Coumarins from Angelica Lucida L. - Antibacterial Activities
Abstract
:Introduction
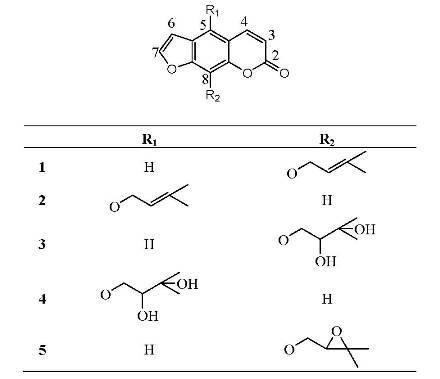
Results and Discussion

Antimicrobial activity
Experimental
General
Plant material
Extraction and isolation
| S. aureus | S. epidermidis | P. aeruginosa | E. cloacae | K. Pneumoniae | E. coli | S. mutans | S. viridans | C. albicans | C. tropicalis | C. glabrata | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| petroleum ether extract | 3.40 ± 0.4 | 3.80 ±0.5 | 3.75 ± 0.4 | 3.64 ± 0.6 | 4.10 ± 0.7 | 2.97 ±0.3 | 2.68 ± 0.3 | 2.35 ±0.4 | inactive | inactive | inactive |
| methanolic extract | 1.97 ± 0.3 | 1.85 ± 0.2 | 2.28 ± 0.3 | 2.90 ±0.4 | 2.76 ±0.2 | 2.39 ±0.3 | 2.20 ±0.2 | 2.47 ±0.2 | inactive | inactive | inactive |
| imperatorin | 0.045 ±0.1 | 0.035 ±0.2 | 0.070 ±0.1 | 0.028 ±0.2 | 0.030 ±0.3 | 0.025 ±0.2 | 0.018 ±0.2 | 0.015 ±0.1 | inactive | inactive | inactive |
| isoimperatorin | 0.040 ±0.2 | 0.032 ±0.1 | 0.070 ±0.3 | 0.025 ±0.2 | 0.030 ±0.2 | 0.023 ±0.1 | 0.015 ±0.1 | 0.012 ±0.2 | inactive | inactive | inactive |
| heraclenol | 0.68 ±0.3 | 0.64 ±0.2 | 0.70 ±0.2 | 0.77 ±0.1 | 0.85 ±0.4 | 0.67 ±0.3 | 0.53 ±0.2 | 0.50 ±0.2 | inactive | inactive | inactive |
| oxypeucedanin hydrate | 0.65 ±0.3 | 0.60 ±0.2 | 0.81 ±0.3 | 0.80 ±0.4 | 0.72 ±0.3 | 0.65 ±0.2 | 0.57 ±0.2 | 0.55 ±0.2 | inactive | inactive | inactive |
| heraclenin | 0.80 ±0.2 | 0.82 ±0.1 | 0.85 ±0.4 | 0.85 ±0.6 | 0.77 ±0.2 | 0.70 ±0.5 | 0.75 ±0.2 | 0.83 ±0.4 | inactive | inactive | inactive |
| netilmicin | 0.004±0.1 | 0.004 ±0.1 | 0.088 ±0.3 | 0.008 ±0.2 | 0.008 ±0.2 | 0.010 ±0.1 | nt | nt | nt | nt | nt |
| amoxicillin with clavulanic acid | 0.003 ±0.2 | 0.003 ±0.1 | 0.003 ±0.2 | 0.042 ±0.2 | 0.048 ±0.3 | 0.005 ±0.1 | nt | nt | nt | nt | nt |
| 5-flucytocine | nt | nt | nt | nt | nt | nt | nt | nt | 0.1x10-3 ±0.1 | 1x10-3 ±0.2 | 10x10-3 ±0.1 |
| amphotericin B | nt | nt | nt | nt | nt | nt | nt | nt | 1x10-3 ±0.3 | 0.5x10-3 ±0.1 | 0.4x10-3 ±0.1 |
| sanguinarine | nt | nt | nt | nt | nt | nt | 0.015 ±0.1 | 0.015 ±0.2 | nt | nt | nt |
Antimicrobial activity assays
Microorganisms
Statistical analysis
Acknowledgements
- Sample Availability: Samples of the compounds are available from the authors.
