Chemical Structures and Bioactivities of Sulfated Polysaccharides from Marine Algae
Abstract
:1. Introduction
2. Structural Diversity of Algal Sulfated Polysaccharides
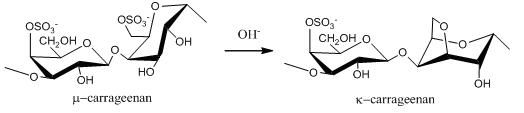
2.1. Carrageenans and Agarans from Red Algae
2.2. Sulfated Polysaccharides from Green Algae
2.3. Fucose-containing Sulfated Polysaccharides from Brown Algae
3. Approaches in Structural Analysis of Algal Sulfated Polysaccharides
3.1. Desulfation and Methylation for Structure Analysis
3.2. Structural Analysis by Using NMR and MS
3.3. Oversulfation of Algal Polysaccharides
3.4. Molecular Size Modification of Algal Sulfated Polysaccharides
4. Bioactivity and Structure-Activity Relationship
4.1. Anticoagulant and Antithrombotic Activities
4.3. Immuno-Inflammatory Activity
4.4. Antioxidant Activities
4.5. Antilipidemic Effects
5. Future Perspectives
Acknowledgments
- Samples Availability: Available from the authors.
References
- Kusaykin, M; Bakunina, I; Sova, V; Ermakova, S; Kuznetsova, T; Besednova, N; Zaporozhets, T; Zvyagintseva, T. Structure, biological activity, and enzymatic transformation of fucoidans from the brown seaweeds. Biotechnol J 2008, 3, 904–915. [Google Scholar]
- Lahaye, M. Developments on gelling algal galactans, their structure and physico-chemistry. J Appl Phycol 2001, 13, 173–184. [Google Scholar]
- Lahaye, M; Robic, A. Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar]
- Li, B; Lu, F; Wei, X; Zhao, R. Fucoidan: structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar]
- Pomin, VH. Structural and functional insights into sulfated galactans: A systematic review. Glycoconj J 2010, 27, 1–12. [Google Scholar]
- Pomin, VH; Mourao, PAS. Structure, biology, evolution, and medical importance of sulfated fucans and galactans. Glycobiology 2008, 18, 1016–1027. [Google Scholar]
- Usov, AI; Bilan, I. Fucoidans-Sulfated polysaccharides of brown algae. Russ Chem Rev 2009, 78, 785–799. [Google Scholar]
- Wijesekara, I; Pangestuti, R; Kim, S-K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr Polym 2010, in press.. [Google Scholar]
- Berteau, O; Mulloy, B. Sulfated fucans, fresh perspectives: structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 2003, 13, 29R–40R. [Google Scholar]
- Knutsen, SH; Myslabodski, DE; Larsen, B; Usov, AI. A modified system of nomenclature for red algal galactans. Bot Mar 1994, 37, 163–170. [Google Scholar]
- Anderson, NS; Dolan, TCS; Rees, DA. Carrageenans. Part VII. Polysaccharides from Eucheuma spinosum and Eucheuma cottonii. The covalent structure of l-carrageenan. J Chem Soc Perkin Trans I 1973, 2173–2176. [Google Scholar]
- Estevez, JM; Ciancia, M; Cerezo, AS. The system of low-molecular-weight carrageenans and agaroids from the room-temperature-extracted fraction of Kappaphycus alvarezii. Carbohydr Res 2000, 325, 287–299. [Google Scholar]
- Funami, T; Hiroe, M; Noda, S; Asai, I; Ikeda, S; Nishinari, K. Influence of molecular structure imaged with atomic force microscopy on the rheological behavior of carrageenan aqueous systems in the presence or absence of cations. Food Hydrocolloids 2007, 21, 617–629. [Google Scholar]
- Zhou, G; Sheng, W; Yao, W; Wang, C. Effect of low molecular [lambda]-carrageenan from Chondrus ocellatus on antitumor H-22 activity of 5-Fu. Pharmacol Res 2006, 53, 129–134. [Google Scholar]
- Doyle, JP; Giannouli, P; Rudolph, B; Morris, ER. Preparation, authentication, rheology and conformation of theta carrageenan. Carbohydr Polym 2010, 80, 648–654. [Google Scholar]
- Yang, B; Yu, G; Zhao, X; Ren, W; Jiao, G; Fang, L; Wang, Y; Du, G; Tiller, C; Girouard, G; Barrow, CJ; Ewart, HS; Zhang, J. Structural characterisation and bioactivities of hybrid carrageenan-like sulphated galactan from red alga Furcellaria lumbricalis. Food Chem 2011, 124, 50–57. [Google Scholar]
- van de Velde, F; Antipova, AS; Rollema, HS; Burova, TV; Grinberg, NV; Pereira, L; Gilsenan, PM; Tromp, RH; Rudolph, B; Grinberg, VY. The structure of kappa/iota-hybrid carrageenans II. Coil-helix transition as a function of chain composition. Carbohydr Res 2005, 340, 1113–1129. [Google Scholar]
- Chopin, T; Kerin, BF; Mazerolle, R. Phycocolloid chemistry as a taxonomic indicator of phylogeny in the Gigartinales, Rhodophyceae: A review and current developments using Fourier transform infrared diffuse reflectance spectroscopy. Pharmacol Res 1999, 47, 167–188. [Google Scholar]
- Hilliou, L; Larotonda, FDS; Abreu, P; Ramos, AM; Sereno, AM; Gonealves, MP. Effect of extraction parameters on the chemical structure and gel properties of kappa/iota-hybrid carrageenans obtained from Mastocarpus stellatus. Biomol Eng 2006, 23, 201–208. [Google Scholar]
- Hilliou, L; Wilhelm, M; Yamanoi, M; Gonclves, MP. Structural and mechanical characterization of [kappa]/[iota]-hybrid carrageenan gels in potassium salt using Fourier Transform rheology. Food Hydrocolloids 2009, 23, 2322–2330. [Google Scholar]
- Jouanneau, D; Guibet, M; Boulenguer, P; Mazoyer, J; Smietana, M; Helbert, W. New insights into the structure of hybrid [kappa]-/[mu]-carrageenan and its alkaline conversion. Food Hydrocolloids 2010, 24, 452–461. [Google Scholar]
- van de Velde, F. Structure and function of hybrid carrageenans. Food Hydrocolloids 2008, 22, 727–734. [Google Scholar]
- Yu, G; Hu, Y; Yang, B; Zhao, X; Wang, P; Ji, G; Wu, J; Guan, H. Extraction, isolation and structural characterization of polysaccharides from a red alga Gloiopeltis furcata. J Ocean Univ China Nat Sci 2010, 9, 193–197. [Google Scholar]
