Published online Jun 14, 2017. doi: 10.3748/wjg.v23.i22.3964
Peer-review started: January 21, 2017
First decision: February 9, 2017
Revised: May 16, 2017
Accepted: June 1, 2017
Article in press: June 1, 2017
Published online: June 14, 2017
Gram-negative bacteria Helicobacter pylori (H. pylori) colonize gastric mucosa in humans and increase the risk of serious diseases such as gastric and duodenal ulcers, stomach cancers and mucosa associated lymphoid tissue lymphoma. The role of H. pylori infection in the pathogenesis of several extragastric diseases has been suggested including immune thrombocytopenic purpura, iron deficiency anemia, vitamin D deficiency, cardiovascular diseases, diabetes mellitus and dermatological disorders. Also neurological diseases and even lung cancer have attracted researchers concern. The relation between H. pylori infection and a growth retardation in children has also been suggested. Many mechanisms of molecular mimicry between H. pylori and the host have been proposed as a pathogen strategy to manipulate the immune system of the host in order to remain unrecognized and avoid eradication. A lot of effort has been put into the demonstration of homologous sequences between H. pylori and host compounds. However, knowledge about how often autoantibodies or autoreactive T lymphocytes induced during H. pylori infections cause pathological disorders is insufficient. This review provides data on H. pylori antigenic mimicry and possible deleterious effects due to the induction of immune response to the components common to these bacteria and the host.
Core tip: Molecular mimicry between Helicobacter pylori (H. pylori) and the host structures has been suggested as an effective mechanism of antibody production, potentially autoreactive. The chronic character of H. pylori infections increases the risk of such production and initiation or maintenance of H. pylori related pathological disorders triggered by the host effector immune mechanisms during infection. The panel of components common to H. pylori and the host is still increasing and thus the risk of autoimmune complications is an open problem.
- Citation: Chmiela M, Gonciarz W. Molecular mimicry in Helicobacter pylori infections. World J Gastroenterol 2017; 23(22): 3964-3977
- URL: https://www.wjgnet.com/1007-9327/full/v23/i22/3964.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i22.3964
Helicobacter pylori (H. pylori) a Gram-negative pathogenic bacterium, which has been described by Warren and Marshall in 1983[1], colonizes the gastric epithelium of humans (on average, 50% of the human population) and induces an excessive inflammatory response with or without symptoms (20% of cases). H. pylori infections possibly lead to different disorders such as: gastric and duodenal ulcers and, chronic gastritis, and even malignant diseases, including: mucosa-associated lymphoid tissue (MALT) lymphoma and gastric cancer[2-6]. Polymorphisms of the host genes encoding interleukins (ILs), including IL-1β, tumor necrosis factor (TNF-α) and cyclooxygenase -2 (COX2) have been suggested to increase the risk of infection and its severe consequences[7]. H. pylori strains have different genes encoding virulence factors that are important for disease development[8-10], which are either secreted, membrane-associated or translocated into cytosol of the host cells via the IV type secretion system, where they can affect the host cell functions[4]. H. pylori strains produce different adhesins, such as blood group antigen - binding adhesin (BabA), sialylated blood group - related adhesin (SabA), adherence - associated lipoprotein (AlpA/B) and outer membrane inflammatory protein (OipA), which promote close contact between the bacteria and the gastric epithelium[8-10]. Soluble factors such as urease and vacuolating cytotoxin (VacA) alter gastric cell survival and intercellular adhesion[11-16].
H. pylori CagA (Cytotoxin - associated gene A) is a highly immunogenic protein, which can trigger inflammatory responses in host gastric tissues, and it may influence the cell morphology, polarity, and proliferation; CagA also modulates the activity of immune cells and increases the risk of severe consequences, such as gastric ulcer and cancer[17-25]. Due to bacterial cell lysis, CagA and other H. pylori virulence factors can also be delivered to the gastric mucosa in a soluble form and affect the host immune cells infiltrating this milieu[24-27]. Moreover, H. pylori continuously produces phospholipid vesicles, which can be distributed by the circulation and function as a secondary extragastric source of CagA and other virulence factors[28-34]. Mucosal recognition of CagA is associated with the stimulation of epithelial cells that produce elevated levels of various cytokines, including IL-1β, IL-6 and IL-8, which is followed by the enhanced infiltration of activated neutrophils and severe mucosal inflammation that increases the risk of gastric cancer[19,35-38].
In addition, flagellin and especially lipopolysaccharide (LPS) were investigated to address their role in H. pylori pathogenesis via activation of NF-κB and chemokine expression[39]. Previous studies showed that H. pylori LPS possesses immunomodulatory properties that diminish the effectiveness of the phagocytosis, cytotoxic activity and the expansion of NK cells and T lymphocytes[40-42].
The interactions of H. pylori with host cells result in adherence, induction of inflammatory responses through cytokine/chemokine release, apoptosis or proliferation, which finally result in persistent colonization, severe inflammation, and disruption of the epithelial barrier function[43,44].
This process can enable the translocation of H. pylori virulence factors and inflammatory mediators into the circulation and promote or intensify the development of systemic inflammatory response and the possible clinical effects of H. pylori infections outside the stomach[45,46].
The role of H. pylori in some hematologic conditions has been considered, such as immune thrombocytopenic purpura (ITP), iron deficiency anemia (IDA), and vitamin B12 deficiency. The possible role of H. pylori infection in other hematologic diseases, such as non-Hodgkin lymphomas of the stomach, monoclonal gammopathy of undetermined significance, megaloblastic anemia and myelodysplastic syndromes, has been suggested[47]. The elevated risk of childhood leukemia and hemorrhage in patients with coagulation disorders due to H. pylori infection has also been considered. The effects of H. pylori on other disorders, such as cardiovascular diseases, diabetes mellitus, dermatological disorders, neurological disorders and even lung cancer, have also attracted attention of researchers[48-53]. Data obtained from these studies showed that the immune response induced by H. pylori may influence the clinical outcome of these disorders. Many seroepidemiological studies have shown that patients with coronary heart disease (CHD) produce anti-H. pylori antibodies[54-57]. A strong immune response triggered by H. pylori CagA - positive strains has been suggested to influence the development of atherosclerosis[58]. Many previous studies have stated that chronic infection with H. pylori has a significant influence on the immune system. Therefore, the possible mechanisms of H. pylori infections in the pathogenesis of the majority of extragastric diseases include chronic local or systemic inflammation and the initiation of autoimmune responses[59].
Molecular mimicry is a common strategy used by infectious agents to adapt to the host organism and avoid its immune response mechanisms. Molecular mimicry is defined as an antigenic and functional similarity between the second-row microbial structures and host molecules that leads to the production of auto-reactive antibodies, which may contribute to the development of autoimmune disorders. Similarities within and between linear amino acid sequences and spatial structures have been identifiedd[60-62].
Streptococcus pyogenes is one of the most intensely studied bacterial pathogens, that can trigger autoimmune diseases in genetically susceptible individuals. S. pyogenes is involved in the development of rheumatic fever and glomerulonephritis due to the induction of antibodies recognizing bacterial M protein and N-acetyl-β-D-glucosamine (GLcNAc) as well as human heart myosin[62,63]. Moreover, infections with Gram-negative bacteria, such as Klebsiella pneumoniae and Campylobacter jejuni, also stimulate the production of crossreactive antibodies that recognize the human leukocyte antigen (HLA)-B27 or gangliosides[62,64]. Additionally, certain viruses, such as the Epstein-Barr virus and the hepatitis B virus, share similar sequences with proteins in the central nervous system[65,66]. Molecular mimicry combined with the ability of T cells to evade the mechanisms of tolerance has been suggested as a potential mechanism implicated in the pathogenesis of various autoimmune diseases, including multiple sclerosis, diabetes mellitus and spondyloarthropaties[60,66-68].
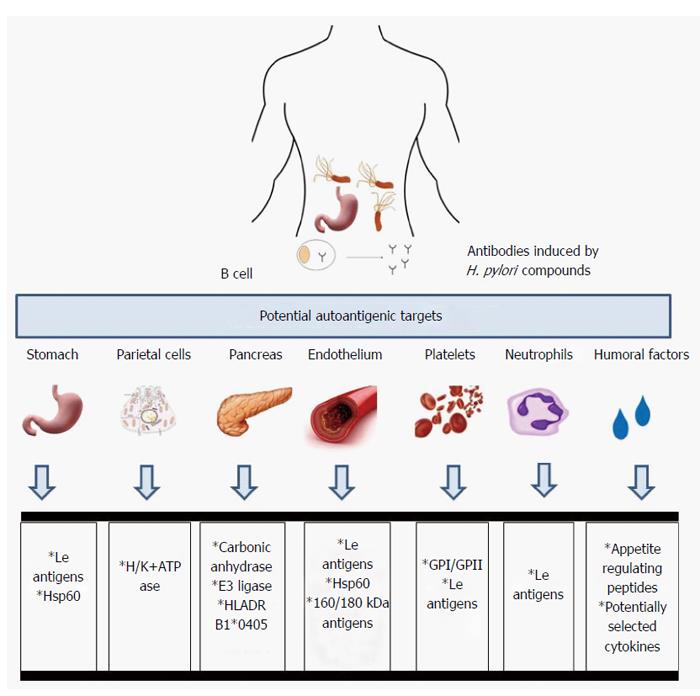
The mechanisms by which H. pylori infections lead to various gastric and potentially extragastric disorders are still poorly understood. One concept indicates the role of autoimmune processes. Chronic exposure to H. pylori compounds may initiate autoimmune gastritis due to molecular mimicry between H. pylori structures and the host tissue. The hypothesis of the induction by H. pylori anti-self reactions was proposed after antibodies with reactivity to the gastric antral mucosa were detected in the sera of infected patients[69-71]. Many mechanisms underlying the molecular mimicry between H. pylori and the host have been proposed and many efforts have been made to identify homologous sequences between H. pylori and host polypeptides, including the P-type adenosine triphosphate (ATP)-ases CopA and CopP that are involved in heavy metal iron transport, 686-bp amino acid ATPase, VacA, and urease beta chain vs gastric H+K+-ATPase[72-75], heat shock protein (Hsp) A vs GroEs, HspB vs 60-kDa host Hsp[76], and hemagglutinin/protease (hap) vs carbonic anhydrase[77]. However, whether and how often the autoantibodies induced in response to H. pylori infection are involved in various post-infectious pathologies due to the pathogen - induced autoreactive T lymphocytes or antibodies is unclear. The examples of potential autoantigenic host targets for anti-H. pylori antibodies are listed in Figure 1.