References
- French, D.H. The Biology & Chemistry of the Umbelliferae; Heywood, V.H., Ed.; Linn. Soc., Academic Press: London, UK, 1971; pp. 385–412. [Google Scholar]
- Murray, R.D.H. Progress in the Biochemistry of Organic Natural Products; Springer: New York, NY, USA, 1978; Vol. 35. [Google Scholar]
- Hoult, J.R.S.; Payá, M. Pharmacological and biochemical actions of simple coumarins: Natural products with therapeutic potential. Gen. Pharmacol. 1996, 27, 713–722. [Google Scholar] [CrossRef]
- Harkar, S.; Razdan, T.K.; Waight, E.S. Steroids, chromone and coumarins from Angelica officinalis. Phytochemistry 1984, 23, 419–426. [Google Scholar]
- Kwon, Y.S.; Kobayashi, A.; Kajiyama, S.I.; Kawazu, T.K.; Kanzaki, H.; Kim, C.M. Antimicrobial constituents of Angelica dahurica roots. Phytochemistry 1997, 44, 887–889. [Google Scholar]
- Thastrup, O.; Lemmich, J. Furocoumarin glucosides of Angelica archangelica subspecies litoralis. Phytochemistry 1983, 22, 2035–2037. [Google Scholar] [CrossRef]
- Lawrence, B.M.; Morton, J.K. Acorenone-B in Angelica lucida oil. Phytochemistry 1974, 13, 528. [Google Scholar] [CrossRef]
- Miftakhova, A.F.; Burasheva, G.S.; Abilov, Z.A.; Ahmad, V.U.; Zahid, M. Coumarins from the aerial part of Halocnemum strobilaceum. Fitoterapia 2001, 72, 319–321. [Google Scholar] [CrossRef]
- Nikonov, G.K.; Pimenov, M.G. Lactones from Angelica ursine fruits. Khim. Prir. Soedin. 1969, 5, 318–319. [Google Scholar]
- An, R.B.; Park, B.Y.; Kim, J.H.; Kwon, O.K.; Lee, J.; Min, B.S.; Ahn, K.S.; Oh, S.R.; Lee, H.K. Coumarins and chromones from Angelica genuflexa. Nat. Prod. Sci. 2005, 11, 79–84. [Google Scholar]
- Milosavljevic, S.; Jeremic, D.; Nevescanin, M.; Radovanovic, G.; Zivanovic, P.; Todorovic, B.; Slavkovska, V.; Vajs, V. Furo- and pyranocoumarins from plant species Angelica silvestris and Peucedanum austriacum. J. Serbian Chem. Soc. 1993, 58, 997–1001. [Google Scholar]
- Schinkovitz, A.; Gibbons, S.; Stavri, M.; Cocksedge, M.J.; Bucar, F. Ostruthin: An antimycobacterial coumarin from the roots of Peucedanum ostruthium. Planta Med. 2003, 69, 369–371. [Google Scholar] [CrossRef] [Green Version]
- Tada, Y.; Shikishima, Y.; Takaishi, Y.; Shibata, H.; Higuti, T.; Honda, G.; Ito, M.; Takeda, Y.; Kodzhimatov, O.; Ashurmetov, O.; Ohmoto, Y. Coumarins and γ-pyrone derivatives form Prangos pabularia antibacterial activity and inhibition of cytokine release. Phytochemistry 2002, 59, 649–654. [Google Scholar] [CrossRef]
- Rosselli, S.; Maggio, A.; Bellone, G.; Formisano, C.; Basile, A.; Cicala, C.; Alfieri, A.; Mascolo, N.; Bruno, M. Antibacterial and Anticoagulant Activities of Coumarins Isolated from the Flowers of Magydaris tomentosa. Planta Med. 2007, 72, 116–120. [Google Scholar]
- Stavri, M.; Gibbons, S. The antimycobacterial constituents of dill (Anethum graveolens). Phytother. Res. 2005, 19, 938–941. [Google Scholar] [CrossRef]
- Passi, S.; Aligiannis, N.; Pratsinis, H.; Skaltsounis, A.L.; Chinou, I.B. Biologically active Triterpenoids of Cephalaria ambrosioides roots. Planta Med. 2009, 75, 163–167. [Google Scholar] [CrossRef]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Widelski, J.; Popova, M.; Graikou, K.; Glowniak, K.; Chinou, I. Coumarins from Angelica Lucida L. - Antibacterial Activities. Molecules 2009, 14, 2729-2734. https://doi.org/10.3390/molecules14082729
Widelski J, Popova M, Graikou K, Glowniak K, Chinou I. Coumarins from Angelica Lucida L. - Antibacterial Activities. Molecules. 2009; 14(8):2729-2734. https://doi.org/10.3390/molecules14082729
Chicago/Turabian StyleWidelski, Jaroslaw, Milena Popova, Konstantia Graikou, Kazimierz Glowniak, and Ioanna Chinou. 2009. "Coumarins from Angelica Lucida L. - Antibacterial Activities" Molecules 14, no. 8: 2729-2734. https://doi.org/10.3390/molecules14082729