- Morrice, LM; McLean, MW; Long, WF; Williamson, FB. Porphyran primary structure. Hydrobiologia 1984, 116–117, 572–575. [Google Scholar]
- Zhang, Q; Qi, H; Zhao, T; Deslandes, E; Ismaeli, NM; Molloy, F; Critchley, AT. Chemical characteristics of a polysaccharide from Porphyra capensis (Rhodophyta). Carbohydr Res 2005, 340, 2447–2450. [Google Scholar]
- Zhang, Z; Zhang, Q; Wang, J; Zhang, H; Niu, X; Li, P. Preparation of the different derivatives of the low-molecular-weight porphyran from Porphyra haitanensis and their antioxidant activities in vitro. Int J Biol Macromol 2009, 45, 22–26. [Google Scholar]
- Zhang, Q; Li, N; Liu, X; Zhao, Z; Li, Z; Xu, Z. The structure of a sulfated galactan from Porphyra haitanensis and its in vivo antioxidant activity. Carbohydr Res 2004, 339, 105–111. [Google Scholar]
- Miller, IJ; Furneaux, RH. The structural determination of the agaroid polysaccharides from four New Zealand algae in the order Ceramiales by means of 13C-NMR Spectroscopy. Bot Mar 1997, 40, 333–340. [Google Scholar]
- Prado, HJ; Ciancia, M; Matulewicz, MC. Agarans from the red seaweed Polysiphonia nigrescens (Rhodomelaceae, Ceramiales). Carbohydr Res 2008, 343, 711–718. [Google Scholar]
- Miller, IJ. Evaluation of the structures of polysaccharides from two New Zealand members of the Ceramiaceae. Bot Mar 2003, 46, 378–385. [Google Scholar]
- Gonçalves, AG; Ducatti, DRB; Duarte, MER; Noseda, MD. Sulfated and pyruvylated disaccharide alditols obtained from a red seaweed galactan: ESIMS and NMR approaches. Carbohydr Res 2002, 337, 2443–2453. [Google Scholar]
- Duarte, MER; Cauduro, JP; Noseda, DG; Noseda, MD; Gonçalves, AG; Pujol, CA; Damonte, EB; Cerezo, AS. The structure of the agaran sulfate from Acanthophora spicifera (Rhodomelaceae, Ceramiales) and its antiviral activity. Relation between structure and antiviral activity in agarans. Carbohydr Res 2004, 339, 335–347. [Google Scholar]
- Stortz, CA; Cerezo, AS. Novel findings in carrageenans, agaroids and “hybrids” red seaweed galactans. Curr Top Phytochem 2000, 4, 121–134. [Google Scholar]
- Cases, MR; Stortz, CA; Cerezo, AS. Structure of the ‘corallinans’-sulfated xylogalactans from Corallina officinalis. Int J Biol Macromol 1994, 16, 93–97. [Google Scholar]
- Navarro, DA; Ricci, AM; Rodríguez, MC; Stortz, CA. Xylogalactans from Lithothamnion heterocladum; a crustose member of the Corallinales (Rhodophyta). Carbohydr Polym. In Press..
- Navarro, DA; Stortz, CA. The system of xylogalactans from the red seaweed Jania rubens (Corallinales, Rhodophyta). Carbohydr Res 2008, 343, 2613–2622. [Google Scholar]
- Martone, PT; Navarro, DA; Stortz, CA; Estevez, JM. Differences in polysaccharide structure between calcified and uncalcified segments in the coralline Calliarthron cheilosporioides (Corallinales, Rhodaphyta). J Phycol 2010, 46, 507–515. [Google Scholar]
- Lim, BL; Ryu, IH. Purification, structural characterization, and antioxidant activity of antioxidant substance from the red seaweed Gloiopeltis tenax. J Med Food 2009, 12, 442–451. [Google Scholar]
- Mandal, P; Pujol, CA; Carlucci, MJ; Chattopadhyay, K; Damonte, EB; Ray, B. Anti-herpetic activity of a sulfated xylomannan from Scinaia hatei. Phytochemistry 2008, 69, 2193–2199. [Google Scholar]
- Lahaye, M; Ray, B. Cell-wall polysaccharides from the marine green alga Ulva rigida (Ulvales, Chlorophyta)-NMR analysis of ulvan oligosaccharides. Carbohydr Res 1996, 283, 161–173. [Google Scholar]
- Percival, E; McDowell, RH. Chemistry and Enzymology of Marine Algal Polysaccharides; Academic Press: New York, NY, USA, 1967; p. 219. [Google Scholar]
- Lahaye, M; Brunel, M; Bonnin, E. Fine chemical structure analysis of oligosaccharides produced by an ulvan-lyase degradation of the water-soluble cell-wall polysaccharides from Ulva sp. (Ulvales, Chlorophyta). Carbohydr Res 1997, 304, 325–333. [Google Scholar]
- Love, J; Percival, E. The polysaccharides of the green seaweed Codium fragile. Part II. The water-soluble sulphated polysaccharides. J Chem Soc 1964, 3338–3345. [Google Scholar]
- Matsubara, K; Matsuura, Y; Bacic, A; Liao, ML; Hori, K; Miyazawa, K. Anticoagulant properties of a sulfated galactan preparation from a marine green alga, Codium cylindricum. Int J Biol Macromol 2001, 28, 395–399. [Google Scholar]
- Farias, EHC; Pomin, VH; Valente, AP; Nader, HB; Rocha, HAO; Mourao, PAS. A preponderantly 4-sulfated, 3-linked galactan from the green alga Codium isthmocladum. Glycobiology 2008, 18, 250–259. [Google Scholar]
- Matsubara, K; Matsuura, Y; Bacic, A; Liao, ML; Hori, K; Miyazawa, K. Anticoagulant properties of a sulfated galactan preparation from a marine green alga, Codium cylindricum. Int J Biol Macromol 2001, 28, 395–399. [Google Scholar]
- Bilan, MI; Vinogradova, EV; Shashkov, AS; Usov, AI. Structure of a highly pyruvylated galactan sulfate from the Pacific green alga Codium yezoense (Bryopsidales, Chlorophyta). Carbohydr Res 2007, 342, 586–596. [Google Scholar]
- Shevchenko, N; Burtseva, Y; Zvyagintseva, T; Makar'eva, T; Sergeeva, O; Zakharenko, A; Isakov, V; Thi Linh, N; Xuan Hoa, N; Minh Ly, B; Van Huyen, P. Polysaccharides and sterols from green algae Caulerpa lentillifera and C sertularioides. Chem Nat Compd 2009, 45, 1–5. [Google Scholar]
- Mao, W; Zang, X; Li, Y; Zhang, H. Sulfated polysaccharides from marine green algae Ulva conglobata and their anticoagulant activity. J Appl Phycol 2006, 18, 9–14. [Google Scholar]