Autoimmune gastritis/pernicious anemia is characterized by two phenomena: atrophy in the corpus and fundus of the stomach and autoantibody production against parietal cells (PC) and their secretory component called an intrinsic factor (IF)[78-80]. Anti- PC antibodies, which target H+/K+ ATPase, a gastric proton pump, have been detected in 60%-85% of patients with autoimmune gastritis, whereas antibodies to IF have been detected in 30%-50% of patients with autoimmune gastritis[81-83]. Chronic autoaggression to H+/K+ ATPase may diminish gastric acid secretion, and cause hypergastrinemia and anemia due to iron deficiency[84,85]. Pernicious anemia is also characterized by a vitamin B12 deficiency. Patients suffering from autoimmune gastritis are predisposed to gastric tumors and adenocarcinomas[86]. In patients with type 1 diabetes or autoimmune thyroid disease, the prevalence of autoimmune gastritis is approximately three-fold higher than that of the general population, in which such autoimmune disorder has the frequency of 2%[87]. CD4+ T lymphocytes, which recognize parietal cell H+/K+ ATPase, have been shown to be involved in the development of autoimmune gastritis. H+/K+ ATPase is released from parietal cells during normal cell turnover and is selectively captured and then processed by antigen - presenting cells[88,89]. Another possibility is that H. pylori infection may initiate the development of autoimmune gastritis and pernicious anemia through the activation of T lymphocytes that are autoreactive to H+/K+ ATPase due to the antigenic mimicry between gastric H+/K+ ATPase and H. pylori at the T cell level[90]. Antibodies to gastric H+/K+ ATPase and their secretory forms are produced by B lymphocytes in cooperation with CD4+ antigen-specific T lymphocytes[91,92]. The deleterious effects of autoantibodies can be a consequence of T cell perforin-dependent cytotoxicity and apoptosis initiated by interaction between the Fas receptor (Fas) an Fas ligand[71]. The role of chronic H. pylori infections in the development of atrophic gastritis has been suggested on the basis of the positive correlation between gastric autoantibodies and antibodies specific to H. pylori antigens in the majority of patients with pernicious anemia[69,75,93-95]. However, this association has not been confirmed in other studies[96,97].
The presence of antibodies that react with the gastric mucosa in patients infected with H. pylori suggested that the autoantibodies induced by this pathogen may play an important role in the H. pylori - associated inflammatory response and cause deleterious gastric effects[75,94,98,99]. These antibodies could be stimulated by various Lewis (Le) antigens (Lex, Ley and Lex/y) that are present in the LPS structure of many H. pylori isolates[99,100], and on human cells including polymorphonuclear leucocytes, gastric epithelial cells and endothelial cells. The LPS O-specific chain of the H. pylori reference strain NCTC (National Collection of Type Cultures) 11637 was found to possess determinants similar to the human Lex blood group antigens, whereas LPS of the H. pylori MO19 strain contains determinants similar to human Ley[101-103]. Other blood group antigens, including H type 1, Lea, Leb, nonfucosylated polylactosoamine (i-antigen), sialyl Lex, and blood group A but not H type 2 have been detected in various H. pylori isolates. Additionally, strains bearing two or three blood group antigens in their LPS have been described[103-106]. The H. pylori LPS phase variation, which is defined as the random reversible change in phenotype in a range of blood group determinants, has been described for both reference and clinical strains[107,108]. During H. pylori infection different environmental and host factors including - gastric juice acidity may promote the selection of bacteria with the best phenotype in terms of virulence[109,110]. In a rhesus monkey model of H. pylori infection, the host Ley phenotype of the gastric mucosa was shown to select the Ley - positive phenotype of H. pylori, and the Lex host gastric phenotype was shown to select the Lex - positive bacteria[109]. Phase variations from Lex to i-Ag and back to Lex, from Lex to Lex plus Ley, and from Lex to Ley and forming Lea have been described[104,111-113]. The molecular mechanism of H. pylori LPS phase variation depends on mutations in the genes encoding α3-fucosylotransferases, the activity of these proteins and their preference for carbohydrate residues that determine antigenic specificity[82,108,114-117].
Experiments performed with the use of anti-Le monoclonal antibodies induced by immunization of mice with H. pylori showed that these antibodies reacted with both murine and human gastric mucosa, foveolar and glandular epithelial cells and parietal cell canaliculi. The anti-Lex monoclonal antibodies provoked by H. pylori were shown to react with polymorphonuclear leukocytes, gastric mucin and H+/K+ ATPase, which all express Le antigens[118]. However, it is unclear how anti-Le antibodies influence H. pylori adhesion and colonization of gastric mucosa in light of the results showing that the attachment of H. pylori to the human gastric epithelium is mediated by blood group antigens, including Leb, Lex and sialylated-Lex[8,119-121]. One possible mechanism underlying this effect is the diversification of H. pylori in the human host through lipopolysaccharide phase variation due to the heterologous expression of the alpha 1,3-fucosyltransferase gene[107,117]. The role of anti-Lex/y antibodies in the pathogenesis of H. pylori - driven deleterious effects is controversial. It has been hypothesized that anti-Le antibodies initiated by H. pylori, if bound to the gastric epithelium, can cause complement - dependent cell lysis promoting an excessive inflammatory response[99]. In early studies, these antibodies were not detected at all or only found in a low number of serum samples from individuals infected with a H. pylori. Other studies, including our previous work, have revealed that humans may produce anti-Lex antibodies, particularly those of the IgM class, in the absence of H. pylori infection or in the context of H. pylori - independent dyspepsia[118,122]. This finding indicates that anti-Le antibodies may be natural antibodies associated with the physiological autoimmunity required for the elimination of self-antigens. However, the incidence of this antibody production increases with age and, can be associated with the history of infections during the life of an individual[123]. The occurrence of anti-Lex antibodies in the sera of subjects not infected H. pylori could be induced by other microorganisms, such as streptococci, Eikenella corrodens or Acinetobacter actinomycetemcomitans bearing Lex determinants[124]. However, the possibility that H. pylori locally induces anti-Lex/y antibodies, which bind directly to gastric mucosal epitopes, and are absent in the serum, cannot be excluded[88,99]. Interestingly, the frequency of anti-Lex/y antibodies in the sera of patients infected with H. pylori and exhibiting gastritis symptoms, as well as in the patients with confirmed ischemic heart disease and H. pylori co-infection, was correlated with the increased occurrence of soluble Lex/y-anti-Lex/y IgG immune complexes[123-125]. It is possible that the deleterious effects of anti-Le antibodies depend on their ability to bind ligands and form rather small immune complexes, which may be deposited locally in both gastric and endothelial tissues where they can promote the inflammatory response. Perhaps the severity of anti-Le antibody production in H. pylori - infected individuals is associated with higher exposure to Le antigens due to inflammation, damage to the gastric epithelium and/or vascular endothelial cells, and the migration and activation of immune cells. Since the expression of Lex/y determinants in H. pylori is related to the cagA status[126], anti-Le antibodies may increase the inflammatory effects on the gastric mucosa in association with H. pylori virulence proteins, such as CagA, VacA and urease. However, Zheng et al[127] showed that peptic ulcer disease was not related to cagA status, iceA (induced by contact with the epithelium) or vacA genotypes, but there was an association with increased expression of a combination of Le antigens in H. pylori. This finding suggests that gastric disorders related to H. pylori infection depend on a specific type of host-pathogen interactions. The bacterial Le determinants may promote the adaptation of bacteria to the host gastric mucosa, which allow them to evade the host immune response and establish a chronic infection, and tissue destruction via the induction of anti-Le autoantibodies. The complex strategy of H. pylori for survival in the gastric mucosa of the host involves both structural modifications of lipid A in LPS to diminish its endotoxic properties and the expression and variation of Le determinants that mimick host components[125].
In 1988 Gasbarrini et al[128], showed that eradication of H. pylori resulted in regression of ITP. There are several potential mechanisms that combain H. pylori infection with ITP. One is molecular mimicry between H. pylori CagA protein and platelet glycoproteins: GPI and GPII[129-131]. In ITP patients infected with CagA positive but not CagA negative H. pylori strains a higher number of B lymphocytes producing anti-CagA antibodies that crossreact with the platelet specific peptides have been detected, which was correlated with the elevated levels of such antibodies in the patients sera[129,131]. A complement dependent mechanism of platelet destruction has been suggested[132,133]. Also Lewis antigenic determinants deposited on the surface of platelets may be recognized in ITP patients by anti-Le antibodies. During H. pylori infection the production of anti-Le antibodies is enhanced in response to Le antigens present in H. pylori LPS[123,130]. The infection can promote platelet aggregation, and the enhancement of expression of phosphatidilserine and p-selectin that may be involved in ITP development[133]. The platelet aggregation is also due to binding the von Willebrand factor by H. pylori[134]. Another possibility is that anti-H.pylori antibodies that link the platelet GP I protein with phagocyte FcRIIa receptors may increase the clearance of platelets during phagocytosis. Bacterial LPS if deposited on the surface of platelets may enhance the immune phagocytosis[135]. Th1 lymphocytes activated during infection by H. pylori antigens are important for the maintenance of ITP[133]. Concerning the host genetic factors the HLA-DQB1*03 haplotype has been proposed as useful marker for prediction of the platelet response in H. pylori infected patients[136].
