after pulsing demonstrating pulse-induced activation (Figure 5
D). The oxidation-reduction peaks seen in the fast sweep rate CVs were greatly reduced after pulsing indicating that a lower amount of charge carriers were available at the interface (Figure 5
E). Mean Qcap values calculated from the slow sweep rate CVs showed a significant increase from 28.8 ± 0.3 to 35.1 ± 1.1 mC/cm2 while values calculated from the fast sweep rate CVs show a significant decrease from 17.6 ± 0.2 to 14.1 ± 0.3 mC/cm2 (Figure 5
F).
Figure 5. Effects of continuously pulsing (200 μs phase duration, 45 μA amplitude, 100 Hz) IrOx-coated electrodes (177 μm2) for 2 h. (A) Mean impedance and (B) phase changed significantly for nearly all frequencies after pulsing. (C) The 1 kHz impedance magnitude increased significantly indicating IrOx delamination. (D) Slow CV measurements show pulse-induced IrOx activation while (E) fast CV measurements show IrOx degradation. (F) These changes in the CV correspond to an increase in Qcap for the slow CV measurements and a decrease in Qcap for fast CV measurements.
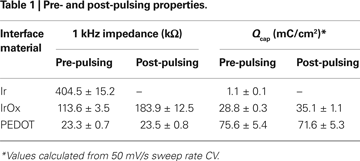
The PEDOT coatings were examined during the same pulsing protocol used to asses the IrOx coatings. Almost identical pre- and post-pulsing impedance magnitude and phase plots were seen at all frequencies below 100 kHz (Figures 6
A,B). Pulsing PEDOT resulted in no significant difference in the 1 kHz impedance magnitude (Figure 6
C). The pre- and post-pulsing CVs displayed slight changes with decreases in the mean Qcap from 75.6 ± 5.4 to 71.6 ± 5.3 mC/cm2 for the slow sweep rate CV and from 178.5 ± 5.4 to 166.5 ± 5.6 mC/cm2 for the fast sweep rate CV (Figures 6
D–F). However, no statistical difference was observed for the Qcap values before and after pulsing. The PEDOT coatings remained electrochemically stable during short-term pulsing. The pre- and post-pulsing values for IrOx- and PEDOT-coated sites are summarized in Table 1
.
Figure 6. Effects of continuously pulsing (200 μs phase duration, 45 μA amplitude, 100 Hz) PEDOT-coated electrodes (177 μm2) for 2 h. (A) Mean impedance magnitude and (B) phase showed no significant change between pre- and post-stimulation values at all frequencies. (C) This is seen by comparing the 1 kHz impedance magnitudes. Both (D) slow and (E) fast CV measurements show little change after pulsing and (F) there is no significant change in the Qcap.
Similar increases in impedance and Qcap were seen for the larger surface area (413 μm2) IrOx-coated sites pulsed at 3.4 mC/cm2 which caused extensive damage to the IrOx coating (Figure 7
). Almost no change was observed with the PEDOT-coated sites pulsed at the same levels. These data confirm that the threshold for IrOx delamination is below 3.4 mC/cm2, which occurs within only 2 h of current pulsing. Alternatively, PEDOT coatings remain stable at electrostimulation levels which cause IrOx damage.
Figure 7. Effects of continuously pulsing (200 μs phase duration, 70 μA amplitude, 100 Hz) IrOx- and PEDOT-coated electrodes (413 μm2) for 2 h characterized by the change in 1 kHz impedance and Qcap. The SEM image of a pulsed IrOx site shows an extensively damaged coating.
IrOx has been compared with metal-based materials such as PtIr (Cogan et al., 2005
) and TiN (Weiland et al., 2002
), and currently is considered the best interface material for neural stimulation. Many studies have suggested the conductive polymer PEDOT as a microelectrode coating for neural stimulation due to its desirable electrical properties (Abidian and Martin, 2008
; Cui and Martin, 2003
; Guimard et al., 2007
), electrochemical stability (Kros et al., 2005
) and ability for biomolecule immobilization (Abidian and Martin, 2008
; Abidian et al., 2006
; Cui and Martin, 2003
; Kim et al., 2007
), but direct comparisons with IrOx coatings have yet to be reported. The results presented in this report suggest PEDOT is a superior micro-neural interface material for electrostimulation compared to IrOx. PEDOT exhibits much lower interfacial impedance across the entire spectrum which has many implications for both recording and stimulating applications. Lower impedance results in higher signal-to-noise ratios for improved electrophysiological recordings and a more effective interface for constant current stimulation.
The large rectangular CV curve for PEDOT-coated electrodes is representative of more charge available for electrical stimulation corresponding with the increased surface area seen in SEM images and indicative of fast ion transport across the PEDOT coating. PEDOT Qcap values are more than double that of IrOx, analogous to more charge available at the interface. Only a small portion of the Qcap is accessible for charge injection during fast current pulsing, but higher Qcap results in higher charge injection capacities corresponding to lower voltage excursions during constant current pulsing. PEDOT voltage excursions are much smaller in amplitude than IrOx and are very ohmic in their shape. The ability to push current at lower voltage amplitudes reduces the formation of toxic species and lowers power consumption of the microstimulator device.
The chronic functionality of neural prostheses is dependent on maintaining a stable electrode–tissue interface. During electrical stimulation, properties of the neural interface material must not degrade or create foreign species. Consistent with previous reports, repetitive pulsing that results in large voltage excursions causes unstable IrOx activation and delamination. This damages the electrode material and will most likely cause tissue damage, greatly compromising the quality of the neural interface. As a result of pulsing, the interfacial impedance is significantly increased after only 2 h. PEDOT displayed a much more stable interface for repetitive pulsing and no changes were observed in the complex impedance spectra. A slight loss in Qcap occurred, but since there was no change in impedance, this is most likely due to rearrangement of the PEDOT and PSS structure after sweeping the voltage during the CV measurement (Cui and Martin, 2003
).
The method of deposition, dopant/counterion used, thickness, and micro/nanoscale morphology and topography for PEDOT and IrOx coatings has a significant effect on electrical, mechanical, and biological interfacing properties. The deposition methods and coating thicknesses used in this study were chosen with the goal to achieve a favorable balance of these properties and any head to head comparison of the electrode coatings should be considered relative. Additionally, the mechanical robustness of conductive polymer coatings remains an area of concern and requires further investigation.
The data presented in this report suggests that PEDOT is better suited than IrOx as a micro-neural interface material for delivering physiologically relevant stimulation. PEDOT exhibits lower interfacial impedance, higher Qcap and lower amplitude voltage excursions which are more ohmic in shape than that exhibited by IrOx. Further, the electrochemical properties of PEDOT coatings remain stable and reliable after short-term repetitive pulsing at current densities IrOx is unable to handle. While these results are promising, long-term stability and in vivo evaluation is still necessary to further suggest PEDOT for chronically implantable and functional coatings.
Sarah M. Richardson-Burns, Jeffrey L. Hendricks and David C. Martin have financial interest in Biotectix, LCC, an innovator in the development and use of conductive polymer materials and coatings. This conflict of interest is managed by the University of Michigan.
This work was supported by the Purdue Research Foundation. The authors would like to thank the Purdue NeuroProstheses Research Laboratory for helping in the preparation of this manuscript, the Center for Neural Communication Technology for providing microelectrode arrays (NIH/NIBIB P41-EB002030), Biotectix, LCC for providing the PEDOT coatings and the Purdue Life Science Microscopy Facility for assisting in acquiring SEM images.