- Ghosh, P; Adhikari, U; Ghosal, PK; Pujol, CA; Carlucci, MJ; Damonte, EB; Ray, B. In vitro anti-herpetic activity of sulfated polysaccharide fractions from Caulerpa racemosa. Phytochemistry 2004, 65, 3151–3157. [Google Scholar]
- Lee, J-B; Koizumi, S; Hayashi, K; Hayashi, T. Structure of rhamnan sulfate from the green alga Monostroma nitidum and its anti-herpetic effect. Carbohydr Polym 2010, in press.. [Google Scholar]
- Ray, B. Polysaccharides from Enteromorpha compressa: Isolation, purification and structural features. Carbohydr Polym 2006, 66, 408–416. [Google Scholar]
- Harada, N; Maeda, M. Chemical structure of antithrombin-active Rhamnan sulfate from Monostrom nitidum. Biosci Biotechnol Biochem 1998, 62, 1647–1652. [Google Scholar]
- Kylin, H. biochemistry of sea algae. Phys Chem 1913, 83, 171–197. [Google Scholar]
- Conchie, J; Percival, EGV. Fucoidin. Part II. The hydrolysis of a methylated fucoidin prepared from Fucus vesiculosus. J Chem Soc 1950, 827–832. [Google Scholar]
- Patankar, MS; Oehninger, S; Barnett, T; Williams, RL; Clark, GF. A revised structure for fucoidan may explain some of its biological activities. J Biol Chem 1993, 268, 21770–21776. [Google Scholar]
- Chevolot, L; Mulloy, B; Ratiskol, J; Foucault, A; Colliec-Jouault, S. A disaccharide repeat unit is the major structure in fucoidans from two species of brown algae. Carbohydr Res 2001, 330, 529–535. [Google Scholar]
- Ushakova, NA; Morozevich, GE; Ustyuzhanina, NE; Bilan, MI; Usov, AI; Nifantiev, NE; Preobrazhenskaya, ME. Anticoagulant activity of fucoidans from brown algae. Biochemistry (Moscow) Supplement Series B: Biomedical Chemistry 2009, 3, 77–83. [Google Scholar]
- Chattopadhyay, N; Ghosh, T; Sinha, S; Chattopadhyay, K; Karmakar, P; Ray, B. Polysaccharides from Turbinaria conoides: Structural features and antioxidant capacity. Food Chem 2010, 118, 823–829. [Google Scholar]
- Bilan, MI; Zakharova, AN; Grachev, AA; Shashkov, AS; Nifant’ev, NE; Usov, AI. Polysaccharides of algae: 60. Fucoidan from the Pacific brown alga Analipus japonicus (Harv.) Winne (Ectocarpales, Scytosiphonaceae). Bioorg Khim 2007, 33, 44–53. [Google Scholar]
- Marais, MF; Joseleau, JP. A fucoidan fraction from Ascophyllum nodosum. Carbohydr Res 2001, 336, 155–159. [Google Scholar]
- Chizhov, AO; Dell, A; Morris, HR; Haslam, SM; McDowell, RA; Shashkov, AS; Nifant’ev, NE; Khatuntseva, EA; Usov, AI. A study of fucoidan from the brown seaweed Chorda filum. Carbohydr Res 1999, 320, 108–119. [Google Scholar]
- Bilan, MI; Grachev, AA; Ustuzhanina, NE; Shashkov, AS; Nifantiev, NE; Usov, AI. A highly regular fraction of a fucoidan from the brown seaweed Fucus distichus L. Carbohydr Res 2004, 339, 511–517. [Google Scholar]
- Bilan, MI; Grachev, AA; Ustuzhanina, NE; Shashkov, AS; Nifantiev, NE; Usov, AI. Structure of a fucoidan from the brown seaweed Fucus evanescens C.Ag. Carbohydr Res 2002, 337, 719–730. [Google Scholar]
- Bilan, MI; Grachev, AA; Shashkov, AS; Nifantiev, NE; Usov, AI. Structure of a fucoidan from the brown seaweed Fucus serratus L. Carbohydr Res 2006, 341, 238–245. [Google Scholar]
- Usov, AI; Smirnova, GP; Bilan, MI; Shashkov, AS. Polysaccharides of algae: 53. Brown alga Laminaria saccharina (L.) Lam. as a source of fucoidan. Bioorg Khim 1998, 24, 382–389. [Google Scholar]
- Adhikari, U; Mateu, CG; Chattopadhyay, K; Pujol, CA; Damonte, EB; Ray, B. Structure and antiviral activity of sulfated fucans from Stoechospermum marginatum. Phytochemistry 2006, 67, 2474–2482. [Google Scholar]
- Nagaoka, M; Shibata, H; Kimura-Takagi, I; Hashimoto, S; Kimura, K; Makino, T; Aiyama, R; Ueyama, S; Yokokura, T. Structural study of fucoidan from Cladosiphon okamuranus TOKIDA. Glycoconj J 1999, 16, 19–26. [Google Scholar]
- Rocha, HAO; Moraes, FA; Trindade, ES; Franco, CRC; Torquato, RJS; Veiga, SS; Valente, AP; Mourao, PAS; Leite, EL; Nader, HB; Dietrich, CP. Structural and hemostatic activities of a sulfated galactofucan from the brown alga Spagtoglossum schroederi: An ideal antithrombotic agent? J Biol Chem 2005, 280, 41278–41288. [Google Scholar]
- Nishino, T; Yokoyama, G; Dobashi, K; Fujihara, M; Nagumo, T. Isolation, purification, and characterization of fucose-containing sulfated polysaccharides from the brown seaweed Ecklonia kurome and their blood-anticoagulant activities. Carbohydr Res 1989, 186, 119–129. [Google Scholar]
- Duarte, MER; Cardoso, MA; Noseda, MD; Cerezo, AS. Structural studies on fucoidans from the brown seaweed Sargassum stenophyllum. Carbohydr Res 2001, 333, 281–293. [Google Scholar]
- Li, B; Wei, XJ; Sun, JL; Xu, SY. Structural investigation of a fucoidan containing a fucose-free core from the brown seaweed Hizikia fusiforme. Carbohydr Res 2006, 341, 1135–1146. [Google Scholar]
- Leite, EL; Medeiros, MGL; Rocha, HAO; Farias, GGM; da Silva, LF; Chavante, SF; de Abreu, LD; Dietrich, CP; Nader, HB. Structure and pharmacological activities of a sulfated xylofucoglucuronan from the alga Spatoglossum schroderi. Plant Sci 1998, 132, 215–228. [Google Scholar]
- Ponce, NMA; Pujol, CA; Damonte, EB; Flores, ML; Stortz, CA. Fucoidans from the brown seaweed Adenocystis utricularis: extraction methods, antiviral activity and structural studies. Carbohydr Res 2003, 338, 153–165. [Google Scholar]
- Teruya, T; Tatemoto, H; Konishi, T; Tako, M. Structural characteristics and in vitro macrophage activation of acetyl fucoidan from Cladosiphon okamuranus. Glycoconj J 2009, 26, 1019–1028. [Google Scholar]
- Bjorndal, H; Hellerqvist, CG; Lindberg, B; Svensson, S. Gas-liquid chromatography and mass spectrometry in methylation analysis of polysaccharides. Angew Chem Int Ed Engl 1970, 9, 610–619. [Google Scholar]