H. pylori infections, especially those with CagA - positive strains, have been suggested to be associated with atherosclerotic vascular disease[58,137]. Patients suffering from CHD were found to be chronically exposed to H. pylori at a higher frequency than non-CHD individuals, which was shown by the high frequency and elevated levels of specific anti-H. pylori IgG and IgA antibodies[54,138,139] and strong inflammatory response[140], upregulation of biochemical markers, coronary lumen reduction, and elevated levels of low density lipoprotein, C-reactive protein, homocysteine, fibrinogen, plasminogen and inflammatory cytokines and the higher incidence of diabetes in H. pylori - infected individuals than in uninfected individuals[141-146]. However, in several studies, no associations between H. pylori seropositivity, exposure to CagA and CHD incidence have been found[46,147]. In the search for links between H. pylori infection and CHD, it has been suggested that H. pylori - induced antibodies with cross-reacting potency towards the host endothelium may play a role in the development and maintenance of atherosclerotic lesions. Autoimmune responses have been shown to participate in the initiation and progression of atherosclerosis[148]. Franceschi et al[149], have investigated whether antibodies against CagA cross-reacted with antigens of normal and atherosclerotic arteries, which would provide a possible link to the disorders observed during atherosclerosis. In this study, anti-CagA antibodies interacted with different parts of smooth muscle cells and endothelial cells present in the thin layer sections of atherosclerotic vessels. The antibodies recognized two vascular antigens of 160 and 180 K, which were present in both normal and atherosclerotic artery lysates and a 130 K protein from H. pylori lysates[149].
All H. pylori isolates produce urease, which can hydrolyze the urea present in the human stomach[150,151]. H. pylori urease is composed of a 26.5 kDa UreA subunit (β chain) and a 61.7 UreB subunit (α chain), which are encoded by the ureA and ureB genes, respectively[152,153]. Although the UreA subunit is the major immunodominant protein the UreB subunit has a higher number of epitopes recognized by anti-urease antibodies[154,155]. The occurrence of anti-urease antibodies was correlated with age and the immunoglobulin class and was linked with the severity of H. pylori - related disease symptoms. Superficial gastritis was correlated with a higher production of anti-urease IgA, whereas athrophy of the gastric epithelium was associated with elevated levels of anti-urease IgG immunoglobulins[156]. Recently, a hypothesis linking atherosclerosis and H. pylori - induced anti-urease antibodies has been suggested[157]. In the study by Arabski et al[158], a significant correlation between the level of antibodies recognizing the 8-mer synthetic peptide corresponding to the UreB minimal flap epitope of H. pylori urease and atherosclerosis symptoms was found. This H. pylori urease region exhibited similarity to the human CCRL1 (CC chemokine receptor-like 1) protein, which is expressed in heart tissue. Antibodies to H. pylori urease initiated during infection might be autoreactive due to the binding of the IKEDV motif in the CCRL1 host receptor. This antigen-antibody interaction may potentially accelerate complement - dependent tissue destruction and the inflammatory response in patients with atherosclerosis lesions and H. pylori infections[157,158].
H. pylori synthesizes the two heat shock proteins: HspA (GroES chaperonin or Hsp 10 homologue) and HspB (GroEL chaperonin or Hsp60 homologue)[76,159,160]. Both antigens reportedly induce autoimmune responses[161,162]. The study by Matusiak et al[139], supports the idea that chronic exposure to H. pylori in patients with CHD may result in an increase in the level of serum lipopolysaccharide - binding protein (LBP) and the production of antibodies against H. pylori Hsp B, which crossreact with human Hsp60. Both LBP and anti-Hsp 60 antibodies may facilitate the inflammation in the vascular endothelium. The pathological role of LBP may depend on the phenotype of the vascular endothelium, which exhibits proinflammatory features such as increased expression of pathogen recognition receptors. The involvement of anti-Hsp60 Igs in CHD-related deleterious processes can be explained by the antigenic mimicry and complement - dependent cell damage, which are possibly induced by these antibodies, similarly to the anti-H. pylori urease antibodies. Because the expression levels of Hsp proteins, including Hsp60, increases as a result of the inflammatory process in atherosclerotic lesions, it can be assumed that these proteins may be a target for anti-Hsp antibodies initiated by an infectious agent[148]. In regard to HspA, the clinical outcomes of H. pylori infection have been shown to be unrelated to HspA antigenicity or amino acid sequence variation[160]. However, age-specific responses to HspA in H. pylori-positive subjects have been found[163].
The association between H. pylori infection and insulin resistance has been suggested[146]. Recently, a significant homology between the human carbonic anhydrase II segment 5-255 and the α-carbonic anhydrase of H. pylori segment 23-239, has been found, with 27% identity and 41% similarity[77]. Anhydrase is a key enzyme for the survival and growth of H. pylori in the gastric mucosa. In humans carbonic anhydrase II coordinates the physiological function of the pancreas. Moreover, the homologous regions contain the binding motifs of the HLA DRB1*0405[77]. These observations support the idea that H. pylori infection can trigger autoimmune pancreatitis in genetically susceptible individuals. In 2009, Frulloni et al[164] showed that in almost all patients with autoimmune pancreatitis there are antibodies against H. pylori plasminogen-binding protein (PBP). This PBP protein shows homology with ubiquitin-protein ligase E3 component n-recognition 2, which is an enzyme highly expressed in the acinar cells of the pancreas. This could be another example of H. pylori and host molecular mimicry triggering autoimmune pancreatitis.
The relationship between H. pylori infections and growth retardation in children is poorly understood. Growth retardation may result from appetite disorders, abnormal metabolism and iron deficiency[165]. Infection with H. pylori causes gastrointestinal bleeding, abnormal absorption of iron due to the impaired gastric acid and insulin secretion, and vitamin C uptake. The mechanism driving anemia in children infected with H. pylori may be antigenic mimicry. H. pylori has an iron-binding protein similar to ferritin, which prevents iron excess. The infection also causes an increase in the concentration of iron-binding lactoferrin in the stomach epithelium[165]. Recent studies indicate the role of the immune system in controlling behaviors related to food intake by producing autoantibodies against peptides and neuropeptides regulating appetite, which may result in a reduction in height and weight[166].
The gastrointestinal microflora, including H. pylori may be a source of antigens, which are similar to appetite - regulating peptides. Thus, the bacterial antigens are potentially able to stimulate the immune system of the gastrointestinal tract to produce autoantibodies that are cross-reactive with many of the appetite -regulating peptides and that modify the actions of these peptides. In the sera of pediatric patients with short stature the autoantibodies against 14 key hormones and peptides regulating appetite such as leptin, ghrelin, orexin, and alpha-melanocyte-stimulating hormone (α-MSH), have been detected[166]. These antibodies are also present in healthy subjects, which suggests physiological role for these antibodies in the regulation of hunger and satiety. A number of common sequences between these peptides and proteins of microorganisms have been identified, including antigenic similarity between leptin and the intestinal microflora proteins of Lactococcus lactis, Escherichia coli, Lactobacillus bacteriophage and representatives of Candida and Aspergillus. The sequence homology of α-MSH and the components of pathogenic E. coli, H. pylori, Clostridium tetani, and Candida albicans has also been demonstrated. Regulatory peptides are signaling molecules, and autoantibody blocking of their sequences may alter their biological activity. A recent study highlighted the impact of H. pylori on the secretion of ghrelin and leptin[167]. Patients infected with H. pylori have been shown to have a significantly lower level of leptin and ghrelin in the plasma. The ghrelin concentration was also lower in the mucous cells of the stomach. After eradication of the infection, the level of ghrelin rose again. However, other authors did not confirm this result[168]. In the studies carried out on a group of Polish children, it was shown that the levels of gastrin in the patients infected with H. pylori were significantly higher, whereas the levels of ghrelin and leptin were lower than those of the controls[169]. Growth failure in children due to anemia occurs more often in patients infected with H. pylori cagA+ than cagA- strains. It has been shown that platelet glycoprotein and the CagA protein are similar and that many patients infected with H. pylori with signs of thrombocytopenia have possess anti-platelet antibodies[165]. These results suggest a role for the CagA protein in the development of systemic pathological processes in children infected with cagA+ H. pylori strains. Further studies are needed to assess the prevalence and the levels of antibodies against the common sequences for CagA protein and peptides regulating appetite in children with short stature. These sequences have been identified by bioinformatic analysis of leptin, ghrelin, visfatin and resistin, which regulate appetite, energy homeostasis, and potentially the immune system[167,170-173]. The release of these proteins is often stimulated by inflammatory processes, growth and gonadal hormones. The results of experiments conducted with serum samples from children with idiopathic short stature and growth hormone deficiency showed that some of the children that were also infected with H. pylori and/or exposed to C. albicans have antibodies against ghrelin, leptin, orexin A and α-MSH, which may potentially disturb the physiological functions of these molecules[174,175]. This result is potentially due to molecular mimicry between antigens of these microbiota and the mentioned peptides. However, further studies are needed to elucidate this suggested relation.
Recently, amino acid identity between additional autoantigens derived from the gastric mucosa and gastric adenocarcinoma cells (AGS) and several H. pylori proteins has been identified. A proteomics investigation of anti-gastric autoantibody profiles in the sera of 300 Korean adults infected with H. pylori, revealed nearly forty autoantigenic proteins, including nicotinamide adenine dinucleotide phosphate (NADP+) alcohol dehydrogenase, alpha enolase, gastrokine-1, gastric triacylglycerol lipase, Hsp70 kDa protein 1, and peroxiredoxin-2. These proteins were detected in the gastric mucosal tissue[59]. The programmed cell death 6 - interacting protein, serum albumin and T-complex protein 1 subunit gamma were identified in the AGS cells. Several proteins such as albumin, alpha-enolase, annexin A3, cytoplasmic actin 1, Hsp - like 71 kDa protein and leukocyte elastase inhibitor, were detected in AGS cells and gastric mucosal tissue. Furthermore, the alpha-enolase, glutathione S-transferase P, Hsp - like 71 kDa protein, Hsp70 kDa protein 1, mitochondrial Hsp60 kDa, peroxiredoxin-2, 78 kDa glucose-regulated protein precursor, tyrosine-protein phosphatase non-receptor type 11 and tryptophan-aspartic acid repeat-containing protein (WD), showed 60% or even higher amino acid positivity[59]. These newly described gastric proteins may have the ability to control and prevention gastroduodenal disorders linked to H. pylori infections, such as chronic gastritis, gastroduodenal ulcers, atrophic gastritis and gastric cancers. However, their role in the pathophysiology of these disorders needs to be examined.