- Bilan, MI; Vinogradova, EV; Tsvetkova, EA; Grachev, AA; Shashkov, AS; Nifantiev, NE; Usov, AI. A sulfated glucuronofucan containing both fucofuranose and fucopyranose residues from the brown alga Chordaria flagelliformis. Carbohydr Res 2008, 343, 2605–2612. [Google Scholar]
- Usov, AI; Adamyants, KS; Miroshnikova, LI; Shaposhnikova, AA; Kochetkov, NK. Solvolytic desulphation of sulphated carbohydrates. Carbohydr Res 1971, 18, 336–338. [Google Scholar]
- Kolender, AA; Matulewicz, MC. Desulfation of sulfated galactans with chlorotrimethylsilane. Characterization of beta-carrageenan by 1H NMR spectroscopy. Carbohydr Res 2004, 339, 1619–1629. [Google Scholar]
- Kantor, TG; Schubert, M. A method for the desulfation of Chondroitin Sulfate1. J Am Chem Soc 1957, 79, 152–153. [Google Scholar]
- Takano, R; Matsuo, M; Kamei-Hayashi, K; Hara, S; Hirase, S. A novel regioselective desulfation method specific to carbohydrate 6-sulfate using silylating reagents. Biosci Biotechnol Biochem 1992, 56, 1577–1580. [Google Scholar]
- Miller, IJ; Blunt, JW. Desulfation of algal galactans. Carbohydr Res 1998, 309, 39–43. [Google Scholar]
- Kusaykin, MI; Chizhov, AO; Grachev, AA; Alekseeva, SA; Bakunina, IY; Nedashkovskaya, OI; Sova, VV; Zvyagintseva, TN. A comparative study of specificity of fucoidanases from marine microorganisms and invertebrates. J Appl Phycol 2006, 18, 369–373. [Google Scholar]
- Ciucanu, I; Kerek, F. A simple and rapid method for the permethylation of carbohydrates. Carbohydr Res 1984, 131, 209–217. [Google Scholar]
- Zibetti, RGM; Duarte, MER; Noseda, MD; Colodi, FG; Ducatti, DRB; Ferreira, LG; Cardoso, MA; Cerezo, AS. Galactans from Cryptonemia species. Part II: Studies on the system of galactans of Cryptonemia seminervis (Halymeniales) and on the structure of major fractions. Carbohydr Res 2009, 344, 2364–2374. [Google Scholar]
- Usov, AI; Yarotsky, SV; Shashkov, AS. 13C-NMR spectroscopy of red algal galactans. Biopolymers 1980, 19, 977–990. [Google Scholar]
- Gonçalves, AG; Ducatti, DRB; Paranha, RG; Eugênia, M; Duarte, R; Noseda, MD. Positional isomers of sulfated oligosaccharides obtained from agarans and carrageenans: preparation and capillary electrophoresis separation. Carbohydr Res 2005, 340, 2123–2134. [Google Scholar]
- Bilan, MI; Grachev, AA; Shashkov, AS; Kelly, M; Sanderson, CJ; Nifantiev, NE; Usov, AI. Further studies on the composition and structure of a fucoidan preparation from the brown alga Saccharina latissima. Carbohydr Res 2010, 345, 2038–2047. [Google Scholar]
- Daniel, R; Chevolot, L; Carrascal, M; Tissot, B; Mourao, PAS; Abian, J. Electrospray ionization mass spectrometry of oligosaccharides derived from fucoidan of Ascophyllum nodosum. Carbohydr Res 2007, 342, 826–834. [Google Scholar]
- Fatema, MK; Nonami, H; Ducatti, DRB; Gonçalves, AG; Duarte, MER; Noseda, MD; Cerezo, AS; Erra-Balsells, R; Matulewicz, MC. Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry analysis of oligosaccharides and oligosaccharide alditols obtained by hydrolysis of agaroses and carrageenans, two important types of red seaweed polysaccharides. Carbohydr Res 2010, 345, 275–283. [Google Scholar]
- Goncalves, AG; Ducatti, DR; Grindley, TB; Duarte, ME; Noseda, MD. ESI-MS differential fragmentation of positional isomers of sulfated oligosaccharides derived from carrageenans and agarans. J Am Soc Mass Spectrom 2010, 21, 1404–1416. [Google Scholar]
- Yang, B; Yu, G; Zhao, X; Jiao, G; Ren, S; Chai, W. Mechanism of mild acid hydrolysis of galactan polysaccharides with highly ordered disaccharide repeats leading to a complete series of exclusively odd-numbered oligosaccharides. FEBS J 2009, 276, 2125–2137. [Google Scholar]
- Nishino, T; Nagumo, T. Anticoagulant and antithrombin activities of oversulfated fucans. Carbohydr Res 1992, 229, 355–362. [Google Scholar]
- Qi, H; Zhang, Q; Zhao, T; Chen, R; Zhang, H; Niu, X; Li, Z. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int J Biol Macromol 2005, 37, 195–199. [Google Scholar]
- Qi, H; Zhang, Q; Zhao, T; Hu, R; Zhang, K; Li, Z. In vitro antioxidant activity of acetylated and benzoylated derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta). Bioorg Med Chem Lett 2006, 16, 2441–2445. [Google Scholar]
- Bhattacharyya, S. Carrageenan-induced innate immune response is modified by enzymes that hydrolyze distinct galactosidic bonds. J Nutr Biochem 2010, 21, 906–913. [Google Scholar]
- Holtkamp, AD; Kelly, S; Ulber, R; Lang, S. Fucoidans and fucoidanases-focus on techniques for molecular structure elucidation and modification of marine polysaccharides. Appl Microbiol Biotechnol 2009, 82, 1–11. [Google Scholar]
- Nardella, A; Chaubet, F; Boisson-Vidal, C; Blondin, C; Durand, P; Jozefonvicz, J. Anticoagulant low molecular weight fucans produced by radical process and ion exchange chromatography of high molecular weight fucans extracted from the brown seaweed Ascophyllum nodosum. Carbohydr Res 1996, 289, 201–208. [Google Scholar]
- Pomin, VH; Valente, AP; Pereira, MS; Mourao, PAS. Mild acid hydrolysis of sulfated fucans: A selective 2-desulfation reaction and an alternative approach for preparing tailored sulfated oligosaccharides. Glycobiology 2005, 15, 1376–1385. [Google Scholar]
- Qiu, X; Amarasekara, A; Doctor, V. Effect of oversulfation on the chemical and biological properties of fucoidan. Carbohydr Polym 2006, 63, 224–228. [Google Scholar]
- Tao, S; Huina, T; Jing, X; Shuo, Z; Xin, X. Degradation and antioxidant activity of kappa-carrageenans. J Appl Phycol 2010, 117, 194–199. [Google Scholar]
- Teruya, T; Takeda, S; Tamaki, Y; Tako, M. Fucoidan isolated from Laminaria angustata var. longissima induced macrophage activation. Biosci Biotechnol Biochem 2010, 74, 1960–1962. [Google Scholar]
- Michel, G; Nyval-Collen, P; Barbeyron, T; Czjzek, M; Helbert, W. Bioconversion of red seaweed galactans: A focus on bacterial agarases and carrageenases. Appl Microbiol Biotechnol 2006, 71, 23–33. [Google Scholar]
- Barbeyron, T; L’Haridon, S; Michel, G; Czjzek, M. Mariniflexile fucanivorans sp. nov., a marine member of the Flavobacteriaceae that degrades sulphated fucans from brown algae. Int J Syst Evol Microbiol 2008, 58, 2107–2113. [Google Scholar]
- Barbeyron, T; Michel, G; Potin, P; Henrissat, B; Kloareg, B. Iota-Carrageenases constitute a novel family of glycoside hydrolases, unrelated to that of kappa-carrageenases. J Biol Chem 2000, 275, 35499–35505. [Google Scholar]
- Burtseva, YV; Kusaikin, MI; Sova, VV; Shevchenko, NM; Skobun, AS; Zvyagintseva, TN. Distribution of fucoidan hydrolases and some glycosidases among marine invertebrates. Russ J Mar Biol 2000, 26, 453–456. [Google Scholar]
- Guibet, M; Colin, S; Barbeyron, T; Genicot, S; Kloareg, B; Michel, G; Helbert, W. Degradation of lambda-carrageenan by Pseudoalteromonas carrageenovora lambda-carrageenase: A new family of glycoside hydrolases unrelated to kappa-and iota-carrageenases. Biochem J 2007, 404, 105–114. [Google Scholar]
- Lemoine, M; Collen, PN; Helbert, W. Physical state of iota-carrageenan modulates the mode of action of iota-carrageenase from Pseudoalteromonas carrageenovora. Biochem J 2009, 419, 545–553. [Google Scholar]
- Colliec-Jouault, S; Millet, J; Helley, D; Sinquin, C; Fischer, AM. Effect of low-molecular-weight fucoidan on experimental arterial thrombosis in the rabbit and rat. J Thromb Haemost 2003, 1, 1114–1115. [Google Scholar]
- Toyama, MH; Toyama, DO; Torres, VM; Pontes, GC; Farias, WRL; Melo, FR; Oliveira, SCB; Fagundes, FHR; Diz Filho, EBS; Cavada, BS. Effects of Low Molecular Weight Sulfated Galactan Fragments From Botryocladia occidentalis on the Pharmacological and Enzymatic Activity of Spla2 From Crotalus Durissus Cascavella. Protein J 2010, 1–5. [Google Scholar]
- Bondu, S; Deslandes, E; Fabre, MS; Berthou, C; Yu, G. Carrageenan from Solieria chordalis (Gigartinales): Structural analysis and immunological activities of the low molecular weight fractions. Carbohydr Polym 2010, 81, 448–460. [Google Scholar]
- Mou, H; Xiaolu, J; Huashi, G. A kappa-carrageenan derived oligosaccharide prepared by enzymatic degradation containing anti-tumor activity. J Appl Phycol 2003, 15, 297–303. [Google Scholar]
- Kitamura, K; Matsuo, M; Yasui, T. Enzymic degradation of fucoidan by fucoidanase from the hepatopancreas of Patinopecten yessoensis. Biosci Biotechnol Biochem 1992, 56, 490–494. [Google Scholar]
- Klarzynski, O; Descamps, V; Plesse, B; Yvin, JC; Kloareg, B; Fritig, B. Sulfated fucan oligosaccharides elicit defense responses in tobacco and local and systemic resistance against tobacco mosaic virus. Mol Plant Microbe Interact 2003, 16, 115–122. [Google Scholar]
- Kim, KJ; Lee, OH; Lee, HH; Lee, BY. A 4-week repeated oral dose toxicity study of fucoidan from the Sporophyll of Undaria pinnatifida in Sprague-Dawley rats. Toxicology 2010, 267, 154–158. [Google Scholar]
- Pereira, MS; Mulloy, B; Mourao, PAS. Structure and anticoagulant activity of sulfated fucans. Comparison between the regular, repetitive, and linear fucans from echinoderms with the more heterogeneous and branched polymers from brown algae. J Biol Chem 1999, 274, 7656–7667. [Google Scholar]
- Bernardi, G; Springer, GF. Properties of highly purified fucan. J Biol Chem 1962, 237, 75–80. [Google Scholar]
- Springer, GF; Wurzel, HA; McNeal, GM, Jr; Ansell, NJ; Doughty, MF. Isolation of anticoagulant fractions from crude fucoidin. Proc Soc Exp Biol Med 1957, 94, 404–409. [Google Scholar]
- Grauffel, V; Kloareg, B; Mabeau, S; Durand, P; Jozefonvicz, J. New natural polysaccharides with potent antithrombic activity: Fucans from brown algae. Biomaterials 1989, 10, 363–368. [Google Scholar]
- Kuznetsova, TA; Besednova, NN; Mamaev, AN; Momot, AP; Shevchenko, NM; Zvyagintseva, TN. Anticoagulant activity of fucoidan from brown algae Fucus evanescens of the Okhotsk Sea. Bull Exp Biol Med 2003, 136, 471–473. [Google Scholar]
- Mauray, S; Sternberg, C; Theveniaux, J; Millet, J; Sinquin, C; Tapon-Bretaudiere, J; Fischer, AM. Venous antithrombotic and anticoagulant activities of a fucoidan fraction. Thromb Haemost 1995, 74, 1280–1285. [Google Scholar]
- Cumashi, A; Ushakova, NA; Preobrazhenskaya, ME; D’Incecco, A; Piccoli, A; Totani, L; Tinari, N; Morozevich, GE; Berman, AE; Bilan, MI; Usov, AI; Ustyuzhanina, NE; Grachev, AA; Sanderson, CJ; Kelly, M; Rabinovich, GA; Iacobelli, S; Nifantiev, NE. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar]
- Nishino, T; Nagumo, T. The sulfate-content dependence of the anticoagulant activity of a fucan sulfate from the brown seaweed Ecklonia kurome. Carbohydr Res 1991, 214, 193–197. [Google Scholar]
- Nishino, T; Kiyohara, H; Yamada, H; Nagumo, T. An anticoagulant fucoidan from the brown seaweed Ecklonia kurome. Phytochemistry 1991, 30, 535–539. [Google Scholar]