Gastric tissue ulceration initiated by H. pylori is related to the elevated production of alarming molecules, including IL-33, which may function as a classic cytokine or transcription factor[176]. IL-33 is suspected to alert the immune system to restore epithelial cell homeostasis. However, IL-33 has also been suspected to play an emerging role in autoimmune diseases[177]. Bioinformatic analysis indicates homology between the amino acid sequences of H. pylori CagA and human Hsp60, as well as IL-33. Hypothetically, both host Hsp60 and IL-33 can be targeted by antibodies induced during H. pylori cagA+ infections, which may affect gastric inflammatory reactions.
Although the homologous sequences of H. pylori and several new host targets have been demonstrated by computer and proteomic analyses, more research is needed to demonstrate the role of these homologous sequences in development of pathological processes due to autoimmune responses initiated by H. pylori components.
In light of this review, we hypothesize that H. pylori possessing antigens that are similar in structure to human cells, tissues and some humoral compounds, which play an important structural and physiological role, through induction of humoral and possible cellular immune responses, may drive tissue destruction and the development a pathological inflammatory response. Chronic exposure of specific memory cells to these H. pylori compounds enables their sustained stimulation and transformation into effector lymphocytes, which may be involved in the autoimmune-mediated tissue destruction. Further studies and deeper analyses are necessary to demonstrate the autoimmune potential of specific H. pylori antigens.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Poland
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Balaban YH, Franceschi F, Vilaichone RK, Yamaoka Y, Youn HS S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
| 1. | Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311-1315. [PubMed] [Cited in This Article: ] |
| 2. | Blaser MJ, Atherton JC. Helicobacter pylori persistence: biology and disease. J Clin Invest. 2004;113:321-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Peek RM, Crabtree JE. Helicobacter infection and gastric neoplasia. J Pathol. 2006;208:233-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 368] [Cited by in F6Publishing: 417] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 4. | Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol. 2010;7:629-641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Posselt G, Backert S, Wessler S. The functional interplay of Helicobacter pylori factors with gastric epithelial cells induces a multi-step process in pathogenesis. Cell Commun Signal. 2013;11:77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 6. | Suzuki N, Murata-Kamiya N, Yanagiya K, Suda W, Hattori M, Kanda H, Bingo A, Fujii Y, Maeda S, Koike K. Mutual reinforcement of inflammation and carcinogenesis by the Helicobacter pylori CagA oncoprotein. Sci Rep. 2015;5:10024. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Machado JC, Figueiredo C, Canedo P, Pharoah P, Carvalho R, Nabais S, Castro Alves C, Campos ML, Van Doorn LJ, Caldas C. A proinflammatory genetic profile increases the risk for chronic atrophic gastritis and gastric carcinoma. Gastroenterology. 2003;125:364-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 8. | Ilver D, Arnqvist A, Ogren J, Frick IM, Kersulyte D, Incecik ET, Berg DE, Covacci A, Engstrand L, Borén T. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 1998;279:373-377. [PubMed] [Cited in This Article: ] |
| 9. | Mahdavi J, Sondén B, Hurtig M, Olfat FO, Forsberg L, Roche N, Angstrom J, Larsson T, Teneberg S, Karlsson KA. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science. 2002;297:573-578. [PubMed] [Cited in This Article: ] |
| 10. | Styer CM, Hansen LM, Cooke CL, Gundersen AM, Choi SS, Berg DE, Benghezal M, Marshall BJ, Peek RM, Borén T. Expression of the BabA adhesin during experimental infection with Helicobacter pylori. Infect Immun. 2010;78:1593-1600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Pai R, Wyle FA, Cover TL, Itani RM, Domek MJ, Tarnawski AS. Helicobacter pylori culture supernatant interferes with epidermal growth factor-activated signal transduction in human gastric KATO III cells. Am J Pathol. 1998;152:1617-1624. [PubMed] [Cited in This Article: ] |
| 12. | Seto K, Hayashi-Kuwabara Y, Yoneta T, Suda H, Tamaki H. Vacuolation induced by cytotoxin from Helicobacter pylori is mediated by the EGF receptor in HeLa cells. FEBS Lett. 1998;431:347-350. [PubMed] [Cited in This Article: ] |
| 13. | Lytton SD, Fischer W, Nagel W, Haas R, Beck FX. Production of ammonium by Helicobacter pylori mediates occludin processing and disruption of tight junctions in Caco-2 cells. Microbiology. 2005;151:3267-3276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Yahiro K, Wada A, Nakayama M, Kimura T, Ogushi K, Niidome T, Aoyagi H, Yoshino K, Yonezawa K, Moss J. Protein-tyrosine phosphatase alpha, RPTP alpha, is a Helicobacter pylori VacA receptor. J Biol Chem. 2003;278:19183-19189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 15. | Yahiro K, Satoh M, Nakano M, Hisatsune J, Isomoto H, Sap J, Suzuki H, Nomura F, Noda M, Moss J. Low-density lipoprotein receptor-related protein-1 (LRP1) mediates autophagy and apoptosis caused by Helicobacter pylori VacA. J Biol Chem. 2012;287:31104-31115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | Cover TL, Blanke SR. Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat Rev Microbiol. 2005;3:320-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 372] [Cited by in F6Publishing: 380] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 17. | Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, Massone A, Papini E, Xiang Z, Figura N. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci USA. 1993;90:5791-5795. [PubMed] [Cited in This Article: ] |
| 18. | Tummuru MK, Cover TL, Blaser MJ. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect Immun. 1993;61:1799-1809. [PubMed] [Cited in This Article: ] |
| 19. | Crabtree JE, Covacci A, Farmery SM, Xiang Z, Tompkins DS, Perry S, Lindley IJ, Rappuoli R. Helicobacter pylori induced interleukin-8 expression in gastric epithelial cells is associated with CagA positive phenotype. J Clin Pathol. 1995;48:41-45. [PubMed] [Cited in This Article: ] |
| 20. | Huang JQ, Zheng GF, Sumanac K, Irvine EJ, Hunt RH. Meta-analysis of the relationship between cagA seropositivity and gastric cancer. Gastroenterology. 2003;125:1636-1644. [PubMed] [Cited in This Article: ] |
| 21. | Backert S, Clyne M, Tegtmeyer N. Molecular mechanisms of gastric epithelial cell adhesion and injection of CagA by Helicobacter pylori. Cell Commun Signal. 2011;9:28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 22. | Higashi H, Tsutsumi R, Muto S, Sugiyama T, Azuma T, Asaka M, Hatakeyama M. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science. 2002;295:683-686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 755] [Cited by in F6Publishing: 758] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 23. | Suzuki M, Mimuro H, Kiga K, Fukumatsu M, Ishijima N, Morikawa H, Nagai S, Koyasu S, Gilman RH, Kersulyte D. Helicobacter pylori CagA phosphorylation-independent function in epithelial proliferation and inflammation. Cell Host Microbe. 2009;5:23-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 245] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 24. | Peek RM, Moss SF, Tham KT, Pérez-Pérez GI, Wang S, Miller GG, Atherton JC, Holt PR, Blaser MJ. Helicobacter pylori cagA+ strains and dissociation of gastric epithelial cell proliferation from apoptosis. J Natl Cancer Inst. 1997;89:863-868. [PubMed] [Cited in This Article: ] |
| 25. | Paziak-Domańska B, Chmiela M, Jarosińska A, Rudnicka W. Potential role of CagA in the inhibition of T cell reactivity in Helicobacter pylori infections. Cell Immunol. 2000;202:136-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Censini S, Lange C, Xiang Z, Crabtree JE, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. Cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648-14653. [PubMed] [Cited in This Article: ] |
| 27. | Backert S, Kwok T, Schmid M, Selbach M, Moese S, Peek RM, König W, Meyer TF, Jungblut PR. Subproteomes of soluble and structure-bound Helicobacter pylori proteins analyzed by two-dimensional gel electrophoresis and mass spectrometry. Proteomics. 2005;5:1331-1345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Olofsson A, Vallström A, Petzold K, Tegtmeyer N, Schleucher J, Carlsson S, Haas R, Backert S, Wai SN, Gröbner G. Biochemical and functional characterization of Helicobacter pylori vesicles. Mol Microbiol. 2010;77:1539-1555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 155] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 29. | Parker H, Chitcholtan K, Hampton MB, Keenan JI. Uptake of Helicobacter pylori outer membrane vesicles by gastric epithelial cells. Infect Immun. 2010;78:5054-5061. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 30. | Fiocca R, Necchi V, Sommi P, Ricci V, Telford J, Cover TL, Solcia E. Release of Helicobacter pylori vacuolating cytotoxin by both a specific secretion pathway and budding of outer membrane vesicles. Uptake of released toxin and vesicles by gastric epithelium. J Pathol. 1999;188:220-226. [PubMed] [Cited in This Article: ] |
| 31. | Heczko U, Smith VC, Mark Meloche R, Buchan AM, Finlay BB. Characteristics of Helicobacter pylori attachment to human primary antral epithelial cells. Microbes Infect. 2000;2:1669-1676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Olofsson A, Nygård Skalman L, Obi I, Lundmark R, Arnqvist A. Uptake of Helicobacter pylori vesicles is facilitated by clathrin-dependent and clathrin-independent endocytic pathways. MBio. 2014;5:e00979-e00914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 33. | Shimoda A, Ueda K, Nishiumi S, Murata-Kamiya N, Mukai SA, Sawada S, Azuma T, Hatakeyama M, Akiyoshi K. Exosomes as nanocarriers for systemic delivery of the Helicobacter pylori virulence factor CagA. Sci Rep. 2016;6:18346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 34. | Akada J, Okuda M, Hiramoto N, Kitagawa T, Zhang X, Kamei S, Ito A, Nakamura M, Uchida T, Hiwatani T. Proteomic characterization of Helicobacter pylori CagA antigen recognized by child serum antibodies and its epitope mapping by peptide array. PLoS One. 2014;9:e104611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Arnold IC, Hitzler I, Engler D, Oertli M, Agger EM, Müller A. The C-terminally encoded, MHC class II-restricted T cell antigenicity of the Helicobacter pylori virulence factor CagA promotes gastric preneoplasia. J Immunol. 2011;186:6165-6172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Shiota S, Matsunari O, Watada M, Yamaoka Y. Serum Helicobacter pylori CagA antibody as a biomarker for gastric cancer in east-Asian countries. Future Microbiol. 2010;5:1885-1893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | Tummuru MK, Sharma SA, Blaser MJ. Helicobacter pylori picB, a homologue of the Bordetella pertussis toxin secretion protein, is required for induction of IL-8 in gastric epithelial cells. Mol Microbiol. 1995;18:867-876. [PubMed] [Cited in This Article: ] |
| 38. | Yamaoka Y, Kita M, Kodama T, Sawai N, Kashima K, Imanishi J. Induction of various cytokines and development of severe mucosal inflammation by cagA gene positive Helicobacter pylori strains. Gut. 1997;41:442-451. [PubMed] [Cited in This Article: ] |
| 39. | Smith MF, Mitchell A, Li G, Ding S, Fitzmaurice AM, Ryan K, Crowe S, Goldberg JB. Toll-like receptor (TLR) 2 and TLR5, but not TLR4, are required for Helicobacter pylori-induced NF-kappa B activation and chemokine expression by epithelial cells. J Biol Chem. 2003;278:32552-32560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 40. | Grebowska A, Moran AP, Matusiak A, Bak-Romaniszyn L, Czkwianianc E, Rechciński T, Walencka M, Płaneta-Małecka I, Rudnicka W, Chmiela M. Anti-phagocytic activity of Helicobacter pylori lipopolysaccharide (LPS)--possible modulation of the innate immune response to these bacteria. Pol J Microbiol. 2008;57:185-192. [PubMed] [Cited in This Article: ] |
| 41. | Grebowska A, Moran AP, Bielanski W, Matusiak A, Rechcinski T, Rudnicka K, Baranowska A, Rudnicka W, Chmiela M. Helicobacter pylori lipopolysaccharide activity in human peripheral blood mononuclear leukocyte cultures. J Physiol Pharmacol. 2010;61:437-442. [PubMed] [Cited in This Article: ] |
| 42. | Rudnicka K, Miszczyk E, Matusiak A, Walencka M, Moran AP, Rudnicka W, Chmiela M. Helicobacter pylori-driven modulation of NK cell expansion, intracellular cytokine expression and cytotoxic activity. Innate Immun. 2015;21:127-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 43. | Suzuki M, Mori M, Miyayama A, Iwai N, Tsunematsu N, Oonuki M, Suzuki H, Hibi T, Ishii H. Enhancement of neutrophil infiltration in the corpus after failure of Helicobacter pylori eradication. J Clin Gastroenterol. 1997;25 Suppl 1:S222-S228. [PubMed] [Cited in This Article: ] |
| 44. | Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164-6172. [PubMed] [Cited in This Article: ] |
| 45. | Wroblewski LE, Shen L, Ogden S, Romero-Gallo J, Lapierre LA, Israel DA, Turner JR, Peek RM. Helicobacter pylori dysregulation of gastric epithelial tight junctions by urease-mediated myosin II activation. Gastroenterology. 2009;136:236-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 46. | Tan HJ, Goh KL. Extragastrointestinal manifestations of Helicobacter pylori infection: facts or myth? A critical review. J Dig Dis. 2012;13:342-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 47. | Papagiannakis P, Michalopoulos C, Papalexi F, Dalampoura D, Diamantidis MD. The role of Helicobacter pylori infection in hematological disorders. Eur J Intern Med. 2013;24:685-690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 48. | Tiwari SK, G M, Khan AA, Habeeb A, Habibullah CM. Chronic Idiopathic Thrombocytopenia Purpura and Helicobacter pylori Eradication: A case study. Gastroenterology Res. 2009;2:57-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 49. | Zhuo WL, Zhu B, Xiang ZL, Zhuo XL, Cai L, Chen ZT. Assessment of the relationship between Helicobacter pylori and lung cancer: a meta-analysis. Arch Med Res. 2009;40:406-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 50. | Yeh JJ, Tsai S, Wu DC, Wu JY, Liu TC, Chen A. P-selectin-dependent platelet aggregation and apoptosis may explain the decrease in platelet count during Helicobacter pylori infection. Blood. 2010;115:4247-4253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 51. | Tamer GS, Tengiz I, Ercan E, Duman C, Alioglu E, Turk UO. Helicobacter pylori seropositivity in patients with acute coronary syndromes. Dig Dis Sci. 2009;54:1253-1256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | El-Eshmawy MM, El-Hawary AK, Abdel Gawad SS, El-Baiomy AA. Helicobacter pylori infection might be responsible for the interconnection between type 1 diabetes and autoimmune thyroiditis. Diabetol Metab Syndr. 2011;3:28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 53. | Deng B, Li Y, Zhang Y, Bai L, Yang P. Helicobacter pylori infection and lung cancer: a review of an emerging hypothesis. Carcinogenesis. 2013;34:1189-1195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Mendall MA, Goggin PM, Molineaux N, Levy J, Toosy T, Strachan D, Camm AJ, Northfield TC. Relation of Helicobacter pylori infection and coronary heart disease. Br Heart J. 1994;71:437-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 409] [Cited by in F6Publishing: 452] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 55. | Danesh J, Peto R. Risk factors for coronary heart disease and infection with Helicobacter pylori: meta-analysis of 18 studies. BMJ. 1998;316:1130-1132. [PubMed] [Cited in This Article: ] |
| 56. | Chmiela M, Kowalewicz-Kulbat M, Miszczak A, Wisniewska M, Rechcinski T, Kolodziej K, Kasprzak J, Wadstrom T, Rudnicka W. A link between Helicobacter pylori and/or Chlamydia spp. infections and atherosclerosis. FEMS Immunol Med Microbiol. 2003;36:187-192. [PubMed] [Cited in This Article: ] |
| 57. | Chmiela M, Gajewski A, Rudnicka K. Helicobacter pylori vs coronary heart disease - searching for connections. World J Cardiol. 2015;7:187-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 58. | Pasceri V, Cammarota G, Patti G, Cuoco L, Gasbarrini A, Grillo RL, Fedeli G, Gasbarrini G, Maseri A. Association of virulent Helicobacter pylori strains with ischemic heart disease. Circulation. 1998;97:1675-1679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 205] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 59. | Park JS, Lee SJ, Kim TH, Yeom J, Park ES, Seo JH, Jun JS, Lim JY, Park CH, Woo HO. Gastric autoantigenic proteins in Helicobacter pylori infection. Yonsei Med J. 2013;54:1342-1352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 60. | Quaratino S, Thorpe CJ, Travers PJ, Londei M. Similar antigenic surfaces, rather than sequence homology, dictate T-cell epitope molecular mimicry. Proc Natl Acad Sci USA. 1995;92:10398-10402. [PubMed] [Cited in This Article: ] |
| 61. | Oldstone MB. Molecular mimicry as a mechanism for the cause and a probe uncovering etiologic agent(s) of autoimmune disease. Curr Top Microbiol Immunol. 1989;145:127-135. [PubMed] [Cited in This Article: ] |
| 62. | Sfriso P, Ghirardello A, Botsios C, Tonon M, Zen M, Bassi N, Bassetto F, Doria A. Infections and autoimmunity: the multifaceted relationship. J Leukoc Biol. 2010;87:385-395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 63. | Whitton JL, Feuer R. Myocarditis, microbes and autoimmunity. Autoimmunity. 2004;37:375-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 64. | Shahrizaila N, Yuki N. Guillain-barré syndrome animal model: the first proof of molecular mimicry in human autoimmune disorder. J Biomed Biotechnol. 2011;2011:829129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 65. | Wandinger K, Jabs W, Siekhaus A, Bubel S, Trillenberg P, Wagner H, Wessel K, Kirchner H, Hennig H. Association between clinical disease activity and Epstein-Barr virus reactivation in MS. Neurology. 2000;55:178-184. [PubMed] [Cited in This Article: ] |
| 66. | Fujinami RS, Oldstone MB. Amino acid homology between the encephalitogenic site of myelin basic protein and virus: mechanism for autoimmunity. Science. 1985;230:1043-1045. [PubMed] [Cited in This Article: ] |
| 67. | Fujinami RS, Oldstone MB, Wroblewska Z, Frankel ME, Koprowski H. Molecular mimicry in virus infection: crossreaction of measles virus phosphoprotein or of herpes simplex virus protein with human intermediate filaments. Proc Natl Acad Sci USA. 1983;80:2346-2350. [PubMed] [Cited in This Article: ] |
| 68. | Ebringer A, Baines M, Ptaszynska T. Spondyloarthritis, uveitis, HLA-B27 and Klebsiella. Immunol Rev. 1985;86:101-116. [PubMed] [Cited in This Article: ] |
| 69. | Negrini R, Savio A, Poiesi C, Appelmelk BJ, Buffoli F, Paterlini A, Cesari P, Graffeo M, Vaira D, Franzin G. Antigenic mimicry between Helicobacter pylori and gastric mucosa in the pathogenesis of body atrophic gastritis. Gastroenterology. 1996;111:655-665. [PubMed] [Cited in This Article: ] |