- Pomin, VH; Pereira, MS; Valente, AP; Tollefsen, DM; Pavao, MSG; Mourao, PAS. Selective cleavage and anticoagulant activity of a sulfated fucan: Stereospecific removal of a 2-sulfate ester from the polysaccharide by mild acid hydrolysis, preparation of oligosaccharides, and heparin cofactor II-dependent anticoagulant activity. Glycobiology 2005, 15, 369–381. [Google Scholar]
- Nishino, T; Aizu, Y; Nagumo, T. The influence of sulfate content and molecular weight of a fucan sulfate from the brown seaweed Ecklonia kurome on its antithrombin activity. Thromb Res 1991, 64, 723–731. [Google Scholar]
- Hayakawa, Y; Hayashi, T; Lee, J-B; Srisomporn, P; Maeda, M; Ozawa, T; Sakuragawa, N. Inhibition of thrombin by sulfated polysaccharides isolated from green algae. Biochim Biophys Acta 2000, 1543, 86–94. [Google Scholar]
- Shanmugam, M; Mody, KH. Heparinoid-active sulphated polysaccharides from marine algae as potential blood anticoagulant agents. Curr Sci 2000, 79, 1672–1683. [Google Scholar]
- Farias, WRL; Valente, AP; Pereira, MS; Mourao, PAS. Structure and anticoagulant activity of sulfated galactans. Isolation of a unique sulfated galactan from the red algae Botryocladia occidentalis and comparison of its anticoagulant action with that of sulfated galactans from invertebrates. J Biol Chem 2000, 275, 29299–29307. [Google Scholar]
- Glauser, BF; Rezende, RM; Melo, FR; Pereira, MS; Francischetti, IMB; Monteiro, RQ; Rezaie, AR; Mourao, PAS. Anticoagulant activity of a sulfated galactan: Serpin-independent effect and specific interaction with factor Xa. Thromb Haemost 2009, 102, 1183–1193. [Google Scholar]
- Pereira, MG; Benevides, NMB; Melo, MRS; Valente, AP; Melo, FR; Mourao, PAS. Structure and anticoagulant activity of a sulfated galactan from the red alga, Gelidium crinale. Is there a specific structural requirement for the anticoagulant action? Carbohydr Res 2005, 340, 2015–2023. [Google Scholar]
- Mourao, PAS; Pereira, MS. Searching for alternatives to heparin: Sulfated fucans from marine invertebrates. Trends Cardiovasc Med 1999, 9, 225–232. [Google Scholar]
- Anderson, JA; Fredenburgh, JC; Stafford, AR; Guo, YS; Hirsh, J; Ghazarossian, V; Weitz, JI. Hypersulfated low molecular weight heparin with reduced affinity for antithrombin acts as an anticoagulant by inhibiting intrinsic tenase and prothrombinase. J Biol Chem 2001, 276, 9755–9761. [Google Scholar]
- Barrow, RT; Parker, ET; Krishnaswamy, S; Lollar, P. Inhibition by heparin of the human blood coagulation intrinsic pathway factor X activator. J Biol Chem 1994, 269, 26796–26800. [Google Scholar]
- Millet, J; Jouault, SC; Mauray, S; Theveniaux, J; Sternberg, C; Vidal, CB; Fischer, AM. Antithrombotic and anticoagulant activities of a low molecular weight fucoidan by the subcutaneous route. Thromb Haemost 1999, 81, 391–395. [Google Scholar]
- Boisson-Vidal, C; Chaubet, F; Chevolot, L; Sinquin, C; Theveniaux, J; Millet, J; Sternberg, C; Mulloy, B; Fischer, AM. Relationship between antithrombotic activities of fucans and their structure. Drug Dev Res 2000, 51, 216–224. [Google Scholar]
- Witvrouw, M; De Clercq, E. Sulfated polysaccharides extracted from sea algae as potential antiviral drugs. Gen Pharmacol 1997, 29, 497–511. [Google Scholar]
- Schaeffer, DJ; Krylov, VS. Anti-HIV activity of extracts and compounds from algae and cyanobacteria. Ecotoxicol Environ Saf 2000, 45, 208–227. [Google Scholar]
- Damonte, EB; Matulewicz, MC; Cerezo, AS. Sulfated seaweed polysaccharides as antiviral agents. Curr Med Chem 2004, 11, 2399–2419. [Google Scholar]
- Luescher-Mattli, M. Algae, A Possible Source for New Drugs in the Treatment of HIV and Other Viral Diseases. Curr Med Chem 2003, 2, 219–225. [Google Scholar]
- Gerber, P; Dutcher, JD; Adams, EV; Sherman, JH. Protective effect of seaweed extracts for chicken embryos infected with influenza B or mumps virus. Proc Soc Exp Biol Med 1958, 99, 590–593. [Google Scholar]
- Nahmias, AJ; Kibrick, S. Inhibitory effect of heparin on herpes simplex virus. J Bacteriol 1964, 87, 1060–1066. [Google Scholar]
- Ghosh, T; Chattopadhyay, K; Marschall, M; Karmakar, P; Mandal, P; Ray, B. Focus on antivirally active sulfated polysaccharides: From structure-activity analysis to clinical evaluation. Glycobiology 2009, 19, 2–15. [Google Scholar]
- Hidari, KIPJ; Takahashi, N; Arihara, M; Nagaoka, M; Morita, K; Suzuki, T. Structure and anti-dengue virus activity of sulfated polysaccharide from a marine alga. Biochem Biophys Res Commun 2008, 376, 91–95. [Google Scholar]
- Talarico, LB; Duarte, MER; Zibetti, RGM; Noseda, MD; Damonte, EB. An algal-derived DL-galactan hybrid is an efficient preventing agent for in vitro dengue virus infection. Planta Med 2007, 73, 1464–1468. [Google Scholar]
- Talarico, LB; Damonte, EB. Interference in dengue virus adsorption and uncoating by carrageenans. Virology 2007, 363, 473–485. [Google Scholar]
- Talarico, LB; Pujol, CA; Zibetti, RGM; Farea, PCS; Noseda, MD; Duarte, MER; Damonte, EB. The antiviral activity of sulfated polysaccharides against dengue virus is dependent on virus serotype and host cell. Antiviral Res 2005, 66, 103–110. [Google Scholar]
- Ghosh, T; Pujol, CA; Damonte, EB; Sinha, S; Ray, B. Sulfated xylomannans from the red seaweed Sebdenia polydactyla: structural features, chemical modification and antiviral activity. Antivir Chem Chemother 2009, 19, 235–242. [Google Scholar]
- Harden, EA; Falshaw, R; Carnachan, SM; Kern, ER; Prichard, MN. Virucidal activity of polysaccharide extracts from four algal species against herpes simplex virus. Antiviral Res 2009, 83, 282–289. [Google Scholar]