| 70. | Negrini R, Savio A, Appelmelk BJ. Autoantibodies to gastric mucosa in Helicobacter pylori infection. Helicobacter. 1997;2 Suppl 1:S13-S16. [PubMed] [Cited in This Article: ] |
| 71. | D’Elios MM, Appelmelk BJ, Amedei A, Bergman MP, Del Prete G. Gastric autoimmunity: the role of Helicobacter pylori and molecular mimicry. Trends Mol Med. 2004;10:316-323. [PubMed] [Cited in This Article: ] |
| 72. | Ge Z, Hiratsuka K, Taylor DE. Nucleotide sequence and mutational analysis indicate that two Helicobacter pylori genes encode a P-type ATPase and a cation-binding protein associated with copper transport. Mol Microbiol. 1995;15:97-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 77] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 73. | Melchers K, Weitzenegger T, Buhmann A, Steinhilber W, Sachs G, Schäfer KP. Cloning and membrane topology of a P type ATPase from Helicobacter pylori. J Biol Chem. 1996;271:446-457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 84] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 74. | Cover TL, Tummuru MK, Cao P, Thompson SA, Blaser MJ. Divergence of genetic sequences for the vacuolating cytotoxin among Helicobacter pylori strains. J Biol Chem. 1994;269:10566-10573. [PubMed] [Cited in This Article: ] |
| 75. | Uibo R, Vorobjova T, Metsküla K, Kisand K, Wadström T, Kivik T. Association of Helicobacter pylori and gastric autoimmunity: a population-based study. FEMS Immunol Med Microbiol. 1995;11:65-68. [PubMed] [Cited in This Article: ] |
| 76. | Kansau I, Guillain F, Thiberge JM, Labigne A. Nickel binding and immunological properties of the C-terminal domain of the Helicobacter pylori GroES homologue (HspA). Mol Microbiol. 1996;22:1013-1023. [PubMed] [Cited in This Article: ] |
| 77. | Guarneri F, Guarneri C, Benvenga S. Helicobacter pylori and autoimmune pancreatitis: role of carbonic anhydrase via molecular mimicry? J Cell Mol Med. 2005;9:741-744. [PubMed] [Cited in This Article: ] |
| 78. | Schwartz M. Intrinsic factor antibody in serum from patients with pernicious anaemia. Lancet. 1960;2:1263-1267. [PubMed] [Cited in This Article: ] |
| 79. | Taylor KB, Roitt IM, Doniach D, Couchman KG, Shapland C. Autoimmune phenomena in pernicious anaemia: gastric antibodies. Br Med J. 1962;2:1347-1352. [PubMed] [Cited in This Article: ] |
| 80. | Strickland RG, Mackay IR. A reappraisal of the nature and significance of chronic atrophic gastritis. Am J Dig Dis. 1973;18:426-440. [PubMed] [Cited in This Article: ] |
| 81. | Toh BH, van Driel IR, Gleeson PA. Pernicious anemia. N Engl J Med. 1997;337:1441-1448. [PubMed] [Cited in This Article: ] |
| 82. | Tomb JF, White O, Kerlavage AR, Clayton RA, Sutton GG, Fleischmann RD, Ketchum KA, Klenk HP, Gill S, Dougherty BA. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539-547. [PubMed] [Cited in This Article: ] |
| 83. | De Block CE, De Leeuw IH, Van Gaal LF. High prevalence of manifestations of gastric autoimmunity in parietal cell antibody-positive type 1 (insulin-dependent) diabetic patients. The Belgian Diabetes Registry. J Clin Endocrinol Metab. 1999;84:4062-4067. [PubMed] [Cited in This Article: ] |
| 84. | Marignani M, Delle Fave G, Mecarocci S, Bordi C, Angeletti S, D’Ambra G, Aprile MR, Corleto VD, Monarca B, Annibale B. High prevalence of atrophic body gastritis in patients with unexplained microcytic and macrocytic anemia: a prospective screening study. Am J Gastroenterol. 1999;94:766-772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 85. | De Block CE, Van Campenhout CM, De Leeuw IH, Keenoy BM, Martin M, Van Hoof V, Van Gaal LF. Soluble transferrin receptor level: a new marker of iron deficiency anemia, a common manifestation of gastric autoimmunity in type 1 diabetes. Diabetes Care. 2000;23:1384-1388. [PubMed] [Cited in This Article: ] |
| 86. | Kokkola A, Sjöblom SM, Haapiainen R, Sipponen P, Puolakkainen P, Järvinen H. The risk of gastric carcinoma and carcinoid tumours in patients with pernicious anaemia. A prospective follow-up study. Scand J Gastroenterol. 1998;33:88-92. [PubMed] [Cited in This Article: ] |
| 87. | De Block CE, De Leeuw IH, Van Gaal LF. Autoimmune gastritis in type 1 diabetes: a clinically oriented review. J Clin Endocrinol Metab. 2008;93:363-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 88. | Claeys D, Faller G, Appelmelk BJ, Negrini R, Kirchner T. The gastric H+,K+-ATPase is a major autoantigen in chronic Helicobacter pylori gastritis with body mucosa atrophy. Gastroenterology. 1998;115:340-347. [PubMed] [Cited in This Article: ] |
| 89. | van Driel IR, Baxter AG, Laurie KL, Zwar TD, La Gruta NL, Judd LM, Scarff KL, Silveira PA, Gleeson PA. Immunopathogenesis, loss of T cell tolerance and genetics of autoimmune gastritis. Autoimmun Rev. 2002;1:290-297. [PubMed] [Cited in This Article: ] |
| 90. | Toh BH, Sentry JW, Alderuccio F. The causative H+/K+ ATPase antigen in the pathogenesis of autoimmune gastritis. Immunol Today. 2000;21:348-354. [PubMed] [Cited in This Article: ] |
| 91. | Amedei A, Bergman MP, Appelmelk BJ, Azzurri A, Benagiano M, Tamburini C, van der Zee R, Telford JL, Vandenbroucke-Grauls CM, D’Elios MM. Molecular mimicry between Helicobacter pylori antigens and H+, K+ --adenosine triphosphatase in human gastric autoimmunity. J Exp Med. 2003;198:1147-1156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 173] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 92. | van Driel IR, Read S, Zwar TD, Gleeson PA. Shaping the T cell repertoire to a bona fide autoantigen: lessons from autoimmune gastritis. Curr Opin Immunol. 2005;17:570-576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 93. | Ma JY, Borch K, Sjöstrand SE, Janzon L, Mårdh S. Positive correlation between H,K-adenosine triphosphatase autoantibodies and Helicobacter pylori antibodies in patients with pernicious anemia. Scand J Gastroenterol. 1994;29:961-965. [PubMed] [Cited in This Article: ] |
| 94. | Faller G, Steininger H, Kränzlein J, Maul H, Kerkau T, Hensen J, Hahn EG, Kirchner T. Antigastric autoantibodies in Helicobacter pylori infection: implications of histological and clinical parameters of gastritis. Gut. 1997;41:619-623. [PubMed] [Cited in This Article: ] |
| 95. | Faller G, Steininger H, Appelmelk B, Kirchner T. Evidence of novel pathogenic pathways for the formation of antigastric autoantibodies in Helicobacter pylori gastritis. J Clin Pathol. 1998;51:244-245. [PubMed] [Cited in This Article: ] |
| 96. | Villako K, Kekki M, Maaroos HI, Sipponen P, Tammur R, Tamm A, Keevallik R. A 12-year follow-up study of chronic gastritis and Helicobacter pylori in a population-based random sample. Scand J Gastroenterol. 1995;30:964-967. [PubMed] [Cited in This Article: ] |
| 97. | Oksanen A, Sipponen P, Karttunen R, Miettinen A, Veijola L, Sarna S, Rautelin H. Atrophic gastritis and Helicobacter pylori infection in outpatients referred for gastroscopy. Gut. 2000;46:460-463. [PubMed] [Cited in This Article: ] |
| 98. | Negrini R, Lisato L, Zanella I, Cavazzini L, Gullini S, Villanacci V, Poiesi C, Albertini A, Ghielmi S. Helicobacter pylori infection induces antibodies cross-reacting with human gastric mucosa. Gastroenterology. 1991;101:437-445. [PubMed] [Cited in This Article: ] |
| 99. | Appelmelk BJ, Simoons-Smit I, Negrini R, Moran AP, Aspinall GO, Forte JG, De Vries T, Quan H, Verboom T, Maaskant JJ. Potential role of molecular mimicry between Helicobacter pylori lipopolysaccharide and host Lewis blood group antigens in autoimmunity. Infect Immun. 1996;64:2031-2040. [PubMed] [Cited in This Article: ] |
| 100. | Simoons-Smit IM, Appelmelk BJ, Verboom T, Negrini R, Penner JL, Aspinall GO, Moran AP, Fei SF, Shi BS, Rudnica W. Typing of Helicobacter pylori with monoclonal antibodies against Lewis antigens in lipopolysaccharide. J Clin Microbiol. 1996;34:2196-2200. [PubMed] [Cited in This Article: ] |
| 101. | Aspinall GO, Monteiro MA, Pang H, Walsh EJ, Moran AP. Lipopolysaccharide of the Helicobacter pylori type strain NCTC 11637 (ATCC 43504): structure of the O antigen chain and core oligosaccharide regions. Biochemistry. 1996;35:2489-2497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 156] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 102. | Aspinall GO, Monteiro MA. Lipopolysaccharides of Helicobacter pylori strains P466 and MO19: structures of the O antigen and core oligosaccharide regions. Biochemistry. 1996;35:2498-2504. [PubMed] [Cited in This Article: ] |
| 103. | Heneghan MA, McCarthy CF, Moran AP. Relationship of blood group determinants on Helicobacter pylori lipopolysaccharide with host Lewis phenotype and inflammatory response. Infect Immun. 2000;68:937-941. [PubMed] [Cited in This Article: ] |
| 104. | Monteiro MA, Chan KH, Rasko DA, Taylor DE, Zheng PY, Appelmelk BJ, Wirth HP, Yang M, Blaser MJ, Hynes SO. Simultaneous expression of type 1 and type 2 Lewis blood group antigens by Helicobacter pylori lipopolysaccharides. Molecular mimicry between h. pylori lipopolysaccharides and human gastric epithelial cell surface glycoforms. J Biol Chem. 1998;273:11533-11543. [PubMed] [Cited in This Article: ] |
| 105. | Monteiro MA, Appelmelk BJ, Rasko DA, Moran AP, Hynes SO, MacLean LL, Chan KH, Michael FS, Logan SM, O’Rourke J. Lipopolysaccharide structures of Helicobacter pylori genomic strains 26695 and J99, mouse model H. pylori Sydney strain, H. pylori P466 carrying sialyl Lewis X, and H. pylori UA915 expressing Lewis B classification of H. pylori lipopolysaccharides into glycotype families. Eur J Biochem. 2000;267:305-320. [PubMed] [Cited in This Article: ] |
| 106. | Monteiro MA, Zheng P, Ho B, Yokota S, Amano K, Pan Z, Berg DE, Chan KH, MacLean LL, Perry MB. Expression of histo-blood group antigens by lipopolysaccharides of Helicobacter pylori strains from asian hosts: the propensity to express type 1 blood-group antigens. Glycobiology. 2000;10:701-713. [PubMed] [Cited in This Article: ] |
| 107. | Ge Z, Chan NW, Palcic MM, Taylor DE. Cloning and heterologous expression of an alpha1,3-fucosyltransferase gene from the gastric pathogen Helicobacter pylori. J Biol Chem. 1997;272:21357-21363. [PubMed] [Cited in This Article: ] |
| 108. | Rasko DA, Wang G, Palcic MM, Taylor DE. Cloning and characterization of the alpha(1,3/4) fucosyltransferase of Helicobacter pylori. J Biol Chem. 2000;275:4988-4994. [PubMed] [Cited in This Article: ] |
| 109. | Wirth HP, Yang M, Peek RM, Tham KT, Blaser MJ. Helicobacter pylori Lewis expression is related to the host Lewis phenotype. Gastroenterology. 1997;113:1091-1098. [PubMed] [Cited in This Article: ] |
| 110. | Moran AP, Knirel YA, Senchenkova SN, Widmalm G, Hynes SO, Jansson PE. Phenotypic variation in molecular mimicry between Helicobacter pylori lipopolysaccharides and human gastric epithelial cell surface glycoforms. Acid-induced phase variation in Lewis(x) and Lewis(y) expression by H. Pylori lipopolysaccharides. J Biol Chem. 2002;277:5785-5795. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 111. | Appelmelk BJ, Shiberu B, Trinks C, Tapsi N, Zheng PY, Verboom T, Maaskant J, Hokke CH, Schiphorst WE, Blanchard D. Phase variation in Helicobacter pylori lipopolysaccharide. Infect Immun. 1998;66:70-76. [PubMed] [Cited in This Article: ] |
| 112. | Appelmelk BJ, Martino MC, Veenhof E, Monteiro MA, Maaskant JJ, Negrini R, Lindh F, Perry M, Del Giudice G, Vandenbroucke-Grauls CM. Phase variation in H type I and Lewis a epitopes of Helicobacter pylori lipopolysaccharide. Infect Immun. 2000;68:5928-5932. [PubMed] [Cited in This Article: ] |
| 113. | Wang G, Rasko DA, Sherburne R, Taylor DE. Molecular genetic basis for the variable expression of Lewis Y antigen in Helicobacter pylori: analysis of the alpha (1,2) fucosyltransferase gene. Mol Microbiol. 1999;31:1265-1274. [PubMed] [Cited in This Article: ] |
| 114. | Berg DE, Hoffman PS, Appelmelk BJ, Kusters JG. The Helicobacter pylori genome sequence: genetic factors for long life in the gastric mucosa. Trends Microbiol. 1997;5:468-474. [PubMed] [Cited in This Article: ] |
| 115. | Saunders NJ, Peden JF, Hood DW, Moxon ER. Simple sequence repeats in the Helicobacter pylori genome. Mol Microbiol. 1998;27:1091-1098. [PubMed] [Cited in This Article: ] |
| 116. | Tannaes T, Dekker N, Bukholm G, Bijlsma JJ, Appelmelk BJ. Phase variation in the Helicobacter pylori phospholipase A gene and its role in acid adaptation. Infect Immun. 2001;69:7334-7340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 117. | Appelmelk BJ, Martin SL, Monteiro MA, Clayton CA, McColm AA, Zheng P, Verboom T, Maaskant JJ, van den Eijnden DH, Hokke CH. Phase variation in Helicobacter pylori lipopolysaccharide due to changes in the lengths of poly(C) tracts in alpha3-fucosyltransferase genes. Infect Immun. 1999;67:5361-5366. [PubMed] [Cited in This Article: ] |
| 118. | Amano K, Hayashi S, Kubota T, Fujii N, Yokota S. Reactivities of Lewis antigen monoclonal antibodies with the lipopolysaccharides of Helicobacter pylori strains isolated from patients with gastroduodenal diseases in Japan. Clin Diagn Lab Immunol. 1997;4:540-544. [PubMed] [Cited in This Article: ] |
| 119. | Borén T, Falk P, Roth KA, Larson G, Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993;262:1892-1895. [PubMed] [Cited in This Article: ] |
| 120. | Gerhard M, Lehn N, Neumayer N, Borén T, Rad R, Schepp W, Miehlke S, Classen M, Prinz C. Clinical relevance of the Helicobacter pylori gene for blood-group antigen-binding adhesin. Proc Natl Acad Sci USA. 1999;96:12778-12783. [PubMed] [Cited in This Article: ] |
| 121. | Edwards NJ, Monteiro MA, Faller G, Walsh EJ, Moran AP, Roberts IS, High NJ. Lewis X structures in the O antigen side-chain promote adhesion of Helicobacter pylori to the gastric epithelium. Mol Microbiol. 2000;35:1530-1539. [PubMed] [Cited in This Article: ] |
| 122. | Chmiela M, Jurkiewicz M, Wiśniewska M, Czkwianianc E, Płaneta-Małecka I, Rechciński T, Rudnicka W. Anti-Lewis X IgM and IgG in H. pylori infections in children and adults. Acta Microbiol Pol. 1999;48:277-281. [PubMed] [Cited in This Article: ] |
| 123. | Chmiela M, Wadstrom T, Folkesson H, Płaneta Małecka I, Czkwianianc E, Rechciński T, Rudnicka W. Anti-Lewis X antibody and Lewis X-anti-Lewis X immune complexes in Helicobacter pylori infection. Immunol Lett. 1998;61:119-125. [PubMed] [Cited in This Article: ] |
| 124. | Hirota K, Kanitani H, Nemoto K, Ono T, Miyake Y. Cross-reactivity between human sialyl Lewis(x) oligosaccharide and common causative oral bacteria of infective endocarditis. FEMS Immunol Med Microbiol. 1995;12:159-164. [PubMed] [Cited in This Article: ] |
| 125. | Chmiela M, Miszczyk E, Rudnicka K. Structural modifications of Helicobacter pylori lipopolysaccharide: an idea for how to live in peace. World J Gastroenterol. 2014;20:9882-9897. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 38] [Cited by in F6Publishing: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 126. | Wirth HP, Yang M, Karita M, Blaser MJ. Expression of the human cell surface glycoconjugates Lewis x and Lewis y by Helicobacter pylori isolates is related to cagA status. Infect Immun. 1996;64:4598-4605. [PubMed] [Cited in This Article: ] |
| 127. | Zheng PY, Hua J, Yeoh KG, Ho B. Association of peptic ulcer with increased expression of Lewis antigens but not cagA, iceA, and vacA in Helicobacter pylori isolates in an Asian population. Gut. 2000;47:18-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 70] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 128. | Gasbarrini A, Franceschi F, Tartaglione R, Landolfi R, Pola P, Gasbarrini G. Regression of autoimmune thrombocytopenia after eradication of Helicobacter pylori. Lancet. 1998;352:878. [PubMed] [Cited in This Article: ] |
| 129. | Takahashi T, Yujiri T, Shinohara K, Inoue Y, Sato Y, Fujii Y, Okubo M, Zaitsu Y, Ariyoshi K, Nakamura Y. Molecular mimicry by Helicobacter pylori CagA protein may be involved in the pathogenesis of H. pylori-associated chronic idiopathic thrombocytopenic purpura. Br J Haematol. 2004;124:91-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 204] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 130. | Stasi R, Provan D. Helicobacter pylori and Chronic ITP. Hematology Am Soc Hematol Educ Program. 2008;206-211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 131. | Cheng YS, Kuang LP, Zhuang CL, Jiang JD, Shi M. Effects of cytotoxin-associated gene A (CagA) positive Helicobacter pylori infection on anti-platelet glycoprotein antibody producing B cells in patients with primary idiopathic thrombocytopenic purpura (ITP). Pak J Med Sci. 2015;31:121-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 132. | Najaoui A, Bakchoul T, Stoy J, Bein G, Rummel MJ, Santoso S, Sachs UJ. Autoantibody-mediated complement activation on platelets is a common finding in patients with immune thrombocytopenic purpura (ITP). Eur J Haematol. 2012;88:167-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 133. | Johnsen J. Pathogenesis in immune thrombocytopenia: new insights. Hematology Am Soc Hematol Educ Program. 2012;2012:306-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 37] [Reference Citation Analysis (0)] |
| 134. | Byrne MF, Kerrigan SW, Corcoran PA, Atherton JC, Murray FE, Fitzgerald DJ, Cox DM. Helicobacter pylori binds von Willebrand factor and interacts with GPIb to induce platelet aggregation. Gastroenterology. 2003;124:1846-1854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 135. | Semple JW, Aslam R, Kim M, Speck ER, Freedman J. Platelet-bound lipopolysaccharide enhances Fc receptor-mediated phagocytosis of IgG-opsonized platelets. Blood. 2007;109:4803-4805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 136. | Kuwana M. Helicobacter pylori-associated immune thrombocytopenia: clinical features and pathogenic mechanisms. World J Gastroenterol. 2014;20:714-723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 53] [Cited by in F6Publishing: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 137. | Rožanković PB, Huzjan AL, Cupić H, Benčić IJ, Bašić S, Demarin V. Influence of CagA-positive Helicobacter pylori strains on atherosclerotic carotid disease. J Neurol. 2011;258:753-761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 138. | Sealy-Jefferson S, Gillespie BW, Aiello AE, Haan MN, Morgenstern LB, Lisabeth LD. Antibody levels to persistent pathogens and incident stroke in Mexican Americans. PLoS One. 2013;8:e65959. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 139. | Matusiak A, Chałubiński M, Broncel M, Rechciński T, Rudnicka K, Miszczyk E, Walencka M, Strapagiel D, Gajewski A, Chmiela M. Putative consequences of exposure to Helicobacter pylori infection in patients with coronary heart disease in terms of humoral immune response and inflammation. Arch Med Sci. 2016;12:45-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 140. | Khodaii Z, Vakili H, Ghaderian SM, Najar RA, Panah AS. Association of Helicobacter pylori infection with acute myocardial infarction. Coron Artery Dis. 2011;22:6-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 141. | Kowalski M, Rees W, Konturek PC, Grove R, Scheffold T, Meixner H, Brunec M, Franz N, Konturek JW, Pieniazek P. Detection of Helicobacter pylori specific DNA in human atheromatous coronary arteries and its association to prior myocardial infarction and unstable angina. Dig Liver Dis. 2002;34:398-402. [PubMed] [Cited in This Article: ] |