- Mohsen, MSA; Mohamed, SF; Ali, FM; El-Sayed, OH. Chemical Structure and Antiviral Activity of Water-soluble Sulfated Polysaccharides from Sargassum latifolium. J Appl Sci Res 2007, 3, 1178–1185. [Google Scholar]
- Carlucci, MJ; Scolaro, LA; Noseda, MD; Cerezo, AS; Damonte, EB. Protective effect of a natural carrageenan on genital herpes simplex virus infection in mice. Antiviral Res 2004, 64, 137–141. [Google Scholar]
- Chen, D; Wu, XZ; Wen, ZY. Sulfated polysaccharides and immune response: promoter or inhibitor? Panminerva Med 2008, 50, 177–183. [Google Scholar]
- Groth, I; Grunewald, N; Alban, S. Pharmacological profiles of animal- and nonanimal-derived sulfated polysaccharides--comparison of unfractionated heparin, the semisynthetic glucan sulfate PS3, and the sulfated polysaccharide fraction isolated from Delesseria sanguinea. Glycobiology 2009, 19, 408–417. [Google Scholar]
- Granert, C; Raud, J; Xie, X; Lindquist, L; Lindbom, L. Inhibition of leukocyte rolling with polysaccharide fucoidin prevents pleocytosis in experimental meningitis in the rabbit. J Clin Invest 1994, 93, 929–936. [Google Scholar]
- Preobrazhenskaya, ME; Berman, AE; Mikhailov, VI; Ushakova, NA; Mazurov, AV; Semenov, AV; Usov, AI; Nifant’ev, NE; Bovin, NV. Fucoidan inhibits leukocyte recruitment in a model peritoneal inflammation in rat and blocks interaction of P-selectin with its carbohydrate ligand. Biochem Mol Biol Int 1997, 43, 443–451. [Google Scholar]
- Senni, K; Gueniche, F; Foucault-Bertaud, A; Igondjo-Tchen, S; Fioretti, F; Colliec-Jouault, S; Durand, P; Guezennec, J; Godeau, G; Letourneur, D. Fucoidan a sulfated polysaccharide from brown algae is a potent modulator of connective tissue proteolysis. Arch Biochem Biophys 2006, 445, 56–64. [Google Scholar]
- Parish, CR; Freeman, C; Hulett, MD. Heparanase: a key enzyme involved in cell invasion. Biochim Biophys Acta 2001, 1471, M99–M108. [Google Scholar]
- Blondin, C; Fischer, E; Boisson-Vidal, C; Kazatchkine, MD; Jozefonvicz, J. Inhibition of complement activation by natural sulfated polysaccharides (fucans) from brown seaweed. Mol Immunol 1994, 31, 247–253. [Google Scholar]
- Clement, MJ; Tissot, B; Chevolot, L; Adjadj, E; Du, Y; Curmi, PA; Daniel, R. NMR characterization and molecular modeling of fucoidan showing the importance of oligosaccharide branching in its anticomplementary activity. Glycobiology 2010, 20, 883–894. [Google Scholar]
- Tissot, B; Daniel, R. Biological properties of sulfated fucans: The potent inhibiting activity of algal fucoidan against the human complement system. Glycobiology 2003, 13, 29G–31G. [Google Scholar]
- Tissot, B; Gonnet, F; Iborra, A; Berthou, C; Thielens, N; Arlaud, GJ; Daniel, R. Mass spectrometry analysis of the oligomeric C1q protein reveals the B chain as the target of trypsin cleavage and interaction with fucoidan. Biochemistry 2005, 44, 2602–2609. [Google Scholar]
- Tissot, B; Montdargent, B; Chevolot, L; Varenne, A; Descroix, S; Gareil, P; Daniel, R. Interaction of fucoidan with the proteins of the complement classical pathway. Biochim Biophys Acta 2003, 1651, 5–16. [Google Scholar]
- Tsuji, RF; Hoshino, K; Noro, Y; Tsuji, NM; Kurokawa, T; Masuda, T; Akira, S; Nowak, B. Suppression of allergic reaction by lambda-carrageenan: toll-like receptor 4/MyD88-dependent and -independent modulation of immunity. Clin Exp Allergy 2003, 33, 249–258. [Google Scholar]
- Maruyama, H; Tamauchi, H; Hashimoto, M; Nakano, T. Suppression of Th2 immune responses by mekabu fucoidan from Undaria pinnatifida sporophylls. Int Arch Allergy Immunol 2005, 137, 289–294. [Google Scholar]
- Leiro, JM; Castro, R; Arranz, JA; Lamas, J. Immunomodulating activities of acidic sulphated polysaccharides obtained from the seaweed Ulva rigida C. Agardh. Int Immunopharmacol 2007, 7, 879–888. [Google Scholar]
- Nakamura, T; Suzuki, H; Wada, Y; Kodama, T; Doi, T. Fucoidan induces nitric oxide production via p38 mitogen-activated protein kinase and NF-kB-dependent signaling pathways through macrophage scavenger receptors. Biochem Biophys Res Commun 2006, 343, 286–294. [Google Scholar]
- Yang, JW; Yoon, SY; Oh, SJ; Kim, SK; Kang, KW. Bifunctional effects of fucoidan on the expression of inducible nitric oxide synthase. Biochem Biophys Res Commun 2006, 346, 345–350. [Google Scholar]
- Do, H; Pyo, S; Sohn, EH. Suppression of iNOS expression by fucoidan is mediated by regulation of p38 MAPK, JAK/STAT, AP-1 and IRF-1, and depends on up-regulation of scavenger receptor B1 expression in TNF-alpha- and IFN-gamma-stimulated C6 glioma cells. J Nutr Biochem 2010, 21, 671. [Google Scholar]
- Choi, EM; Kim, AJ; Kim, YO; Hwang, JK. Immunomodulating activity of arabinogalactan and focoidan in vitro. J Med Food 2005, 8, 446–453. [Google Scholar]
- Kim, M-H; Joo, H-G. Immunostimulatory effects of fucoidan on bone marrow-derived dendritic cells. Immunol Lett 2008, 115, 138–143. [Google Scholar]
- Zhou, G; Sun, Y; Xin, H; Zhang, Y; Li, Z; Xu, Z. In vivo antitumor and immunomodulation activities of different molecular weight lambda-carrageenans from Chondrus ocellatus. Pharmacol Res 2004, 50, 47–53. [Google Scholar]
- Ruparez, P; Ahrazem, O; Leal, JA. Potential antioxidant capacity of sulfated polysaccharides from the edible marine brown seaweed Fucus vesiculosus. J Agric Food Chem 2002, 50, 840–845. [Google Scholar]
- Rocha de Souza, M; Marques, C; Guerra Dore, C; Ferreira da Silva, F; Oliveira Rocha, H; Leite, E. Antioxidant activities of sulfated polysaccharides from brown and red seaweeds. J Appl Phycol 2007, 19, 153–160. [Google Scholar]
- Zhao, X; Xue, C; Cai, Y; Wang, D; Fang, Y. Study of antioxidant activities of fucoidan from Laminaria japonica. High Tech Lett 2005, 11, 91–94. [Google Scholar]
- Costa, LS; Fidelis, GP; Cordeiro, SL; Oliveira, RM; Sabry, DA; Ciara, RBG; Nobre, LTDB; Costa, MSSP; Almeida-Lima, J; Farias, EHC; Leite, EL; Rocha, HAO. Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed Pharmacother 2010, 64, 21–28. [Google Scholar]
- Wang, J; Zhang, Q; Zhang, Z; Song, H; Li, P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int J Biol Macromol 2010, 46, 6–12. [Google Scholar]
- Wang, J; Liu, L; Zhang, Q; Zhang, Z; Qi, H; Li, P. Synthesized oversulphated, acetylated and benzoylated derivatives of fucoidan extracted from Laminaria japonica and their potential antioxidant activity in vitro. Food Chem 2009, 114, 1285–1290. [Google Scholar]
- Vaquez-Freire, MJ; Lamela, M; Calleja, JM. Hypolipidaemic activity of a polysaccharide extract from Fucus vesiculosus. Phytother Res 1996, 10, 647–650. [Google Scholar]
- Huang, L; Wen, K; Gao, X; Liu, Y. Hypolipidemic effect of fucoidan from Laminaria japonica in hyperlipidemic rats. Pharm Biol 2010, 48, 422–426. [Google Scholar]
- Pengzhan, Y; Ning, L; Xiguang, L; Gefei, Z; Quanbin, Z; Pengcheng, L. Antihyperlipidemic effects of different molecular weight sulfated polysaccharides from Ulva pertusa (Chlorophyta). Pharmacol Res 2003, 48, 543–549. [Google Scholar]
- Yokota, T; Nagashima, M; Ghazizadeh, M; Kawanami, O. Increased effect of fucoidan on lipoprotein lipase secretion in adipocytes. Life Sci 2009, 84, 523–529. [Google Scholar]
- Raghavendran, HR; Sathivel, A; Devaki, T. Effect of Sargassum polycystum (Phaeophyceae)-sulphated polysaccharide extract against acetaminophen-induced hyperlipidemia during toxic hepatitis in experimental rats. Mol Cell Biochem 2005, 276, 89–96. [Google Scholar]
- Josephine, A; Veena, CK; Amudha, G; Preetha, SP; Varalakshmi, P. Protective role of sulphated polysaccharides in abating the hyperlipidemic nephropathy provoked by cyclosporine A. Arch Toxicol 2007, 81, 371–379. [Google Scholar]
- Bourgougnon, N; Lahaye, M; Quemener, B; Chermann, JC; Rimbert, M; Cormaci, M; Furnari, G; Kornprobst, JM. Annual variation in composition and in vitro anti-HIV-1 activity of the sulfated glucuronogalactan from Schizymenia dubyi (Rhodophyta, Gigartinales). J Appl Phycol 1996, 8, 155–161. [Google Scholar]
- Hiebert, LM. Oral heparins. Clin Lab 2002, 48, 111–116. [Google Scholar]
- Irhimeh, MR; Fitton, JH; Lowenthal, RM. Pilot clinical study to evaluate the anticoagulant activity of fucoidan. Blood Coagul Fibrin 2009, 20, 607–610. [Google Scholar]
- Ewart, HS; Bloch, O; Girouard, GS; Kralovec, J; Barrow, CJ; Ben-Yehudah, G; Suárez, ER; Rapoport, MJ. Stimulation of cytokine production in human peripheral blood mononuclear cells by an aqueous Chlorella extract. Planta Med 2007, 73, 762–768. [Google Scholar]
- Fitton, JH; Irimeh, M; Falk, N. Macroalgal fucoidan extracts: a new opportunity for marine cosmetics. Cosmet Toiletries 2007, 122, 55–64. [Google Scholar]



| Species | Fucoidan structures | Reference |
|---|---|---|
| Analipus japonicus | 3 (4Fucp) and 1 (2Fucp) per ten (1→3)-α-L-Fucp(2/4SO3−) residues | Bilan et al., 2007 [60] |
| Ascophyllum nodosum | →3)-α-L-Fucp(2SO3−)-(1→4)-α-L-Fucp(2,3-diSO3−)-(1→ | Chevolot et al., 2001 [57] |
| A. nodosum | (1→3)-α-L-Fucp and a few (1→4)-α-L-Fucp with (1→3)-α-L-(2 and/or 4 Fucp) | Marais et al., 2001 [61] |
| Chorda filum | -[→3)-α-L-Fucp-(1-]3→3)-α-L-Fucp(2Fucp)-(1→ | Chizhov et al., 1999 [62] |
| Fucus distichus L. | →3)-α-L-Fucp-(2,4-diSO3−)-(1→4)-α-L-Fucp-(2SO3−)-(1→ | Bilan et al., 2004 [63] |
| F. evanescens | →3)-α-L-Fucp(2SO3−)-(1→4)-α-L-Fucp(2SO3−)-(1→ | Bilan et al., 2002 [64] |
| F. serratus L | →3)-α-L-Fucp(2R1,4R2)-(1→4)-α-L-Fucp(2SO3−)-(1→ a. (∼50%): R1 = SO3−, R2 = H b. (∼50%): R1 = H, R2 = α-L-Fucp-(1→4)-α-L-Fucp(2SO3−)-(1→3)-α-L-Fucp(2SO3−)-(1→ | Bilan et al., 2006 [65] |
| Laminaria saccharina | →3)-α-L-Fucp(4SO3−)-(1→ and additionally →3)-α-L-Fucp(4SO3− or 2Fucp)-(1→ | Usov et al., 1998 [66] |
| Stoechospermum marginatum | →3)-α-L-Fucp(2/4SO3−)-(1→ and →4)-α-L-Fucp(2SO3−)-(1→ | Adhikari et al., 2006 [67] |
| Modification methods | Source | Applications | Reference |
|---|---|---|---|
| Chemical | Ascophyllum nodosum | Antithrombotic activity | Colliec-Jouault et al., 2003 [109] |
| Botryocladia occidentalis | Anti-venom activity | Toyama et al., 2010 [110] | |
| Furcellaria lumbricalis | Immunostimulation activity | Yang et al., 2011 [16] | |
| Solieria chordalis | Immunostimulation activity | Bondu et al., 2010 [111] | |
| Enzymatic | Chondrus ocellatus | Anti-tumor activity | Mou et al., 2003 [112] |
| Nemacystus decipieus | Anticoagulant activity | Kitamura et al., 1992 [113] | |
| Pelvetia canaliculata | Antiviral activity | Klarzynski et al., 2003 [114] | |
| Undaria pinnatifida | Anticoagulant activity | Kim et al., 2010 [115] |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical Structures and Bioactivities of Sulfated Polysaccharides from Marine Algae. Mar. Drugs 2011, 9, 196-223. https://doi.org/10.3390/md9020196
Jiao G, Yu G, Zhang J, Ewart HS. Chemical Structures and Bioactivities of Sulfated Polysaccharides from Marine Algae. Marine Drugs. 2011; 9(2):196-223. https://doi.org/10.3390/md9020196
Chicago/Turabian StyleJiao, Guangling, Guangli Yu, Junzeng Zhang, and H. Stephen Ewart. 2011. "Chemical Structures and Bioactivities of Sulfated Polysaccharides from Marine Algae" Marine Drugs 9, no. 2: 196-223. https://doi.org/10.3390/md9020196