| 142. | Akbas HS, Basyigit S, Suleymanlar I, Kemaloglu D, Koc S, Davran F, Demir I, Suleymanlar G. The assessment of carotid intima media thickness and serum paraoxonase-1 activity in Helicobacter pylori positive subjects. Lipids Health Dis. 2010;9:92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 143. | Satoh H, Saijo Y, Yoshioka E, Tsutsui H. Helicobacter Pylori infection is a significant risk for modified lipid profile in Japanese male subjects. J Atheroscler Thromb. 2010;17:1041-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 144. | Wierzbicki AS. Homocysteine and cardiovascular disease: a review of the evidence. Diab Vasc Dis Res. 2007;4:143-150. [PubMed] [Cited in This Article: ] |
| 145. | Lazzerini PE, Capecchi PL, Selvi E, Lorenzini S, Bisogno S, Galeazzi M, Laghi Pasini F. Hyperhomocysteinemia: a cardiovascular risk factor in autoimmune diseases? Lupus. 2007;16:852-862. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 146. | Polyzos SA, Kountouras J, Zavos C, Deretzi G. The association between Helicobacter pylori infection and insulin resistance: a systematic review. Helicobacter. 2011;16:79-88. [PubMed] [Cited in This Article: ] |
| 147. | Christodoulou DK, Milionis HJ, Pappa P, Katsanos KH, Sigounas D, Florentin M, Elisaf M, Tsianos EV. Association of Helicobacter pylori infection with cardiovascular disease--is it just a myth? Eur J Intern Med. 2011;22:191-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 148. | George J, Afek A, Gilburd B, Harats D, Shoenfeld Y. Autoimmunity in atherosclerosis: lessons from experimental models. Lupus. 2000;9:223-227. [PubMed] [Cited in This Article: ] |
| 149. | Franceschi F, Sepulveda AR, Gasbarrini A, Pola P, Silveri NG, Gasbarrini G, Graham DY, Genta RM. Cross-reactivity of anti-CagA antibodies with vascular wall antigens: possible pathogenic link between Helicobacter pylori infection and atherosclerosis. Circulation. 2002;106:430-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 105] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 150. | Dunn BE, Phadnis SH. Structure, function and localization of Helicobacter pylori urease. Yale J Biol Med. 1998;71:63-73. [PubMed] [Cited in This Article: ] |
| 151. | Schoep TD, Fulurija A, Good F, Lu W, Himbeck RP, Schwan C, Choi SS, Berg DE, Mittl PR, Benghezal M. Surface properties of Helicobacter pylori urease complex are essential for persistence. PLoS One. 2010;5:e15042. [PubMed] [Cited in This Article: ] |
| 152. | Mobley HL, Island MD, Hausinger RP. Molecular biology of microbial ureases. Microbiol Rev. 1995;59:451-480. [PubMed] [Cited in This Article: ] |
| 153. | Mobley HL, Hausinger RP. Microbial ureases: significance, regulation, and molecular characterization. Microbiol Rev. 1989;53:85-108. [PubMed] [Cited in This Article: ] |
| 154. | Leal-Herrera Y, Torres J, Perez-Perez G, Gomez A, Monath T, Tapia-Conyer R, Muñoz O. Serologic IgG response to urease in Helicobacter pylori-infected persons from Mexico. Am J Trop Med Hyg. 1999;60:587-592. [PubMed] [Cited in This Article: ] |
| 155. | Burnie JP, al-Dughaym A. The application of epitope mapping in the development of a new serological test for Helicobacter pylori infection. J Immunol Methods. 1996;194:85-94. [PubMed] [Cited in This Article: ] |
| 156. | Futagami S, Takahashi H, Norose Y, Kobayashi M. Systemic and local immune responses against Helicobacter pylori urease in patients with chronic gastritis: distinct IgA and IgG productive sites. Gut. 1998;43:168-175. [PubMed] [Cited in This Article: ] |
| 157. | Konieczna I, Zarnowiec P, Kwinkowski M, Kolesinska B, Fraczyk J, Kaminski Z, Kaca W. Bacterial urease and its role in long-lasting human diseases. Curr Protein Pept Sci. 2012;13:789-806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 158. | Arabski M, Konieczna I, Sołowiej D, Rogoń A, Kolesińska B, Kamiński Z, Kaca W. Are anti-Helicobacter pylori urease antibodies involved in atherosclerotic diseases? Clin Biochem. 2010;43:115-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 159. | Kansau I, Labigne A. Heat shock proteins of Helicobacter pylori. Aliment Pharmacol Ther. 1996;10 Suppl 1:51-56. [PubMed] [Cited in This Article: ] |
| 160. | Ng EK, Thompson SA, Pérez-Pérez GI, Kansau I, van der Ende A, Labigne A, Sung JJ, Chung SC, Blaser MJ. Helicobacter pylori heat shock protein A: serologic responses and genetic diversity. Clin Diagn Lab Immunol. 1999;6:377-382. [PubMed] [Cited in This Article: ] |
| 161. | Birnie DH, Holme ER, McKay IC, Hood S, McColl KE, Hillis WS. Association between antibodies to heat shock protein 65 and coronary atherosclerosis. Possible mechanism of action of Helicobacter pylori and other bacterial infections in increasing cardiovascular risk. Eur Heart J. 1998;19:387-394. [PubMed] [Cited in This Article: ] |
| 162. | Pockley AG, De Faire U, Kiessling R, Lemne C, Thulin T, Frostegård J. Circulating heat shock protein and heat shock protein antibody levels in established hypertension. J Hypertens. 2002;20:1815-1820. [PubMed] [Cited in This Article: ] |
| 163. | Eamranond PP, Torres J, Muñoz O, Pérez-Pérez GI. Age-specific immune response to HspA in Helicobacter pylori-positive persons in Mexico. Clin Diagn Lab Immunol. 2004;11:983-985. [PubMed] [Cited in This Article: ] |
| 164. | Frulloni L, Lunardi C, Simone R, Dolcino M, Scattolini C, Falconi M, Benini L, Vantini I, Corrocher R, Puccetti A. Identification of a novel antibody associated with autoimmune pancreatitis. N Engl J Med. 2009;361:2135-2142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 272] [Cited by in F6Publishing: 279] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 165. | Pacifico L, Osborn JF, Tromba V, Romaggioli S, Bascetta S, Chiesa C. Helicobacter pylori infection and extragastric disorders in children: a critical update. World J Gastroenterol. 2014;20:1379-1401. [PubMed] [DOI] [Cited in This Article: ] |
| 166. | Fetissov SO, Hamze Sinno M, Coëffier M, Bole-Feysot C, Ducrotté P, Hökfelt T, Déchelotte P. Autoantibodies against appetite-regulating peptide hormones and neuropeptides: putative modulation by gut microflora. Nutrition. 2008;24:348-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 167. | Paoluzi OA, Blanco del VG, Caruso R, Monteleone I, Monteleone G, Pallone F. Impairment of ghrelin synthesis in Helicobacter pylori-colonized stomach: new clues for the pathogenesis of H. pylori-related gastric inflammation. World J Gastroenterol. 2014;20:639-646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 20] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 168. | Roper J, Francois F, Shue PL, Mourad MS, Pei Z, Olivares de Perez AZ, Perez-Perez GI, Tseng CH, Blaser MJ. Leptin and ghrelin in relation to Helicobacter pylori status in adult males. J Clin Endocrinol Metab. 2008;93:2350-2357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 169. | Plonka M, Bielanski W, Konturek SJ, Targosz A, Sliwowski Z, Dobrzanska M, Kaminska A, Sito E, Konturek PC, Brzozowski T. Helicobacter pylori infection and serum gastrin, ghrelin and leptin in children of Polish shepherds. Dig Liver Dis. 2006;38:91-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 170. | Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86:4753-4758. [PubMed] [Cited in This Article: ] |
| 171. | Konturek PC, Brzozowski T, Pajdo R, Nikiforuk A, Kwiecien S, Harsch I, Drozdowicz D, Hahn EG, Konturek SJ. Ghrelin-a new gastroprotective factor in gastric mucosa. J Physiol Pharmacol. 2004;55:325-336. [PubMed] [Cited in This Article: ] |
| 172. | Kusminski CM, McTernan PG, Kumar S. Role of resistin in obesity, insulin resistance and Type II diabetes. Clin Sci (Lond). 2005;109:243-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 203] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 173. | Al-Suhaimi EA, Shehzad A. Leptin, resistin and visfatin: the missing link between endocrine metabolic disorders and immunity. Eur J Med Res. 2013;18:12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 174. | Stawerska R, Czkwianianc E, Matusiak A, Smyczyńska J, Hilczer M, Chmiela M, Lewiński A. Assessment of ghrelin, leptin, orexin A and alpha-MSH serum concentrations and the levels of the autoantibodies against the aforementioned peptides in relation to Helicobacter pylori infections and Candida albicans colonization in children with short stature. Pediatr Endocrinol Diabetes Metab. 2016;21:102-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 175. | Stawerska R, Czkwianianc E, Matusiak A, Smyczyńska J, Hilczer M, Chmiela M, Lewiński A. Prevalence of autoantibodies against some selected growth and appetite-regulating neuropeptides in serum of short children exposed to Candida albicans colonization and/or Helicobacter pylori infection: the molecular mimicry phenomenon. Neuro Endocrinol Lett. 2015;36:458-464. [PubMed] [Cited in This Article: ] |
| 176. | Buzzelli JN, Chalinor HV, Pavlic DI, Sutton P, Menheniott TR, Giraud AS, Judd LM. IL33 Is a Stomach Alarmin That Initiates a Skewed Th2 Response to Injury and Infection. Cell Mol Gastroenterol Hepatol. 2015;1:203-221.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 177. | Pei C, Barbour M, Fairlie-Clarke KJ, Allan D, Mu R, Jiang HR. Emerging role of interleukin-33 in autoimmune diseases. Immunology. 2014;141:9-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |